 Found 2362 hits with Last Name = 'chen' and Initial = 'ch'
Found 2362 hits with Last Name = 'chen' and Initial = 'ch' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aurora kinase A
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His6-tagged Aurora A (1 to 403 residues) (unknown origin) expressed in baculovirus expression system |
Eur J Med Chem 124: 186-199 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.026
BindingDB Entry DOI: 10.7270/Q2668G5M |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His6-tagged Aurora C (1 to 309 residues) (unknown origin) expressed in baculovirus expression system |
Eur J Med Chem 124: 186-199 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.026
BindingDB Entry DOI: 10.7270/Q2668G5M |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50004205

(MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)[nH]n2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His6-tagged Aurora B (62 to 344 residues) (unknown origin) expressed in baculovirus expression system |
Eur J Med Chem 124: 186-199 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.026
BindingDB Entry DOI: 10.7270/Q2668G5M |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50448790
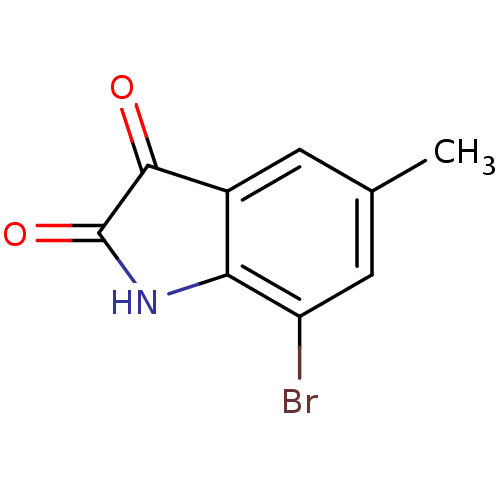
(CHEMBL3128208)Show InChI InChI=1S/C9H6BrNO2/c1-4-2-5-7(6(10)3-4)11-9(13)8(5)12/h2-3H,1H3,(H,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Noncompetitive/mixed type inhibition of human ALDH1A1 by Lineweaver-Burk plot analysis in presence of NAD+ |
J Med Chem 57: 714-22 (2014)
Article DOI: 10.1021/jm401377v
BindingDB Entry DOI: 10.7270/Q21V5GF2 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase, dimeric NADP-preferring
(Homo sapiens (Human)) | BDBM50448790

(CHEMBL3128208)Show InChI InChI=1S/C9H6BrNO2/c1-4-2-5-7(6(10)3-4)11-9(13)8(5)12/h2-3H,1H3,(H,11,12,13) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH3A1 using benzaldehyde as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 714-22 (2014)
Article DOI: 10.1021/jm401377v
BindingDB Entry DOI: 10.7270/Q21V5GF2 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase, dimeric NADP-preferring
(Homo sapiens (Human)) | BDBM50448790

(CHEMBL3128208)Show InChI InChI=1S/C9H6BrNO2/c1-4-2-5-7(6(10)3-4)11-9(13)8(5)12/h2-3H,1H3,(H,11,12,13) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH3A1 by Lineweaver-Burk plot analysis in presence of NADP+ |
J Med Chem 57: 714-22 (2014)
Article DOI: 10.1021/jm401377v
BindingDB Entry DOI: 10.7270/Q21V5GF2 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50448790

(CHEMBL3128208)Show InChI InChI=1S/C9H6BrNO2/c1-4-2-5-7(6(10)3-4)11-9(13)8(5)12/h2-3H,1H3,(H,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of human ALDH1A1 using propionaldehyde as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 714-22 (2014)
Article DOI: 10.1021/jm401377v
BindingDB Entry DOI: 10.7270/Q21V5GF2 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase, mitochondrial
(Homo sapiens (Human)) | BDBM50448790

(CHEMBL3128208)Show InChI InChI=1S/C9H6BrNO2/c1-4-2-5-7(6(10)3-4)11-9(13)8(5)12/h2-3H,1H3,(H,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH2 using propionaldehyde as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 714-22 (2014)
Article DOI: 10.1021/jm401377v
BindingDB Entry DOI: 10.7270/Q21V5GF2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldehyde dehydrogenase, dimeric NADP-preferring
(Homo sapiens (Human)) | BDBM22794

(1-benzyl-2,3-dihydro-1H-indole-2,3-dione | Isatin-...)Show InChI InChI=1S/C15H11NO2/c17-14-12-8-4-5-9-13(12)16(15(14)18)10-11-6-2-1-3-7-11/h1-9H,10H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH3A1 using benzaldehyde as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 714-22 (2014)
Article DOI: 10.1021/jm401377v
BindingDB Entry DOI: 10.7270/Q21V5GF2 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase, mitochondrial
(Homo sapiens (Human)) | BDBM50448790

(CHEMBL3128208)Show InChI InChI=1S/C9H6BrNO2/c1-4-2-5-7(6(10)3-4)11-9(13)8(5)12/h2-3H,1H3,(H,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH2 by Lineweaver-Burk plot analysis in presence of NAD+ |
J Med Chem 57: 714-22 (2014)
Article DOI: 10.1021/jm401377v
BindingDB Entry DOI: 10.7270/Q21V5GF2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldehyde dehydrogenase, dimeric NADP-preferring
(Homo sapiens (Human)) | BDBM22794

(1-benzyl-2,3-dihydro-1H-indole-2,3-dione | Isatin-...)Show InChI InChI=1S/C15H11NO2/c17-14-12-8-4-5-9-13(12)16(15(14)18)10-11-6-2-1-3-7-11/h1-9H,10H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH3A1 by Lineweaver-Burk plot analysis in presence of NADP+ |
J Med Chem 57: 714-22 (2014)
Article DOI: 10.1021/jm401377v
BindingDB Entry DOI: 10.7270/Q21V5GF2 |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM26658
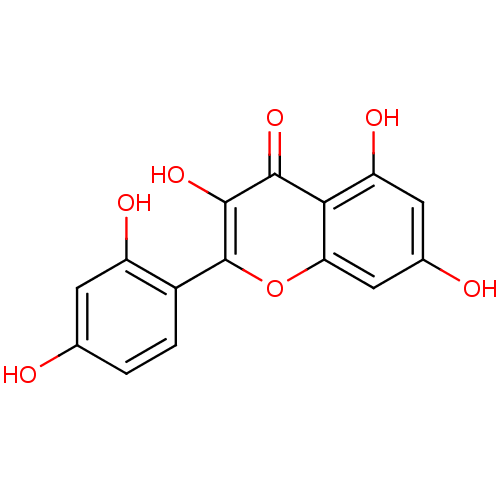
(2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...)Show InChI InChI=1S/C15H10O7/c16-6-1-2-8(9(18)3-6)15-14(21)13(20)12-10(19)4-7(17)5-11(12)22-15/h1-5,16-19,21H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Chinese University of Hong Kong
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1-mediated urate uptake in HEK293 cells by competitive inhibition assay |
Drug Metab Dispos 35: 981-6 (2007)
Article DOI: 10.1124/dmd.106.012187
BindingDB Entry DOI: 10.7270/Q2KK9CPP |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50292544

(3,4,6,7-tetrahydroxyxanthone | CHEMBL477740)Show InChI InChI=1S/C13H8O6/c14-7-2-1-5-11(17)6-3-8(15)9(16)4-10(6)19-13(5)12(7)18/h1-4,14-16,18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ACE by Lineweaver-Burk plot |
J Nat Prod 55: 691-695 (1992)
Article DOI: 10.1021/np50083a025
BindingDB Entry DOI: 10.7270/Q21C1WXZ |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase, mitochondrial
(Homo sapiens (Human)) | BDBM22794

(1-benzyl-2,3-dihydro-1H-indole-2,3-dione | Isatin-...)Show InChI InChI=1S/C15H11NO2/c17-14-12-8-4-5-9-13(12)16(15(14)18)10-11-6-2-1-3-7-11/h1-9H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of human ALDH2 using propionaldehyde as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 714-22 (2014)
Article DOI: 10.1021/jm401377v
BindingDB Entry DOI: 10.7270/Q21V5GF2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldehyde dehydrogenase, mitochondrial
(Homo sapiens (Human)) | BDBM22794

(1-benzyl-2,3-dihydro-1H-indole-2,3-dione | Isatin-...)Show InChI InChI=1S/C15H11NO2/c17-14-12-8-4-5-9-13(12)16(15(14)18)10-11-6-2-1-3-7-11/h1-9H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Noncompetitive/mixed type inhibition of human ALDH2 by Lineweaver-Burk plot analysis in presence of NAD+ |
J Med Chem 57: 714-22 (2014)
Article DOI: 10.1021/jm401377v
BindingDB Entry DOI: 10.7270/Q21V5GF2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50292547

(1,3,5,6-tetrahydroxyxanthone | 1,3,5,6-tetrahydrox...)Show InChI InChI=1S/C13H8O6/c14-5-3-8(16)10-9(4-5)19-13-6(11(10)17)1-2-7(15)12(13)18/h1-4,14-16,18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ACE by Lineweaver-Burk plot |
J Nat Prod 55: 691-695 (1992)
Article DOI: 10.1021/np50083a025
BindingDB Entry DOI: 10.7270/Q21C1WXZ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50292546

(3,4,5,6-tetrahydroxyxanthone | CHEMBL477921 | US91...)Show InChI InChI=1S/C13H8O6/c14-7-3-1-5-9(16)6-2-4-8(15)11(18)13(6)19-12(5)10(7)17/h1-4,14-15,17-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ACE by Lineweaver-Burk plot |
J Nat Prod 55: 691-695 (1992)
Article DOI: 10.1021/np50083a025
BindingDB Entry DOI: 10.7270/Q21C1WXZ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50155447
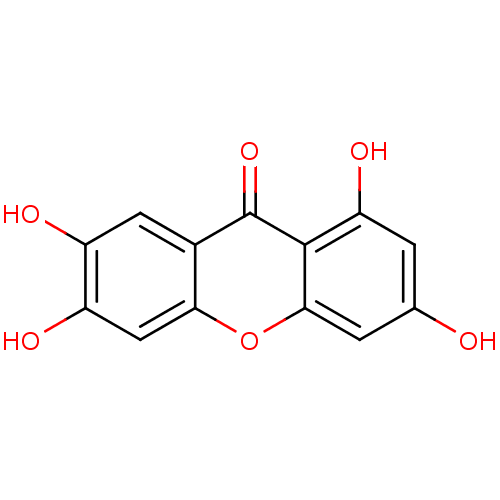
(1,3,6,7-Tetrahydroxy-xanthen-9-one | 1,3,6,7-tetra...)Show InChI InChI=1S/C13H8O6/c14-5-1-9(17)12-11(2-5)19-10-4-8(16)7(15)3-6(10)13(12)18/h1-4,14-17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of ACE by Lineweaver-Burk plot |
J Nat Prod 55: 691-695 (1992)
Article DOI: 10.1021/np50083a025
BindingDB Entry DOI: 10.7270/Q21C1WXZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50530623
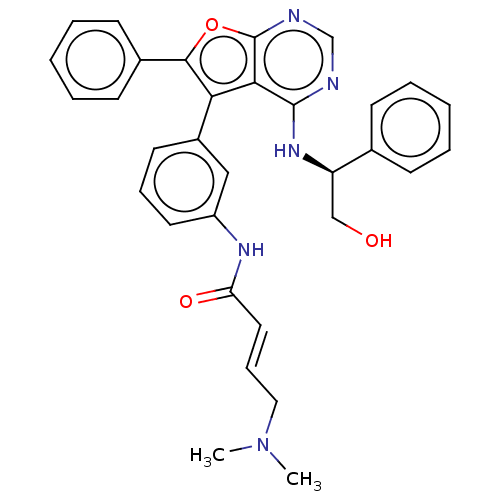
(CHEMBL4521381)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)-c1c(oc2ncnc(N[C@H](CO)c3ccccc3)c12)-c1ccccc1 |r| Show InChI InChI=1S/C32H31N5O3/c1-37(2)18-10-17-27(39)35-25-16-9-15-24(19-25)28-29-31(36-26(20-38)22-11-5-3-6-12-22)33-21-34-32(29)40-30(28)23-13-7-4-8-14-23/h3-17,19,21,26,38H,18,20H2,1-2H3,(H,35,39)(H,33,34,36)/b17-10+/t26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50530623

(CHEMBL4521381)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)-c1c(oc2ncnc(N[C@H](CO)c3ccccc3)c12)-c1ccccc1 |r| Show InChI InChI=1S/C32H31N5O3/c1-37(2)18-10-17-27(39)35-25-16-9-15-24(19-25)28-29-31(36-26(20-38)22-11-5-3-6-12-22)33-21-34-32(29)40-30(28)23-13-7-4-8-14-23/h3-17,19,21,26,38H,18,20H2,1-2H3,(H,35,39)(H,33,34,36)/b17-10+/t26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50175305
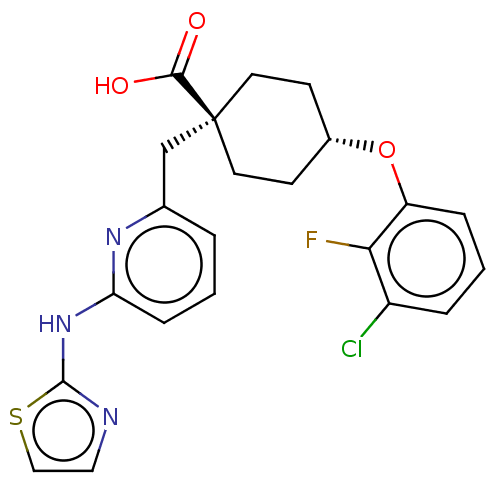
(CHEMBL3600873)Show SMILES OC(=O)[C@@]1(Cc2cccc(Nc3nccs3)n2)CC[C@@H](CC1)Oc1cccc(Cl)c1F |r,wU:3.2,wD:19.24,(2.4,1.65,;1.33,2.27,;1.34,3.5,;,1.54,;-1.33,2.27,;-2.67,1.5,;-2.64,-.04,;-3.96,-.84,;-5.31,-.09,;-5.34,1.45,;-6.69,2.19,;-8,1.39,;-8.11,-.13,;-9.61,-.48,;-10.41,.83,;-9.4,2,;-4.02,2.24,;1.33,.77,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;0,-3.08,;1.34,-3.85,;2.67,-3.07,;4.01,-3.84,;4.01,-5.38,;2.68,-6.15,;2.68,-7.39,;1.34,-5.39,;.28,-6.01,)| Show InChI InChI=1S/C22H21ClFN3O3S/c23-16-4-2-5-17(19(16)24)30-15-7-9-22(10-8-15,20(28)29)13-14-3-1-6-18(26-14)27-21-25-11-12-31-21/h1-6,11-12,15H,7-10,13H2,(H,28,29)(H,25,26,27)/t15-,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged Aurora A expressed in Escherichia coli using RRR(GLRRASLG)4R-NH2 as substrate after 40 mins in presence of... |
Eur J Med Chem 124: 186-199 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.026
BindingDB Entry DOI: 10.7270/Q2668G5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50468247
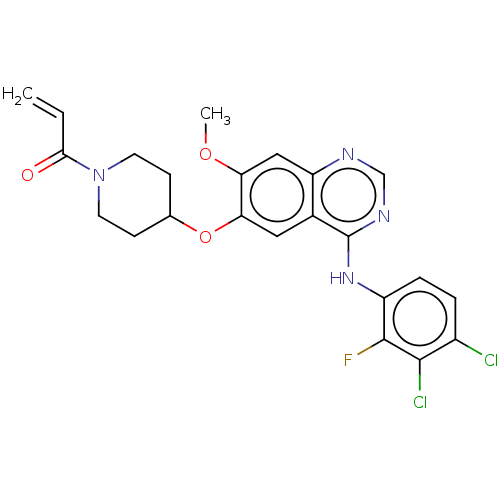
(HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...)Show SMILES COc1cc2ncnc(Nc3ccc(Cl)c(Cl)c3F)c2cc1OC1CCN(CC1)C(=O)C=C Show InChI InChI=1S/C23H21Cl2FN4O3/c1-3-20(31)30-8-6-13(7-9-30)33-19-10-14-17(11-18(19)32-2)27-12-28-23(14)29-16-5-4-15(24)21(25)22(16)26/h3-5,10-13H,1,6-9H2,2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50468247

(HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...)Show SMILES COc1cc2ncnc(Nc3ccc(Cl)c(Cl)c3F)c2cc1OC1CCN(CC1)C(=O)C=C Show InChI InChI=1S/C23H21Cl2FN4O3/c1-3-20(31)30-8-6-13(7-9-30)33-19-10-14-17(11-18(19)32-2)27-12-28-23(14)29-16-5-4-15(24)21(25)22(16)26/h3-5,10-13H,1,6-9H2,2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519062

(CHEMBL4442196)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](OC(C)=O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:41:57.58:38,THB:62:43:57.58:38,45:43:57.58:38,56:57:43.41.44:38,32:37:45.57.56:41| Show InChI InChI=1S/C46H56O13/c1-25(2)42-22-31(23-53-39(49)29-17-11-8-12-18-29)45-34-37(42)57-46(58-42,59-45)32(54-28(5)48)21-15-7-6-10-16-26(3)33-27(4)36(55-40(50)30-19-13-9-14-20-30)44(52,35(33)45)41(51)43(24-47)38(34)56-43/h8-9,11-14,17-20,26-27,31-38,41,47,51-52H,1,6-7,10,15-16,21-24H2,2-5H3/t26-,27+,31+,32-,33+,34-,35-,36+,37-,38+,41-,42-,43+,44-,45-,46?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50206389
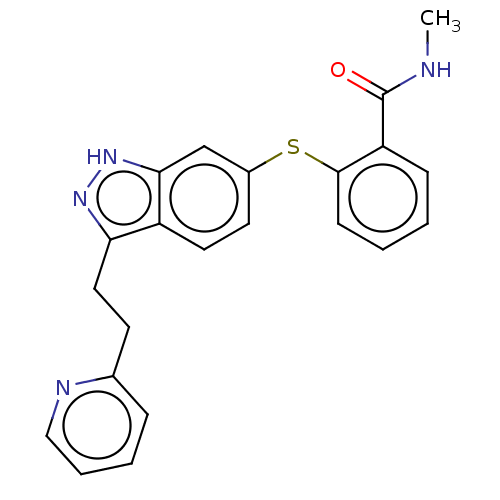
(CHEMBL3939307)Show SMILES CNC(=O)c1ccccc1Sc1ccc2c(CCc3ccccn3)n[nH]c2c1 Show InChI InChI=1S/C22H20N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-8,10-11,13-14H,9,12H2,1H3,(H,23,27)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR3 (unknown origin) |
Eur J Med Chem 124: 186-199 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.026
BindingDB Entry DOI: 10.7270/Q2668G5M |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50206389

(CHEMBL3939307)Show SMILES CNC(=O)c1ccccc1Sc1ccc2c(CCc3ccccn3)n[nH]c2c1 Show InChI InChI=1S/C22H20N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-8,10-11,13-14H,9,12H2,1H3,(H,23,27)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR1 (unknown origin) |
Eur J Med Chem 124: 186-199 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.026
BindingDB Entry DOI: 10.7270/Q2668G5M |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519087
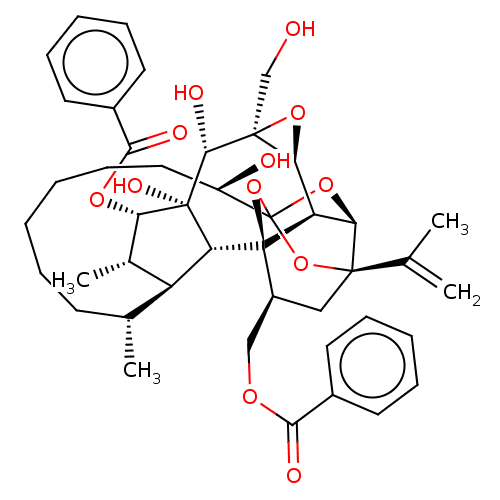
(CHEMBL3741746)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:38:35:54.55,THB:55:34:38:54.53.42,32:34:38:54.53.42,53:54:40.38.41:35,42:40:35:54.55,56:54:40.38.41:35| Show InChI InChI=1S/C44H54O12/c1-24(2)40-21-29(22-51-37(47)27-16-10-7-11-17-27)43-32-35(40)54-44(55-40,56-43)30(46)20-14-6-5-9-15-25(3)31-26(4)34(52-38(48)28-18-12-8-13-19-28)42(50,33(31)43)39(49)41(23-45)36(32)53-41/h7-8,10-13,16-19,25-26,29-36,39,45-46,49-50H,1,5-6,9,14-15,20-23H2,2-4H3/t25-,26+,29+,30-,31+,32-,33-,34+,35-,36+,39-,40-,41+,42-,43-,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50530623

(CHEMBL4521381)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)-c1c(oc2ncnc(N[C@H](CO)c3ccccc3)c12)-c1ccccc1 |r| Show InChI InChI=1S/C32H31N5O3/c1-37(2)18-10-17-27(39)35-25-16-9-15-24(19-25)28-29-31(36-26(20-38)22-11-5-3-6-12-22)33-21-34-32(29)40-30(28)23-13-7-4-8-14-23/h3-17,19,21,26,38H,18,20H2,1-2H3,(H,35,39)(H,33,34,36)/b17-10+/t26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50530623

(CHEMBL4521381)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)-c1c(oc2ncnc(N[C@H](CO)c3ccccc3)c12)-c1ccccc1 |r| Show InChI InChI=1S/C32H31N5O3/c1-37(2)18-10-17-27(39)35-25-16-9-15-24(19-25)28-29-31(36-26(20-38)22-11-5-3-6-12-22)33-21-34-32(29)40-30(28)23-13-7-4-8-14-23/h3-17,19,21,26,38H,18,20H2,1-2H3,(H,35,39)(H,33,34,36)/b17-10+/t26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519068
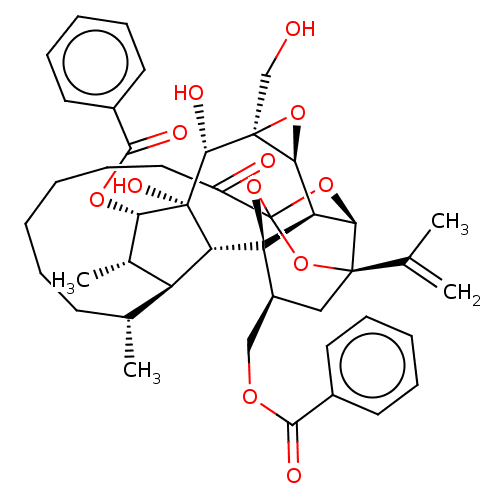
(CHEMBL4575056)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCCC(=O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:38:54.55:35,THB:59:40:54.55:35,42:40:54.55:35,53:54:40.38.41:35,32:34:42.54.53:38| Show InChI InChI=1S/C44H52O12/c1-24(2)40-21-29(22-51-37(47)27-16-10-7-11-17-27)43-32-35(40)54-44(55-40,56-43)30(46)20-14-6-5-9-15-25(3)31-26(4)34(52-38(48)28-18-12-8-13-19-28)42(50,33(31)43)39(49)41(23-45)36(32)53-41/h7-8,10-13,16-19,25-26,29,31-36,39,45,49-50H,1,5-6,9,14-15,20-23H2,2-4H3/t25-,26+,29+,31+,32-,33-,34+,35-,36+,39-,40-,41+,42-,43-,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50530618
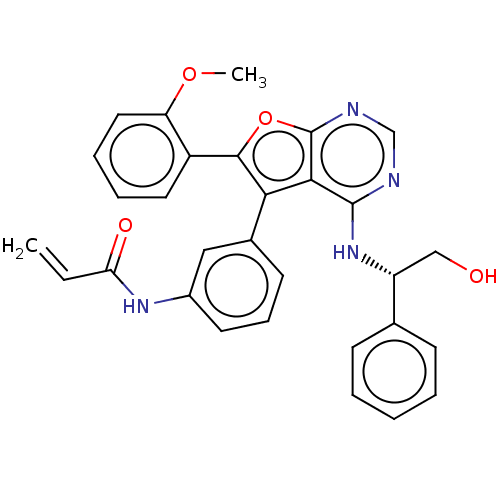
(CHEMBL4559807)Show SMILES COc1ccccc1-c1oc2ncnc(N[C@H](CO)c3ccccc3)c2c1-c1cccc(NC(=O)C=C)c1 |r| Show InChI InChI=1S/C30H26N4O4/c1-3-25(36)33-21-13-9-12-20(16-21)26-27-29(34-23(17-35)19-10-5-4-6-11-19)31-18-32-30(27)38-28(26)22-14-7-8-15-24(22)37-2/h3-16,18,23,35H,1,17H2,2H3,(H,33,36)(H,31,32,34)/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50530618

(CHEMBL4559807)Show SMILES COc1ccccc1-c1oc2ncnc(N[C@H](CO)c3ccccc3)c2c1-c1cccc(NC(=O)C=C)c1 |r| Show InChI InChI=1S/C30H26N4O4/c1-3-25(36)33-21-13-9-12-20(16-21)26-27-29(34-23(17-35)19-10-5-4-6-11-19)31-18-32-30(27)38-28(26)22-14-7-8-15-24(22)37-2/h3-16,18,23,35H,1,17H2,2H3,(H,33,36)(H,31,32,34)/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519060
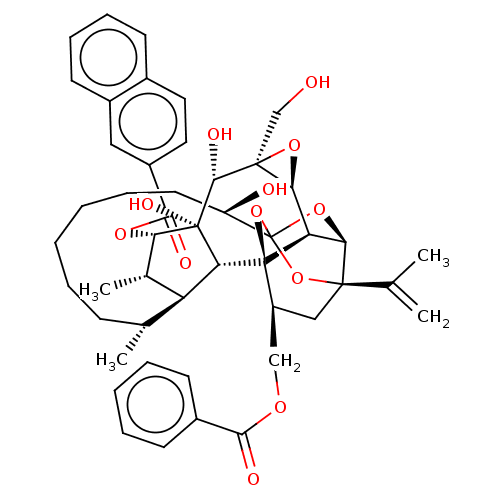
(CHEMBL4528495)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccc4ccccc4c3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:42:58.59:39,THB:63:44:58.59:39,36:38:46.58.57:42,46:44:58.59:39,57:58:44.42.45:39| Show InChI InChI=1S/C48H56O12/c1-26(2)44-23-33(24-55-41(51)30-16-9-7-10-17-30)47-36-39(44)58-48(59-44,60-47)34(50)19-11-6-5-8-14-27(3)35-28(4)38(46(54,37(35)47)43(53)45(25-49)40(36)57-45)56-42(52)32-21-20-29-15-12-13-18-31(29)22-32/h7,9-10,12-13,15-18,20-22,27-28,33-40,43,49-50,53-54H,1,5-6,8,11,14,19,23-25H2,2-4H3/t27-,28+,33+,34-,35+,36-,37-,38+,39-,40+,43-,44-,45+,46-,47-,48?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50235402
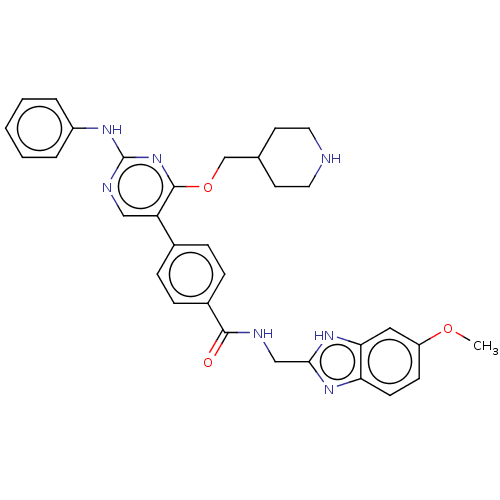
(CHEMBL4077934)Show SMILES COc1ccc2nc(CNC(=O)c3ccc(cc3)-c3cnc(Nc4ccccc4)nc3OCC3CCNCC3)[nH]c2c1 Show InChI InChI=1S/C32H33N7O3/c1-41-25-11-12-27-28(17-25)38-29(37-27)19-34-30(40)23-9-7-22(8-10-23)26-18-35-32(36-24-5-3-2-4-6-24)39-31(26)42-20-21-13-15-33-16-14-21/h2-12,17-18,21,33H,13-16,19-20H2,1H3,(H,34,40)(H,37,38)(H,35,36,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK (unknown origin) containing kinase domain and UBA domain using STK S1 as substrate after 20 mins by HTRF assay |
J Med Chem 60: 2155-2161 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00033
BindingDB Entry DOI: 10.7270/Q2MC928T |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519083

(CHEMBL4538431)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccc(OC)cc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:40:56.57:37,THB:61:42:56.57:37,44:42:56.57:37,55:56:42.40.43:37,34:36:44.56.55:40| Show InChI InChI=1S/C45H56O13/c1-24(2)41-21-29(22-53-38(48)27-14-10-8-11-15-27)44-33-36(41)56-45(57-41,58-44)31(47)16-12-7-6-9-13-25(3)32-26(4)35(54-39(49)28-17-19-30(52-5)20-18-28)43(51,34(32)44)40(50)42(23-46)37(33)55-42/h8,10-11,14-15,17-20,25-26,29,31-37,40,46-47,50-51H,1,6-7,9,12-13,16,21-23H2,2-5H3/t25-,26+,29+,31-,32+,33-,34-,35+,36-,37+,40-,41-,42+,43-,44-,45?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519084
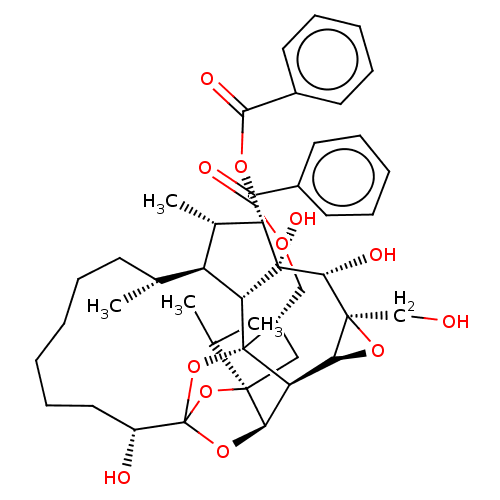
(CHEMBL4443190)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccccc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)C)[C@@]13[H] |r,TLB:1:38:54.55:35,THB:59:40:54.55:35,42:40:54.55:35,53:54:40.38.41:35,32:34:42.54.53:38| Show InChI InChI=1S/C44H56O12/c1-24(2)40-21-29(22-51-37(47)27-16-10-7-11-17-27)43-32-35(40)54-44(55-40,56-43)30(46)20-14-6-5-9-15-25(3)31-26(4)34(52-38(48)28-18-12-8-13-19-28)42(50,33(31)43)39(49)41(23-45)36(32)53-41/h7-8,10-13,16-19,24-26,29-36,39,45-46,49-50H,5-6,9,14-15,20-23H2,1-4H3/t25-,26+,29+,30-,31+,32-,33-,34+,35-,36+,39-,40-,41+,42-,43-,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50206389

(CHEMBL3939307)Show SMILES CNC(=O)c1ccccc1Sc1ccc2c(CCc3ccccn3)n[nH]c2c1 Show InChI InChI=1S/C22H20N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-8,10-11,13-14H,9,12H2,1H3,(H,23,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiwan National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
Eur J Med Chem 124: 186-199 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.026
BindingDB Entry DOI: 10.7270/Q2668G5M |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50321894
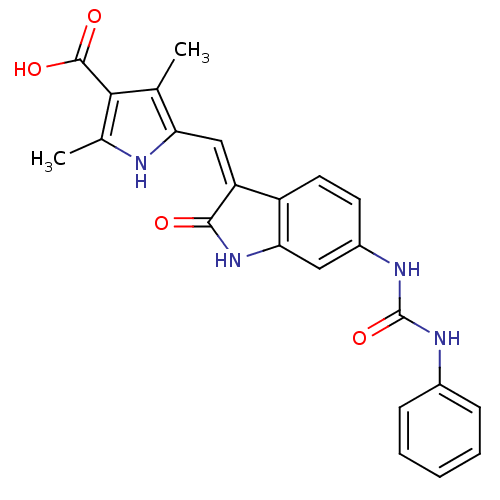
((Z)-2,4-Dimethyl-5-[2-oxo-6-(3-phenylureido)-1,2-d...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3cc(NC(=O)Nc4ccccc4)ccc23)c(C)c1C(O)=O Show InChI InChI=1S/C23H20N4O4/c1-12-18(24-13(2)20(12)22(29)30)11-17-16-9-8-15(10-19(16)27-21(17)28)26-23(31)25-14-6-4-3-5-7-14/h3-11,24H,1-2H3,(H,27,28)(H,29,30)(H2,25,26,31)/b17-11- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50530622

(CHEMBL4514452)Show SMILES C[C@@H](Nc1ncnc2oc(c(-c3cccc(NC(=O)C=C)c3)c12)-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C29H24N4O2/c1-3-24(34)33-23-16-10-15-22(17-23)25-26-28(32-19(2)20-11-6-4-7-12-20)30-18-31-29(26)35-27(25)21-13-8-5-9-14-21/h3-19H,1H2,2H3,(H,33,34)(H,30,31,32)/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50530622

(CHEMBL4514452)Show SMILES C[C@@H](Nc1ncnc2oc(c(-c3cccc(NC(=O)C=C)c3)c12)-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C29H24N4O2/c1-3-24(34)33-23-16-10-15-22(17-23)25-26-28(32-19(2)20-11-6-4-7-12-20)30-18-31-29(26)35-27(25)21-13-8-5-9-14-21/h3-19H,1H2,2H3,(H,33,34)(H,30,31,32)/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50468247

(HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...)Show SMILES COc1cc2ncnc(Nc3ccc(Cl)c(Cl)c3F)c2cc1OC1CCN(CC1)C(=O)C=C Show InChI InChI=1S/C23H21Cl2FN4O3/c1-3-20(31)30-8-6-13(7-9-30)33-19-10-14-17(11-18(19)32-2)27-12-28-23(14)29-16-5-4-15(24)21(25)22(16)26/h3-5,10-13H,1,6-9H2,2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.218 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50468247

(HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...)Show SMILES COc1cc2ncnc(Nc3ccc(Cl)c(Cl)c3F)c2cc1OC1CCN(CC1)C(=O)C=C Show InChI InChI=1S/C23H21Cl2FN4O3/c1-3-20(31)30-8-6-13(7-9-30)33-19-10-14-17(11-18(19)32-2)27-12-28-23(14)29-16-5-4-15(24)21(25)22(16)26/h3-5,10-13H,1,6-9H2,2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.218 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519069
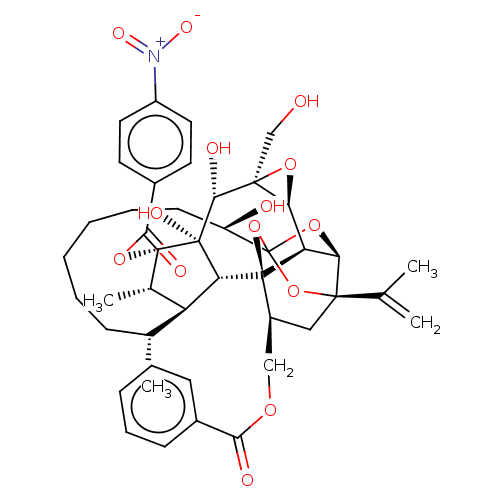
(CHEMBL4435580)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccc(cc3)[N+]([O-])=O)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:41:57.58:38,THB:62:43:57.58:38,45:43:57.58:38,56:57:43.41.44:38,35:37:45.57.56:41| Show InChI InChI=1S/C44H53NO14/c1-23(2)40-20-28(21-54-37(48)26-13-9-7-10-14-26)43-32-35(40)57-44(58-40,59-43)30(47)15-11-6-5-8-12-24(3)31-25(4)34(55-38(49)27-16-18-29(19-17-27)45(52)53)42(51,33(31)43)39(50)41(22-46)36(32)56-41/h7,9-10,13-14,16-19,24-25,28,30-36,39,46-47,50-51H,1,5-6,8,11-12,15,20-22H2,2-4H3/t24-,25+,28+,30-,31+,32-,33-,34+,35-,36+,39-,40-,41+,42-,43-,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50427934
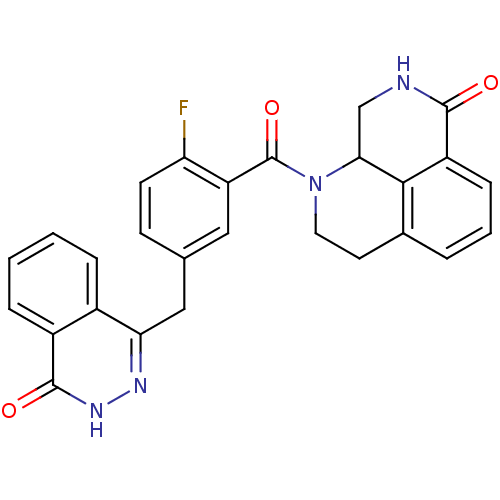
(CHEMBL2322618)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCc2cccc3C(=O)NCC1c23 Show InChI InChI=1S/C27H21FN4O3/c28-21-9-8-15(13-22-17-5-1-2-6-18(17)26(34)31-30-22)12-20(21)27(35)32-11-10-16-4-3-7-19-24(16)23(32)14-29-25(19)33/h1-9,12,23H,10-11,13-14H2,(H,29,33)(H,31,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 after 1 hr by ELISA |
J Med Chem 56: 2885-903 (2013)
Article DOI: 10.1021/jm301825t
BindingDB Entry DOI: 10.7270/Q26M385C |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50519063
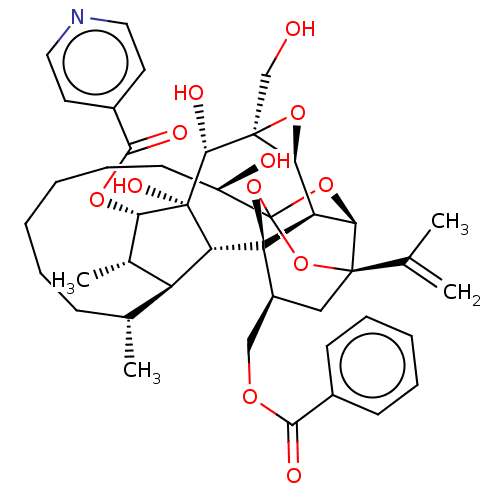
(CHEMBL4587471)Show SMILES [H][C@@]12O[C@]1(CO)[C@@H](O)[C@]1(O)[C@@H](OC(=O)c3ccncc3)[C@@H](C)[C@]3([H])[C@H](C)CCCCCC[C@@H](O)C45O[C@]6([H])[C@@]2([H])[C@](O4)([C@H](COC(=O)c2ccccc2)C[C@@]6(O5)C(C)=C)[C@@]13[H] |r,TLB:1:38:54.55:35,THB:59:40:54.55:35,42:40:54.55:35,53:54:40.38.41:35,32:34:42.54.53:38| Show InChI InChI=1S/C43H53NO12/c1-23(2)39-20-28(21-51-36(47)26-13-9-7-10-14-26)42-31-34(39)54-43(55-39,56-42)29(46)15-11-6-5-8-12-24(3)30-25(4)33(52-37(48)27-16-18-44-19-17-27)41(50,32(30)42)38(49)40(22-45)35(31)53-40/h7,9-10,13-14,16-19,24-25,28-35,38,45-46,49-50H,1,5-6,8,11-12,15,20-22H2,2-4H3/t24-,25+,28+,29-,30+,31-,32-,33+,34-,35+,38-,39-,40+,41-,42-,43?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Agonist activity at Protein kinase C in human MT4 cells infected with HIV-1 NL4-3 assessed as inhibition of viral replication measured on day 3 post-... |
J Med Chem 62: 6958-6971 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00339
BindingDB Entry DOI: 10.7270/Q2959MZJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.421 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.421 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50321892

((Z)-5-{6-[3-(4-Methoxyphenyl)-ureido]-2-oxo-1,2-di...)Show SMILES COc1ccc(NC(=O)Nc2ccc3\C(=C\c4[nH]cc(C(O)=O)c4C)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C23H20N4O5/c1-12-18(22(29)30)11-24-19(12)10-17-16-8-5-14(9-20(16)27-21(17)28)26-23(31)25-13-3-6-15(32-2)7-4-13/h3-11,24H,1-2H3,(H,27,28)(H,29,30)(H2,25,26,31)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50321892

((Z)-5-{6-[3-(4-Methoxyphenyl)-ureido]-2-oxo-1,2-di...)Show SMILES COc1ccc(NC(=O)Nc2ccc3\C(=C\c4[nH]cc(C(O)=O)c4C)C(=O)Nc3c2)cc1 Show InChI InChI=1S/C23H20N4O5/c1-12-18(22(29)30)11-24-19(12)10-17-16-8-5-14(9-20(16)27-21(17)28)26-23(31)25-13-3-6-15(32-2)7-4-13/h3-11,24H,1-2H3,(H,27,28)(H,29,30)(H2,25,26,31)/b17-10- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Bioorg Med Chem 18: 4674-86 (2011)
Article DOI: 10.1016/j.bmc.2010.05.021
BindingDB Entry DOI: 10.7270/Q2QF8TVS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50530618

(CHEMBL4559807)Show SMILES COc1ccccc1-c1oc2ncnc(N[C@H](CO)c3ccccc3)c2c1-c1cccc(NC(=O)C=C)c1 |r| Show InChI InChI=1S/C30H26N4O4/c1-3-25(36)33-21-13-9-12-20(16-21)26-27-29(34-23(17-35)19-10-5-4-6-11-19)31-18-32-30(27)38-28(26)22-14-7-8-15-24(22)37-2/h3-16,18,23,35H,1,17H2,2H3,(H,33,36)(H,31,32,34)/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.636 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data