 Found 2805 hits with Last Name = 'christ' and Initial = 'd'
Found 2805 hits with Last Name = 'christ' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM50227856
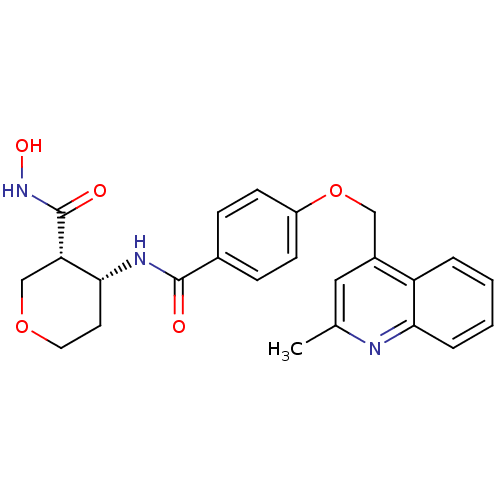
((3R,4R)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2CCOC[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C24H25N3O5/c1-15-12-17(19-4-2-3-5-21(19)25-15)13-32-18-8-6-16(7-9-18)23(28)26-22-10-11-31-14-20(22)24(29)27-30/h2-9,12,20,22,30H,10-11,13-14H2,1H3,(H,26,28)(H,27,29)/t20-,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of pig TACE |
Bioorg Med Chem Lett 18: 241-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.093
BindingDB Entry DOI: 10.7270/Q2JM2BGZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19423

(HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...)Show InChI InChI=1S/C19H17N3O2S/c1-12(23)21-15-7-4-13(5-8-15)19(24)22-17-11-14(6-9-16(17)20)18-3-2-10-25-18/h2-11H,20H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| <0.200 | <-55.4 | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT
| Assay Description
Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... |
ACS Chem Biol 11: 363-74 (2016)
Article DOI: 10.1021/acschembio.5b00640
BindingDB Entry DOI: 10.7270/Q2BZ64T2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50039026
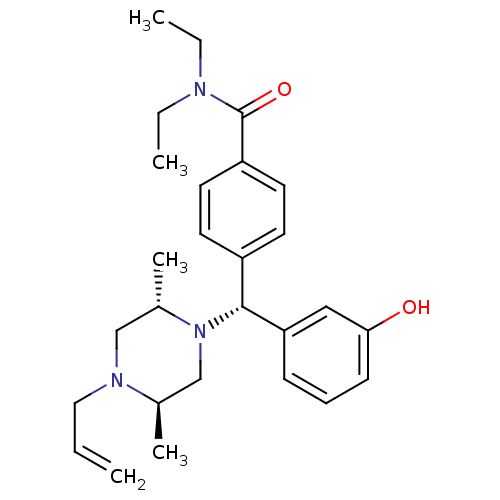
(4-((R)-((2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1C[C@@H](C)N(CC=C)C[C@@H]1C)c1cccc(O)c1 Show InChI InChI=1S/C27H37N3O2/c1-6-16-29-18-21(5)30(19-20(29)4)26(24-10-9-11-25(31)17-24)22-12-14-23(15-13-22)27(32)28(7-2)8-3/h6,9-15,17,20-21,26,31H,1,7-8,16,18-19H2,2-5H3/t20-,21+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor in CHO cells |
J Med Chem 51: 5893-6 (2008)
Article DOI: 10.1021/jm8008986
BindingDB Entry DOI: 10.7270/Q26W9C0D |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11551
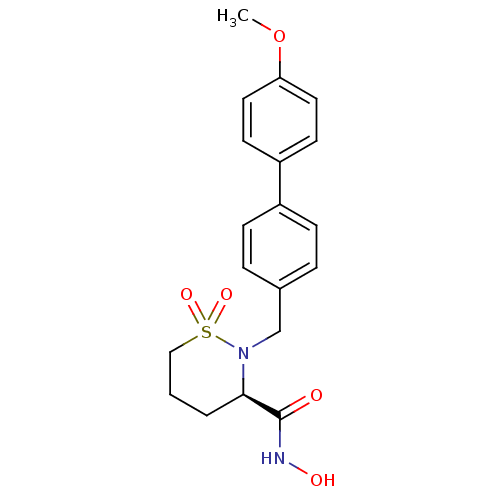
((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...)Show SMILES COc1ccc(cc1)-c1ccc(CN2[C@H](CCCS2(=O)=O)C(=O)NO)cc1 |r| Show InChI InChI=1S/C19H22N2O5S/c1-26-17-10-8-16(9-11-17)15-6-4-14(5-7-15)13-21-18(19(22)20-23)3-2-12-27(21,24)25/h4-11,18,23H,2-3,12-13H2,1H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... |
J Med Chem 47: 2981-3 (2004)
Article DOI: 10.1021/jm049833g
BindingDB Entry DOI: 10.7270/Q29W0CQB |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50084878
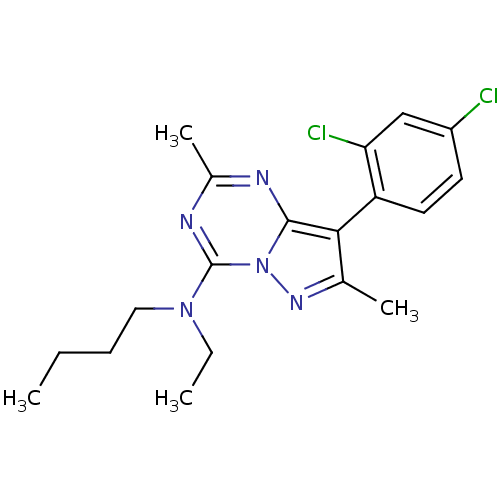
(Butyl-[8-(2,4-dichloro-phenyl)-2,7-dimethyl-pyrazo...)Show SMILES CCCCN(CC)c1nc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl |(6.56,7.35,;6.07,5.89,;4.59,5.55,;4.12,4.1,;2.61,3.75,;1.57,4.9,;2.03,6.37,;2.13,2.3,;.64,1.96,;.17,.5,;-1.34,.16,;1.22,-.62,;2.71,-.3,;3.96,-1.2,;5.2,-.3,;6.65,-.77,;4.72,1.18,;3.18,1.16,;3.96,-2.74,;2.64,-3.51,;2.64,-5.05,;3.96,-5.82,;3.96,-7.36,;5.3,-5.05,;5.3,-3.51,;6.62,-2.73,)| Show InChI InChI=1S/C19H23Cl2N5/c1-5-7-10-25(6-2)19-23-13(4)22-18-17(12(3)24-26(18)19)15-9-8-14(20)11-16(15)21/h8-9,11H,5-7,10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-tyrosine-ovine-CRF from human Corticotropin releasing factor receptor 1 |
J Med Chem 43: 449-56 (2000)
BindingDB Entry DOI: 10.7270/Q2542P9M |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50252953
(CHEMBL494479 | N,N-Diethyl-4-(6-hydroxyspiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccc(O)cc12 |t:14| Show InChI InChI=1S/C24H28N2O3/c1-3-26(4-2)23(28)18-7-5-17(6-8-18)21-16-24(11-13-25-14-12-24)29-22-10-9-19(27)15-20(21)22/h5-10,15-16,25,27H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor in CHO cells |
J Med Chem 51: 5893-6 (2008)
Article DOI: 10.1021/jm8008986
BindingDB Entry DOI: 10.7270/Q26W9C0D |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104967
(CHEMBL419751 | N*4*-Hydroxy-2-(4-hydroxy-benzyl)-N...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@@H](CC(=O)NO)Cc1ccc(O)cc1 Show InChI InChI=1S/C22H27N3O6/c1-23-22(29)19(12-15-5-9-18(31-2)10-6-15)24-21(28)16(13-20(27)25-30)11-14-3-7-17(26)8-4-14/h3-10,16,19,26,30H,11-13H2,1-2H3,(H,23,29)(H,24,28)(H,25,27)/t16-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-8 |
J Med Chem 44: 3347-50 (2001)
BindingDB Entry DOI: 10.7270/Q2057F7H |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-8 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM26526
((2R)-N-hydroxy-2-[(3S)-3-methyl-3-{4-[(2-methylqui...)Show SMILES C[C@@H](N1CC[C@](C)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N3O4/c1-16-14-18(21-6-4-5-7-22(21)26-16)15-32-20-10-8-19(9-11-20)25(3)12-13-28(24(25)30)17(2)23(29)27-31/h4-11,14,17,31H,12-13,15H2,1-3H3,(H,27,29)/t17-,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Affinity for Tumor necrosis factor alpha converting enzyme (TACE) |
J Med Chem 45: 4954-7 (2002)
BindingDB Entry DOI: 10.7270/Q2XP7497 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50084876

(CHEMBL344223 | [8-(2,4-Dichloro-phenyl)-2,7-dimeth...)Show SMILES CCCN(CCC)c1nc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl |(6.07,5.89,;4.59,5.55,;4.12,4.1,;2.61,3.75,;1.57,4.9,;2.03,6.37,;3.54,6.69,;2.13,2.3,;.64,1.96,;.17,.5,;-1.34,.16,;1.22,-.62,;2.71,-.3,;3.96,-1.2,;5.2,-.3,;6.65,-.77,;4.72,1.18,;3.18,1.16,;3.96,-2.74,;2.64,-3.51,;2.64,-5.05,;3.96,-5.82,;3.96,-7.36,;5.3,-5.05,;5.3,-3.51,;6.62,-2.73,)| Show InChI InChI=1S/C19H23Cl2N5/c1-5-9-25(10-6-2)19-23-13(4)22-18-17(12(3)24-26(18)19)15-8-7-14(20)11-16(15)21/h7-8,11H,5-6,9-10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-tyrosine-ovine-CRF from human Corticotropin releasing factor receptor 1 |
J Med Chem 43: 449-56 (2000)
BindingDB Entry DOI: 10.7270/Q2542P9M |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50084880
(CHEMBL140894 | Cyclopropylmethyl-[8-(2,4-dichloro-...)Show SMILES CCCN(CC1CC1)c1nc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl |(6.07,5.89,;4.59,5.55,;4.12,4.1,;2.61,3.75,;1.57,4.9,;.08,4.58,;-1.4,5.04,;-1.08,3.53,;2.13,2.3,;.64,1.96,;.17,.5,;-1.34,.16,;1.22,-.62,;2.71,-.3,;3.96,-1.2,;5.2,-.3,;6.65,-.77,;4.72,1.18,;3.18,1.16,;3.96,-2.74,;2.64,-3.51,;2.64,-5.05,;3.96,-5.82,;3.96,-7.36,;5.3,-5.05,;5.3,-3.51,;6.62,-2.73,)| Show InChI InChI=1S/C20H23Cl2N5/c1-4-9-26(11-14-5-6-14)20-24-13(3)23-19-18(12(2)25-27(19)20)16-8-7-15(21)10-17(16)22/h7-8,10,14H,4-6,9,11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-tyrosine-ovine-CRF from human Corticotropin releasing factor receptor 1 |
J Med Chem 43: 449-56 (2000)
BindingDB Entry DOI: 10.7270/Q2542P9M |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104969
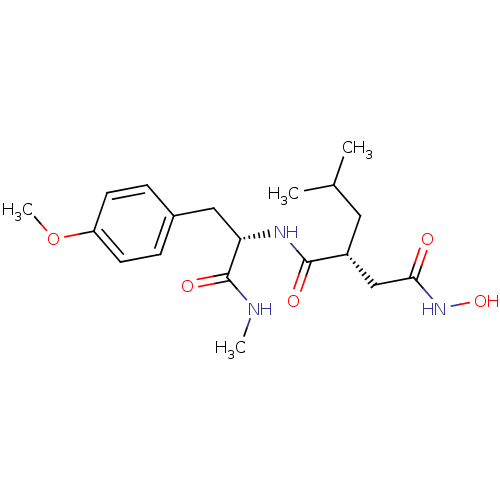
((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C19H29N3O5/c1-12(2)9-14(11-17(23)22-26)18(24)21-16(19(25)20-3)10-13-5-7-15(27-4)8-6-13/h5-8,12,14,16,26H,9-11H2,1-4H3,(H,20,25)(H,21,24)(H,22,23)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-8 |
J Med Chem 44: 3347-50 (2001)
BindingDB Entry DOI: 10.7270/Q2057F7H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50583624
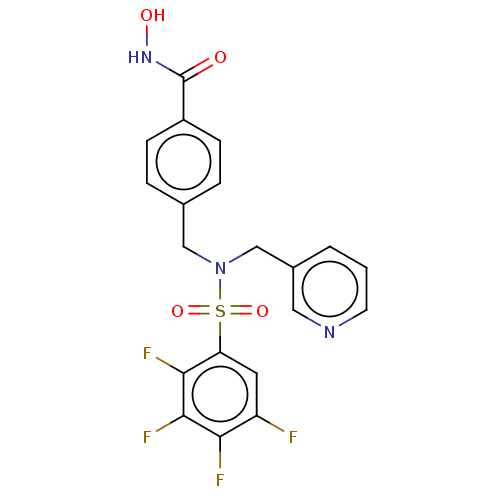
(CHEMBL5075135)Show SMILES ONC(=O)c1ccc(CN(Cc2cccnc2)S(=O)(=O)c2cc(F)c(F)c(F)c2F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human HDAC6 by jump dilution assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01922
BindingDB Entry DOI: 10.7270/Q2NC6530 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-7 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50252954
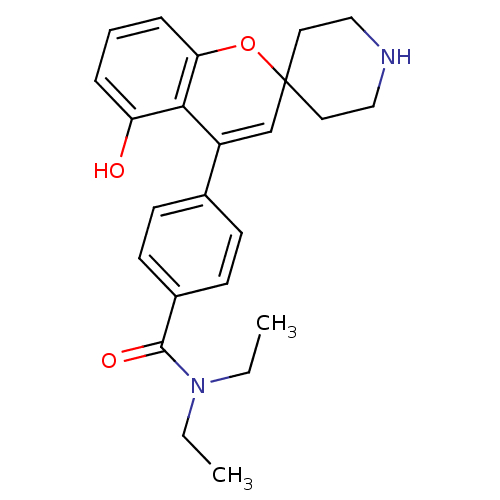
(ADL-5859 | CHEMBL494480 | N,N-diethyl-4-(5-hydroxy...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2cccc(O)c12 |t:14| Show InChI InChI=1S/C24H28N2O3/c1-3-26(4-2)23(28)18-10-8-17(9-11-18)19-16-24(12-14-25-15-13-24)29-21-7-5-6-20(27)22(19)21/h5-11,16,25,27H,3-4,12-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor in CHO cells |
J Med Chem 51: 5893-6 (2008)
Article DOI: 10.1021/jm8008986
BindingDB Entry DOI: 10.7270/Q26W9C0D |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM11551

((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...)Show SMILES COc1ccc(cc1)-c1ccc(CN2[C@H](CCCS2(=O)=O)C(=O)NO)cc1 |r| Show InChI InChI=1S/C19H22N2O5S/c1-26-17-10-8-16(9-11-17)15-6-4-14(5-7-15)13-21-18(19(22)20-23)3-2-12-27(21,24)25/h4-11,18,23H,2-3,12-13H2,1H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... |
J Med Chem 47: 2981-3 (2004)
Article DOI: 10.1021/jm049833g
BindingDB Entry DOI: 10.7270/Q29W0CQB |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-9. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11554

((3R)-N-hydroxy-1,1-dioxo-2-{[4-(pyridin-4-yl)pheny...)Show SMILES ONC(=O)[C@H]1CCCS(=O)(=O)N1Cc1ccc(cc1)-c1ccncc1 |r| Show InChI InChI=1S/C17H19N3O4S/c21-17(19-22)16-2-1-11-25(23,24)20(16)12-13-3-5-14(6-4-13)15-7-9-18-10-8-15/h3-10,16,22H,1-2,11-12H2,(H,19,21)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... |
J Med Chem 47: 2981-3 (2004)
Article DOI: 10.1021/jm049833g
BindingDB Entry DOI: 10.7270/Q29W0CQB |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50102630
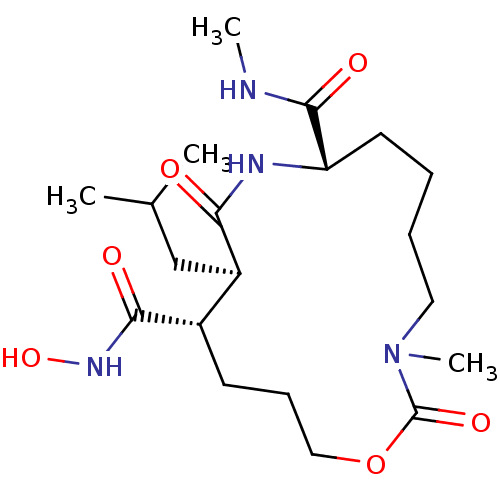
(11-Isobutyl-3-methyl-2,10-dioxo-1-oxa-3,9-diaza-cy...)Show SMILES CNC(=O)[C@@H]1CCCCN(C)C(=O)OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO Show InChI InChI=1S/C20H36N4O6/c1-13(2)12-15-14(18(26)23-29)8-7-11-30-20(28)24(4)10-6-5-9-16(19(27)21-3)22-17(15)25/h13-16,29H,5-12H2,1-4H3,(H,21,27)(H,22,25)(H,23,26)/t14-,15+,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50076993

((2S,11S,12R)-12-Isobutyl-13-oxo-1,7diaza-cyclotrid...)Show SMILES CNC(=O)[C@@H]1CCCCNCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO Show InChI InChI=1S/C18H34N4O4/c1-12(2)11-14-13(17(24)22-26)7-6-10-20-9-5-4-8-15(18(25)19-3)21-16(14)23/h12-15,20,26H,4-11H2,1-3H3,(H,19,25)(H,21,23)(H,22,24)/t13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50102631
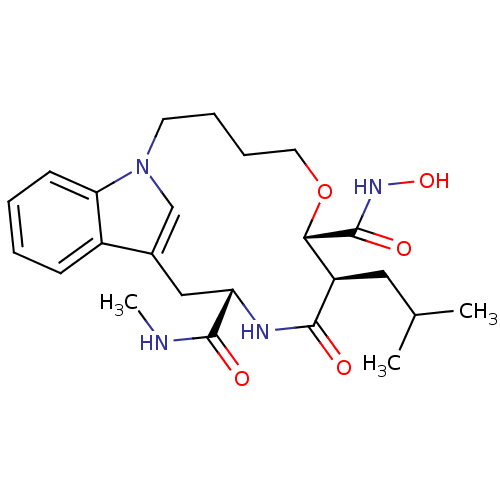
(8-Isobutyl-9-oxo-6-oxa-1,10-diaza-tricyclo[11.6.1....)Show SMILES CNC(=O)[C@@H]1Cc2cn(CCCCO[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)c1ccccc21 Show InChI InChI=1S/C24H34N4O5/c1-15(2)12-18-21(24(31)27-32)33-11-7-6-10-28-14-16(17-8-4-5-9-20(17)28)13-19(23(30)25-3)26-22(18)29/h4-5,8-9,14-15,18-19,21,32H,6-7,10-13H2,1-3H3,(H,25,30)(H,26,29)(H,27,31)/t18-,19+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50076991
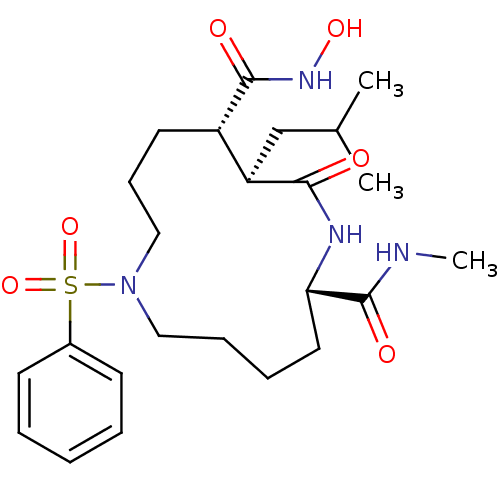
((2S,11S,12R)-7-Benzenesulfonyl-12-isobutyl-13-oxo-...)Show SMILES CNC(=O)[C@@H]1CCCCN(CCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C24H38N4O6S/c1-17(2)16-20-19(23(30)27-32)12-9-15-28(35(33,34)18-10-5-4-6-11-18)14-8-7-13-21(24(31)25-3)26-22(20)29/h4-6,10-11,17,19-21,32H,7-9,12-16H2,1-3H3,(H,25,31)(H,26,29)(H,27,30)/t19-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-9. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50064340
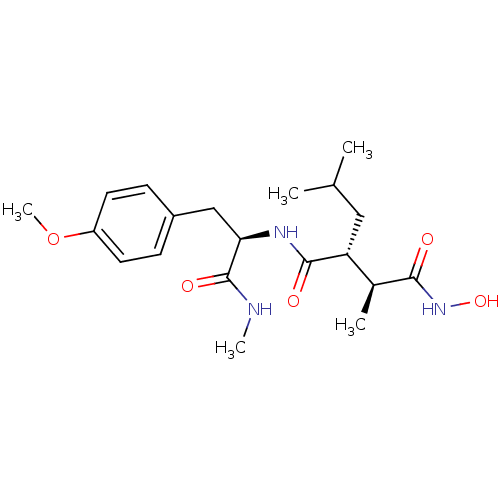
((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-[(R)-2-(4-met...)Show SMILES CNC(=O)[C@@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO Show InChI InChI=1S/C20H31N3O5/c1-12(2)10-16(13(3)18(24)23-27)19(25)22-17(20(26)21-4)11-14-6-8-15(28-5)9-7-14/h6-9,12-13,16-17,27H,10-11H2,1-5H3,(H,21,26)(H,22,25)(H,23,24)/t13-,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50064340

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-[(R)-2-(4-met...)Show SMILES CNC(=O)[C@@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO Show InChI InChI=1S/C20H31N3O5/c1-12(2)10-16(13(3)18(24)23-27)19(25)22-17(20(26)21-4)11-14-6-8-15(28-5)9-7-14/h6-9,12-13,16-17,27H,10-11H2,1-5H3,(H,21,26)(H,22,25)(H,23,24)/t13-,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-9. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50102600

(12-Isobutyl-4-methyl-3,11-dioxo-1-oxa-4,10-diaza-c...)Show SMILES CNC(=O)[C@@H]1CCCCN(C)C(=O)CO[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO Show InChI InChI=1S/C18H32N4O6/c1-11(2)9-12-15(18(26)21-27)28-10-14(23)22(4)8-6-5-7-13(17(25)19-3)20-16(12)24/h11-13,15,27H,5-10H2,1-4H3,(H,19,25)(H,20,24)(H,21,26)/t12-,13+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50076995
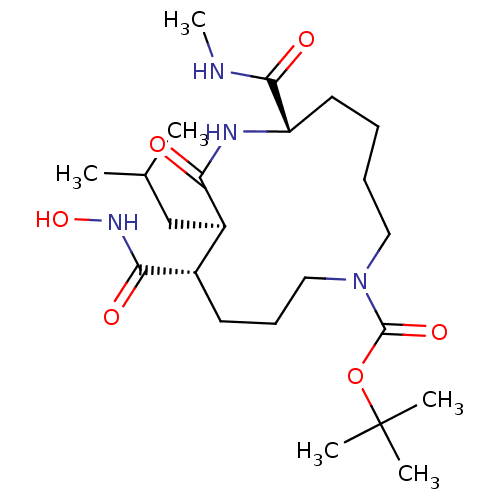
((6S,9R,10S)-10-Hydroxycarbamoyl-9-isobutyl-6-methy...)Show SMILES CNC(=O)[C@@H]1CCCCN(CCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)C(=O)OC(C)(C)C Show InChI InChI=1S/C23H42N4O6/c1-15(2)14-17-16(20(29)26-32)10-9-13-27(22(31)33-23(3,4)5)12-8-7-11-18(21(30)24-6)25-19(17)28/h15-18,32H,7-14H2,1-6H3,(H,24,30)(H,25,28)(H,26,29)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-9. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-9. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50076991

((2S,11S,12R)-7-Benzenesulfonyl-12-isobutyl-13-oxo-...)Show SMILES CNC(=O)[C@@H]1CCCCN(CCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C24H38N4O6S/c1-17(2)16-20-19(23(30)27-32)12-9-15-28(35(33,34)18-10-5-4-6-11-18)14-8-7-13-21(24(31)25-3)26-22(20)29/h4-6,10-11,17,19-21,32H,7-9,12-16H2,1-3H3,(H,25,31)(H,26,29)(H,27,30)/t19-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50102604
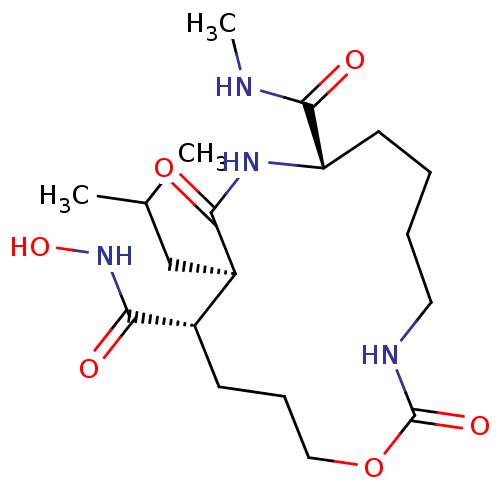
(11-Isobutyl-2,10-dioxo-1-oxa-3,9-diaza-cyclopentad...)Show SMILES CNC(=O)[C@@H]1CCCCNC(=O)OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO Show InChI InChI=1S/C19H34N4O6/c1-12(2)11-14-13(17(25)23-28)7-6-10-29-19(27)21-9-5-4-8-15(18(26)20-3)22-16(14)24/h12-15,28H,4-11H2,1-3H3,(H,20,26)(H,21,27)(H,22,24)(H,23,25)/t13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50102630

(11-Isobutyl-3-methyl-2,10-dioxo-1-oxa-3,9-diaza-cy...)Show SMILES CNC(=O)[C@@H]1CCCCN(C)C(=O)OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO Show InChI InChI=1S/C20H36N4O6/c1-13(2)12-15-14(18(26)23-29)8-7-11-30-20(28)24(4)10-6-5-9-16(19(27)21-3)22-17(15)25/h13-16,29H,5-12H2,1-4H3,(H,21,27)(H,22,25)(H,23,26)/t14-,15+,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-9. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50076995

((6S,9R,10S)-10-Hydroxycarbamoyl-9-isobutyl-6-methy...)Show SMILES CNC(=O)[C@@H]1CCCCN(CCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)C(=O)OC(C)(C)C Show InChI InChI=1S/C23H42N4O6/c1-15(2)14-17-16(20(29)26-32)10-9-13-27(22(31)33-23(3,4)5)12-8-7-11-18(21(30)24-6)25-19(17)28/h15-18,32H,7-14H2,1-6H3,(H,24,30)(H,25,28)(H,26,29)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50102610
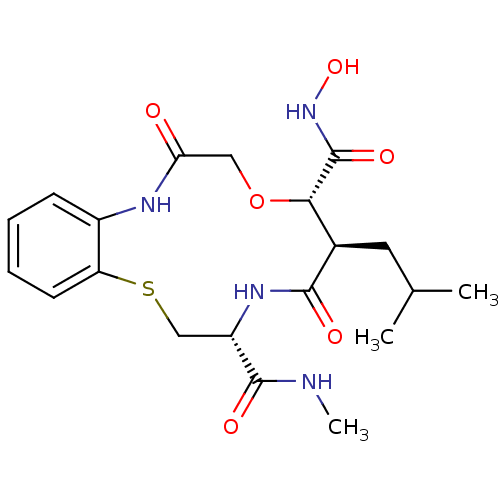
(10-Isobutyl-6,11-dioxo-6,7,9,10,11,12,13,14-octahy...)Show SMILES CNC(=O)[C@@H]1CSc2ccccc2NC(=O)CO[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO Show InChI InChI=1S/C20H28N4O6S/c1-11(2)8-12-17(20(28)24-29)30-9-16(25)22-13-6-4-5-7-15(13)31-10-14(19(27)21-3)23-18(12)26/h4-7,11-12,14,17,29H,8-10H2,1-3H3,(H,21,27)(H,22,25)(H,23,26)(H,24,28)/t12-,14+,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50107571
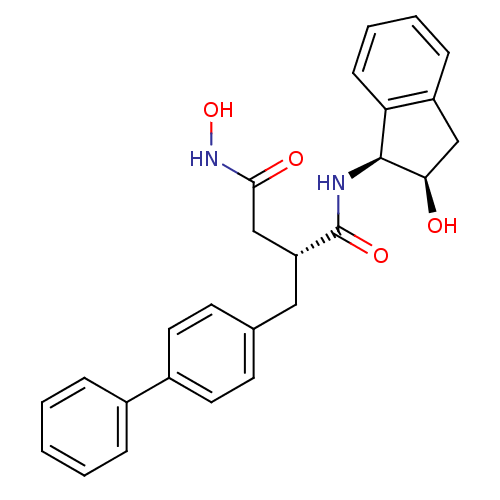
((R)-2-Biphenyl-4-ylmethyl-N*4*-hydroxy-N*1*-((1S,2...)Show SMILES ONC(=O)C[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C26H26N2O4/c29-23-15-20-8-4-5-9-22(20)25(23)27-26(31)21(16-24(30)28-32)14-17-10-12-19(13-11-17)18-6-2-1-3-7-18/h1-13,21,23,25,29,32H,14-16H2,(H,27,31)(H,28,30)/t21-,23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharma Company
Curated by ChEMBL
| Assay Description
Binding affinity for human gelatinase A (MMP-2) |
Bioorg Med Chem Lett 12: 101-4 (2001)
BindingDB Entry DOI: 10.7270/Q2R49Q1C |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50104969

((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C19H29N3O5/c1-12(2)9-14(11-17(23)22-26)18(24)21-16(19(25)20-3)10-13-5-7-15(27-4)8-6-13/h5-8,12,14,16,26H,9-11H2,1-4H3,(H,20,25)(H,21,24)(H,22,23)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 |
J Med Chem 44: 3347-50 (2001)
BindingDB Entry DOI: 10.7270/Q2057F7H |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104969

((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C19H29N3O5/c1-12(2)9-14(11-17(23)22-26)18(24)21-16(19(25)20-3)10-13-5-7-15(27-4)8-6-13/h5-8,12,14,16,26H,9-11H2,1-4H3,(H,20,25)(H,21,24)(H,22,23)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3347-50 (2001)
BindingDB Entry DOI: 10.7270/Q2057F7H |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50104969

((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C19H29N3O5/c1-12(2)9-14(11-17(23)22-26)18(24)21-16(19(25)20-3)10-13-5-7-15(27-4)8-6-13/h5-8,12,14,16,26H,9-11H2,1-4H3,(H,20,25)(H,21,24)(H,22,23)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-1 |
J Med Chem 44: 3347-50 (2001)
BindingDB Entry DOI: 10.7270/Q2057F7H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50102608
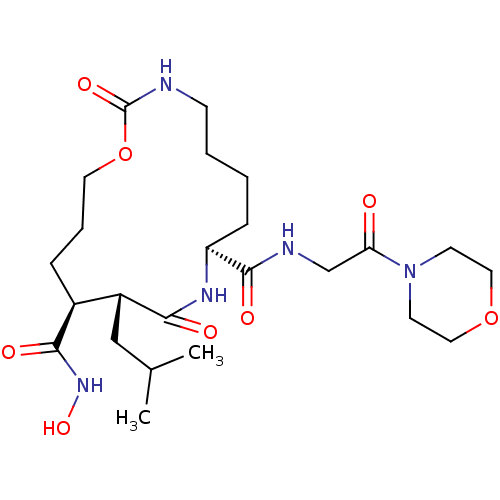
(11-Isobutyl-2,10-dioxo-1-oxa-3,9-diaza-cyclopentad...)Show SMILES CC(C)C[C@@H]1[C@H](CCCOC(=O)NCCCC[C@H](NC1=O)C(=O)NCC(=O)N1CCOCC1)C(=O)NO Show InChI InChI=1S/C24H41N5O8/c1-16(2)14-18-17(22(32)28-35)6-5-11-37-24(34)25-8-4-3-7-19(27-21(18)31)23(33)26-15-20(30)29-9-12-36-13-10-29/h16-19,35H,3-15H2,1-2H3,(H,25,34)(H,26,33)(H,27,31)(H,28,32)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-8 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50084873
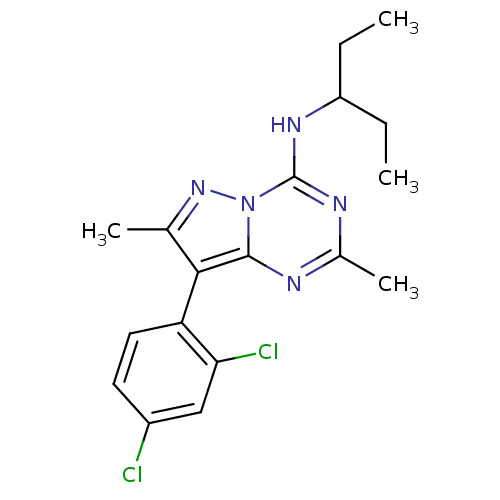
(CHEMBL336678 | [8-(2,4-Dichloro-phenyl)-2,7-dimeth...)Show SMILES CCC(CC)Nc1nc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl |(10.17,.44,;8.83,1.19,;7.5,.42,;6.27,1.35,;4.86,.77,;7.11,-1.07,;6.63,-2.54,;5.13,-2.87,;4.67,-4.34,;3.15,-4.68,;5.71,-5.47,;7.21,-5.15,;8.46,-6.05,;9.7,-5.15,;11.17,-5.61,;9.21,-3.67,;7.67,-3.68,;8.46,-7.59,;7.14,-8.37,;7.14,-9.91,;8.46,-10.68,;8.46,-12.23,;9.81,-9.91,;9.81,-8.37,;11.13,-7.57,)| Show InChI InChI=1S/C18H21Cl2N5/c1-5-13(6-2)23-18-22-11(4)21-17-16(10(3)24-25(17)18)14-8-7-12(19)9-15(14)20/h7-9,13H,5-6H2,1-4H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-tyrosine-ovine-CRF from human Corticotropin releasing factor receptor 1 |
J Med Chem 43: 449-56 (2000)
BindingDB Entry DOI: 10.7270/Q2542P9M |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50102604

(11-Isobutyl-2,10-dioxo-1-oxa-3,9-diaza-cyclopentad...)Show SMILES CNC(=O)[C@@H]1CCCCNC(=O)OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO Show InChI InChI=1S/C19H34N4O6/c1-12(2)11-14-13(17(25)23-28)7-6-10-29-19(27)21-9-5-4-8-15(18(26)20-3)22-16(14)24/h12-15,28H,4-11H2,1-3H3,(H,20,26)(H,21,27)(H,22,24)(H,23,25)/t13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-9. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50102608

(11-Isobutyl-2,10-dioxo-1-oxa-3,9-diaza-cyclopentad...)Show SMILES CC(C)C[C@@H]1[C@H](CCCOC(=O)NCCCC[C@H](NC1=O)C(=O)NCC(=O)N1CCOCC1)C(=O)NO Show InChI InChI=1S/C24H41N5O8/c1-16(2)14-18-17(22(32)28-35)6-5-11-37-24(34)25-8-4-3-7-19(27-21(18)31)23(33)26-15-20(30)29-9-12-36-13-10-29/h16-19,35H,3-15H2,1-2H3,(H,25,34)(H,26,33)(H,27,31)(H,28,32)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50084869
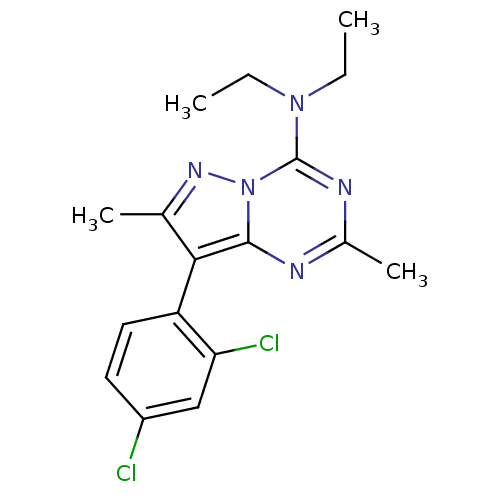
(CHEMBL337768 | [8-(2,4-Dichloro-phenyl)-2,7-dimeth...)Show SMILES CCN(CC)c1nc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl |(2.03,6.37,;1.57,4.9,;2.61,3.75,;4.12,4.1,;4.59,5.55,;2.13,2.3,;.64,1.96,;.17,.5,;-1.34,.16,;1.22,-.62,;2.71,-.3,;3.96,-1.2,;5.2,-.3,;6.65,-.77,;4.72,1.18,;3.18,1.16,;3.96,-2.74,;2.64,-3.51,;2.64,-5.05,;3.96,-5.82,;3.96,-7.36,;5.3,-5.05,;5.3,-3.51,;6.62,-2.73,)| Show InChI InChI=1S/C17H19Cl2N5/c1-5-23(6-2)17-21-11(4)20-16-15(10(3)22-24(16)17)13-8-7-12(18)9-14(13)19/h7-9H,5-6H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-tyrosine-ovine-CRF from human Corticotropin releasing factor receptor 1 |
J Med Chem 43: 449-56 (2000)
BindingDB Entry DOI: 10.7270/Q2542P9M |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50084877

(CHEMBL140143 | [8-(2,4-Dichloro-phenyl)-2,7-dimeth...)Show SMILES CCCC(CC)Nc1nc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl |(15.31,1.18,;13.83,.79,;12.75,1.88,;11.26,1.46,;10.18,2.56,;8.69,2.14,;10.88,-.04,;10.39,-1.49,;8.9,-1.84,;8.44,-3.28,;6.93,-3.64,;9.48,-4.41,;10.97,-4.09,;12.22,-4.98,;13.45,-4.09,;14.92,-4.57,;12.97,-2.61,;11.43,-2.64,;12.22,-6.52,;10.91,-7.29,;10.91,-8.83,;12.22,-9.6,;12.22,-11.14,;13.57,-8.83,;13.57,-7.29,;14.89,-6.52,)| Show InChI InChI=1S/C19H23Cl2N5/c1-5-7-14(6-2)24-19-23-12(4)22-18-17(11(3)25-26(18)19)15-9-8-13(20)10-16(15)21/h8-10,14H,5-7H2,1-4H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]-tyrosine-ovine-CRF from human Corticotropin releasing factor receptor 1 |
J Med Chem 43: 449-56 (2000)
BindingDB Entry DOI: 10.7270/Q2542P9M |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM11551

((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...)Show SMILES COc1ccc(cc1)-c1ccc(CN2[C@H](CCCS2(=O)=O)C(=O)NO)cc1 |r| Show InChI InChI=1S/C19H22N2O5S/c1-26-17-10-8-16(9-11-17)15-6-4-14(5-7-15)13-21-18(19(22)20-23)3-2-12-27(21,24)25/h4-11,18,23H,2-3,12-13H2,1H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
The enzymatic activities of MMPs were determined with recombinant human version catalytic domains and a fluorogenic peptide substrate. Fluorescence m... |
J Med Chem 47: 2981-3 (2004)
Article DOI: 10.1021/jm049833g
BindingDB Entry DOI: 10.7270/Q29W0CQB |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50039029

((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1C[C@@H](C)N(CC=C)C[C@@H]1C)c1cccc(OC)c1 |r| Show InChI InChI=1S/C28H39N3O2/c1-7-17-30-19-22(5)31(20-21(30)4)27(25-11-10-12-26(18-25)33-6)23-13-15-24(16-14-23)28(32)29(8-2)9-3/h7,10-16,18,21-22,27H,1,8-9,17,19-20H2,2-6H3/t21-,22+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor in CHO cells |
J Med Chem 51: 5893-6 (2008)
Article DOI: 10.1021/jm8008986
BindingDB Entry DOI: 10.7270/Q26W9C0D |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50064341
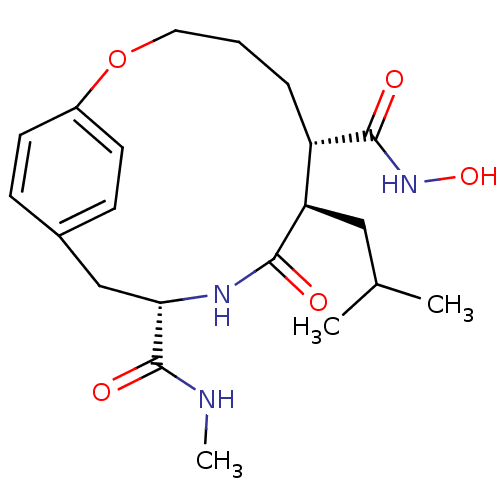
((6S,7R,10S)-7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[1...)Show SMILES CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C21H31N3O5/c1-13(2)11-17-16(20(26)24-28)5-4-10-29-15-8-6-14(7-9-15)12-18(21(27)22-3)23-19(17)25/h6-9,13,16-18,28H,4-5,10-12H2,1-3H3,(H,22,27)(H,23,25)(H,24,26)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50102608

(11-Isobutyl-2,10-dioxo-1-oxa-3,9-diaza-cyclopentad...)Show SMILES CC(C)C[C@@H]1[C@H](CCCOC(=O)NCCCC[C@H](NC1=O)C(=O)NCC(=O)N1CCOCC1)C(=O)NO Show InChI InChI=1S/C24H41N5O8/c1-16(2)14-18-17(22(32)28-35)6-5-11-37-24(34)25-8-4-3-7-19(27-21(18)31)23(33)26-15-20(30)29-9-12-36-13-10-29/h16-19,35H,3-15H2,1-2H3,(H,25,34)(H,26,33)(H,27,31)(H,28,32)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-9. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50102640
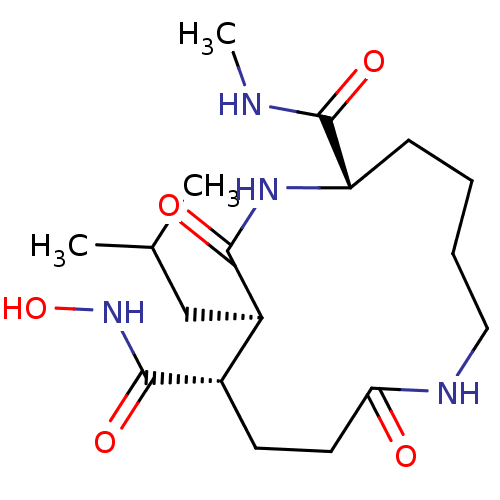
(12-Isobutyl-8,13-dioxo-1,7diaza-cyclotridecane-2,1...)Show SMILES CNC(=O)[C@@H]1CCCCNC(=O)CC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO Show InChI InChI=1S/C18H32N4O5/c1-11(2)10-13-12(17(25)22-27)7-8-15(23)20-9-5-4-6-14(18(26)19-3)21-16(13)24/h11-14,27H,4-10H2,1-3H3,(H,19,26)(H,20,23)(H,21,24)(H,22,25)/t12-,13+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data