 Found 336 hits with Last Name = 'cirillo' and Initial = 'p'
Found 336 hits with Last Name = 'cirillo' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50240404
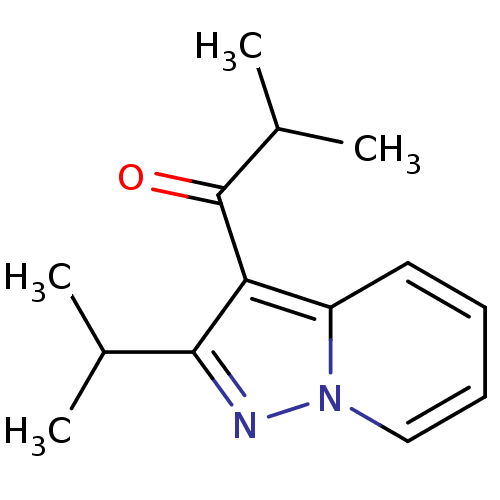
((Ibudilast)1-(2-Isopropyl-pyrazolo[1,5-a]pyridin-3...)Show InChI InChI=1S/C14H18N2O/c1-9(2)13-12(14(17)10(3)4)11-7-5-6-8-16(11)15-13/h5-10H,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 3.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Non-competitive inhibition of recombinant human MIF assessed as inhibition constant using varying levels of p-hydroxyphenylpyruvate as substrate by L... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00351
BindingDB Entry DOI: 10.7270/Q2CJ8J3J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50215054
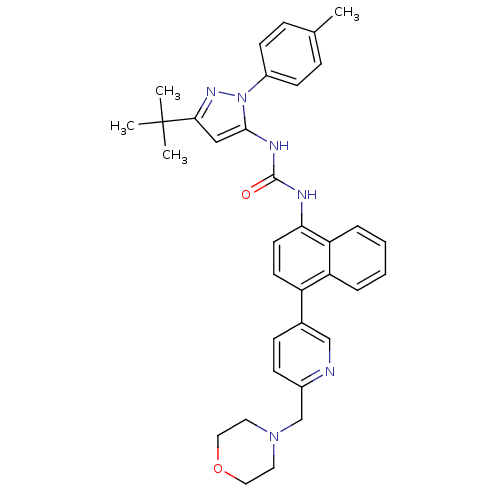
(1-(3-tert-butyl-1-p-tolyl-1H-pyrazol-5-yl)-3-(4-(6...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(-c2ccc(CN3CCOCC3)nc2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C35H38N6O2/c1-24-9-13-27(14-10-24)41-33(21-32(39-41)35(2,3)4)38-34(42)37-31-16-15-28(29-7-5-6-8-30(29)31)25-11-12-26(36-22-25)23-40-17-19-43-20-18-40/h5-16,21-22H,17-20,23H2,1-4H3,(H2,37,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of Jnk2 |
Bioorg Med Chem Lett 17: 4242-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.042
BindingDB Entry DOI: 10.7270/Q27S7NHZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of JNK2alpha2 (unknown origin) by by exchange curve binding kinetic analysis |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218689
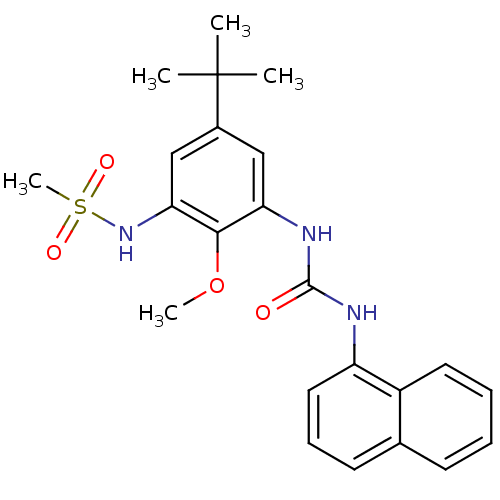
(CHEMBL245230 | N-(5-tert-butyl-2-methoxy-3-(3-naph...)Show SMILES COc1c(NC(=O)Nc2cccc3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C23H27N3O4S/c1-23(2,3)16-13-19(21(30-4)20(14-16)26-31(5,28)29)25-22(27)24-18-12-8-10-15-9-6-7-11-17(15)18/h6-14,26H,1-5H3,(H2,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against c-Jun N-terminal kinase 2-alpha 2 protein kinase |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50458539
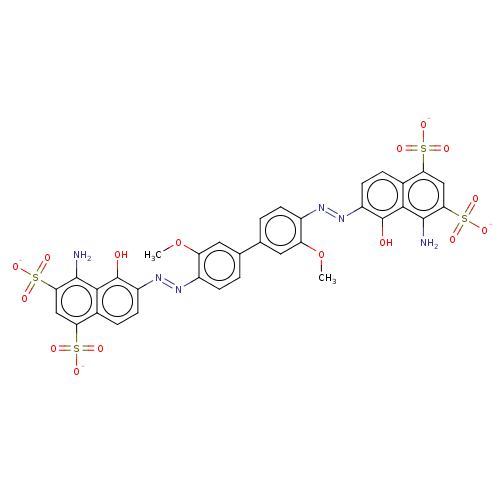
(CHICAGO SKY BLUE SODIUM | Chicago Sky Blue | Chica...)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[Na;v0+].[#6]-[#8]-c1cc(ccc1\[#7]=[#7]\c1ccc2c(cc(c(-[#7])c2c1-[#8])S([#8-])(=O)=O)S([#8-])(=O)=O)-c1ccc(\[#7]=[#7]\c2ccc3c(cc(c(-[#7])c3c2-[#8])S([#8-])(=O)=O)S([#8-])(=O)=O)c(-[#8]-[#6])c1 Show InChI InChI=1S/C34H28N6O16S4/c1-55-23-11-15(3-7-19(23)37-39-21-9-5-17-25(57(43,44)45)13-27(59(49,50)51)31(35)29(17)33(21)41)16-4-8-20(24(12-16)56-2)38-40-22-10-6-18-26(58(46,47)48)14-28(60(52,53)54)32(36)30(18)34(22)42/h3-14,41-42H,35-36H2,1-2H3,(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)/p-4/b39-37+,40-38+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant biotinylated human MIF tautomerase activity expressed in Escherichia coli BL21 DE3 assessed as reduction in tautomerization... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00351
BindingDB Entry DOI: 10.7270/Q2CJ8J3J |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50458539

(CHICAGO SKY BLUE SODIUM | Chicago Sky Blue | Chica...)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[Na;v0+].[#6]-[#8]-c1cc(ccc1\[#7]=[#7]\c1ccc2c(cc(c(-[#7])c2c1-[#8])S([#8-])(=O)=O)S([#8-])(=O)=O)-c1ccc(\[#7]=[#7]\c2ccc3c(cc(c(-[#7])c3c2-[#8])S([#8-])(=O)=O)S([#8-])(=O)=O)c(-[#8]-[#6])c1 Show InChI InChI=1S/C34H28N6O16S4/c1-55-23-11-15(3-7-19(23)37-39-21-9-5-17-25(57(43,44)45)13-27(59(49,50)51)31(35)29(17)33(21)41)16-4-8-20(24(12-16)56-2)38-40-22-10-6-18-26(58(46,47)48)14-28(60(52,53)54)32(36)30(18)34(22)42/h3-14,41-42H,35-36H2,1-2H3,(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)/p-4/b39-37+,40-38+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MIF expressed in Escherichia coli BL21 DE3 binding to recombinant soluble human CD74 receptor ectodomain (73 to 232 r... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00351
BindingDB Entry DOI: 10.7270/Q2CJ8J3J |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50215054

(1-(3-tert-butyl-1-p-tolyl-1H-pyrazol-5-yl)-3-(4-(6...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(-c2ccc(CN3CCOCC3)nc2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C35H38N6O2/c1-24-9-13-27(14-10-24)41-33(21-32(39-41)35(2,3)4)38-34(42)37-31-16-15-28(29-7-5-6-8-30(29)31)25-11-12-26(36-22-25)23-40-17-19-43-20-18-40/h5-16,21-22H,17-20,23H2,1-4H3,(H2,37,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of cRaf |
Bioorg Med Chem Lett 17: 4242-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.042
BindingDB Entry DOI: 10.7270/Q27S7NHZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of Abl (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50458539

(CHICAGO SKY BLUE SODIUM | Chicago Sky Blue | Chica...)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[Na;v0+].[#6]-[#8]-c1cc(ccc1\[#7]=[#7]\c1ccc2c(cc(c(-[#7])c2c1-[#8])S([#8-])(=O)=O)S([#8-])(=O)=O)-c1ccc(\[#7]=[#7]\c2ccc3c(cc(c(-[#7])c3c2-[#8])S([#8-])(=O)=O)S([#8-])(=O)=O)c(-[#8]-[#6])c1 Show InChI InChI=1S/C34H28N6O16S4/c1-55-23-11-15(3-7-19(23)37-39-21-9-5-17-25(57(43,44)45)13-27(59(49,50)51)31(35)29(17)33(21)41)16-4-8-20(24(12-16)56-2)38-40-22-10-6-18-26(58(46,47)48)14-28(60(52,53)54)32(36)30(18)34(22)42/h3-14,41-42H,35-36H2,1-2H3,(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)/p-4/b39-37+,40-38+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant biotinylated human MIF expressed in Escherichia coli BL21 DE3 binding to immobilized soluble human CD74 receptor ectodomain... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00351
BindingDB Entry DOI: 10.7270/Q2CJ8J3J |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of craf (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50547499
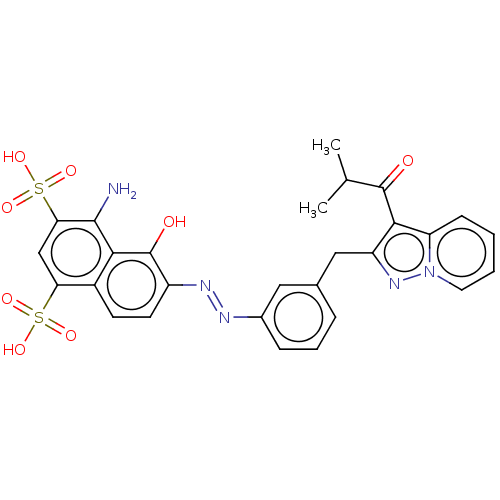
(CHEMBL4785454)Show SMILES CC(C)C(=O)c1c(Cc2cccc(c2)\N=N\c2ccc3c(cc(c(N)c3c2O)S(O)(=O)=O)S(O)(=O)=O)nn2ccccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MIF expressed in Escherichia coli BL21 DE3 binding to recombinant soluble human CD74 receptor ectodomain (73 to 232 r... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00351
BindingDB Entry DOI: 10.7270/Q2CJ8J3J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50215054

(1-(3-tert-butyl-1-p-tolyl-1H-pyrazol-5-yl)-3-(4-(6...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(-c2ccc(CN3CCOCC3)nc2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C35H38N6O2/c1-24-9-13-27(14-10-24)41-33(21-32(39-41)35(2,3)4)38-34(42)37-31-16-15-28(29-7-5-6-8-30(29)31)25-11-12-26(36-22-25)23-40-17-19-43-20-18-40/h5-16,21-22H,17-20,23H2,1-4H3,(H2,37,38,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of Src |
Bioorg Med Chem Lett 17: 4242-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.042
BindingDB Entry DOI: 10.7270/Q27S7NHZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50215054

(1-(3-tert-butyl-1-p-tolyl-1H-pyrazol-5-yl)-3-(4-(6...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(-c2ccc(CN3CCOCC3)nc2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C35H38N6O2/c1-24-9-13-27(14-10-24)41-33(21-32(39-41)35(2,3)4)38-34(42)37-31-16-15-28(29-7-5-6-8-30(29)31)25-11-12-26(36-22-25)23-40-17-19-43-20-18-40/h5-16,21-22H,17-20,23H2,1-4H3,(H2,37,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 4242-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.042
BindingDB Entry DOI: 10.7270/Q27S7NHZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50215054

(1-(3-tert-butyl-1-p-tolyl-1H-pyrazol-5-yl)-3-(4-(6...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(-c2ccc(CN3CCOCC3)nc2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C35H38N6O2/c1-24-9-13-27(14-10-24)41-33(21-32(39-41)35(2,3)4)38-34(42)37-31-16-15-28(29-7-5-6-8-30(29)31)25-11-12-26(36-22-25)23-40-17-19-43-20-18-40/h5-16,21-22H,17-20,23H2,1-4H3,(H2,37,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of Lyn |
Bioorg Med Chem Lett 17: 4242-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.042
BindingDB Entry DOI: 10.7270/Q27S7NHZ |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against RAF proto-oncogene serine/threonine-protein kinase (c-Raf-1) |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of Lyn (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218677
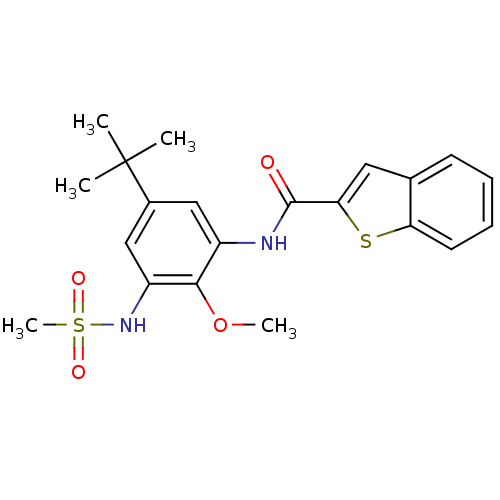
(CHEMBL242005 | N-(5-tert-butyl-2-methoxy-3-(methyl...)Show SMILES COc1c(NC(=O)c2cc3ccccc3s2)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C21H24N2O4S2/c1-21(2,3)14-11-15(19(27-4)16(12-14)23-29(5,25)26)22-20(24)18-10-13-8-6-7-9-17(13)28-18/h6-12,23H,1-5H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of HEK (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50458539

(CHICAGO SKY BLUE SODIUM | Chicago Sky Blue | Chica...)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[Na;v0+].[#6]-[#8]-c1cc(ccc1\[#7]=[#7]\c1ccc2c(cc(c(-[#7])c2c1-[#8])S([#8-])(=O)=O)S([#8-])(=O)=O)-c1ccc(\[#7]=[#7]\c2ccc3c(cc(c(-[#7])c3c2-[#8])S([#8-])(=O)=O)S([#8-])(=O)=O)c(-[#8]-[#6])c1 Show InChI InChI=1S/C34H28N6O16S4/c1-55-23-11-15(3-7-19(23)37-39-21-9-5-17-25(57(43,44)45)13-27(59(49,50)51)31(35)29(17)33(21)41)16-4-8-20(24(12-16)56-2)38-40-22-10-6-18-26(58(46,47)48)14-28(60(52,53)54)32(36)30(18)34(22)42/h3-14,41-42H,35-36H2,1-2H3,(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)/p-4/b39-37+,40-38+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MIF-induced IL-8 secretion in human foreskin fibroblasts incubated for 30 mins by ELISA |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00351
BindingDB Entry DOI: 10.7270/Q2CJ8J3J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50277627
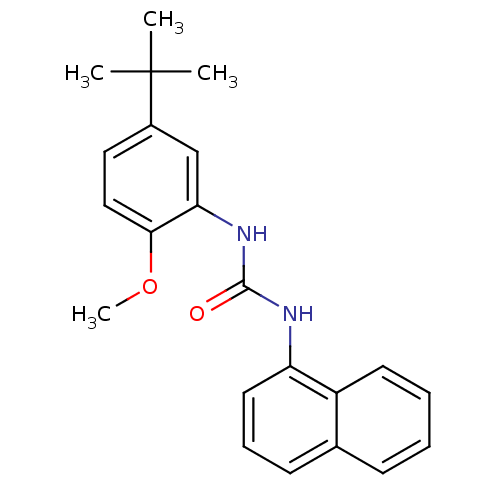
(1-(5-tert-butyl-2-methoxyphenyl)-3-(naphthalen-1-y...)Show InChI InChI=1S/C22H24N2O2/c1-22(2,3)16-12-13-20(26-4)19(14-16)24-21(25)23-18-11-7-9-15-8-5-6-10-17(15)18/h5-14H,1-4H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218695
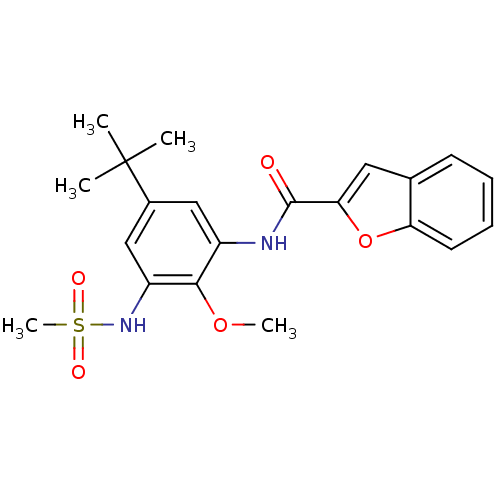
(CHEMBL390254 | N-(5-tert-butyl-2-methoxy-3-(methyl...)Show SMILES COc1c(NC(=O)c2cc3ccccc3o2)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C21H24N2O5S/c1-21(2,3)14-11-15(19(27-4)16(12-14)23-29(5,25)26)22-20(24)18-10-13-8-6-7-9-17(13)28-18/h6-12,23H,1-5H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50547499

(CHEMBL4785454)Show SMILES CC(C)C(=O)c1c(Cc2cccc(c2)\N=N\c2ccc3c(cc(c(N)c3c2O)S(O)(=O)=O)S(O)(=O)=O)nn2ccccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MIF-induced IL-8 secretion in human foreskin fibroblasts incubated for 30 mins by ELISA |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00351
BindingDB Entry DOI: 10.7270/Q2CJ8J3J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of ECK (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of PKBalpha/Akt1 (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of bRaf (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50547499

(CHEMBL4785454)Show SMILES CC(C)C(=O)c1c(Cc2cccc(c2)\N=N\c2ccc3c(cc(c(N)c3c2O)S(O)(=O)=O)S(O)(=O)=O)nn2ccccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant biotinylated human MIF tautomerase activity expressed in Escherichia coli BL21 DE3 assessed as reduction in tautomerization... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00351
BindingDB Entry DOI: 10.7270/Q2CJ8J3J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50277663

(CHEMBL519511 | N-(5-tert-butyl-2-methoxyphenyl)ben...)Show InChI InChI=1S/C20H21NO2S/c1-20(2,3)14-9-10-16(23-4)15(12-14)21-19(22)18-11-13-7-5-6-8-17(13)24-18/h5-12H,1-4H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50277662
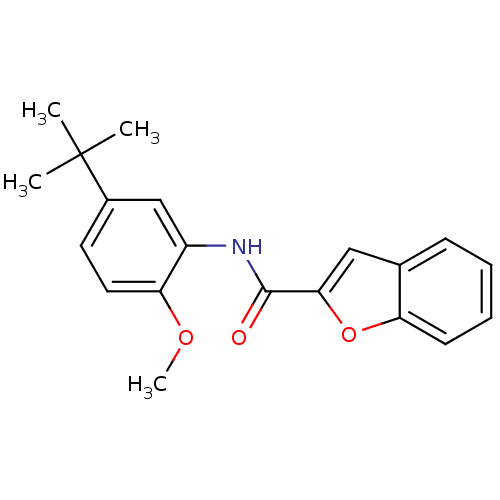
(CHEMBL484405 | N-(5-tert-butyl-2-methoxyphenyl)ben...)Show InChI InChI=1S/C20H21NO3/c1-20(2,3)14-9-10-17(23-4)15(12-14)21-19(22)18-11-13-7-5-6-8-16(13)24-18/h5-12H,1-4H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against Zeta-chain (TCR) associated protein kinase 70 kDa (ZAP70) |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against HER2 kinase |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against Syk protein tyrosine kinase |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against Epidermal growth factor receptor |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against Extracellular signal-regulated kinase 1 (Erk-1) |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against p59 Fyn tyrosine kinase |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against p56 Lck tyrosine kinase |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against I-kappa-B-kinase beta |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50042912
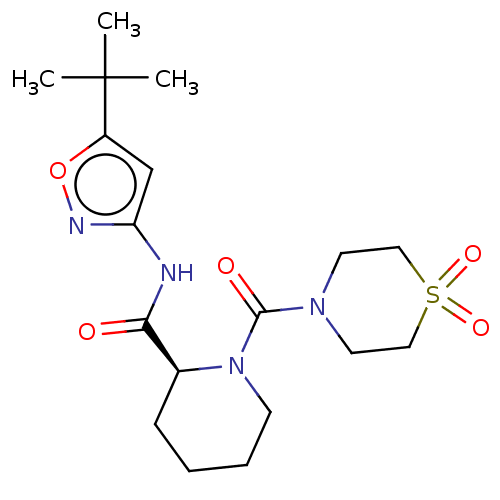
(CHEMBL3354553)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCCN2C(=O)N2CCS(=O)(=O)CC2)no1 |r| Show InChI InChI=1S/C18H28N4O5S/c1-18(2,3)14-12-15(20-27-14)19-16(23)13-6-4-5-7-22(13)17(24)21-8-10-28(25,26)11-9-21/h12-13H,4-11H2,1-3H3,(H,19,20,23)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 25: 587-92 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.031
BindingDB Entry DOI: 10.7270/Q2QZ2CM5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50341008
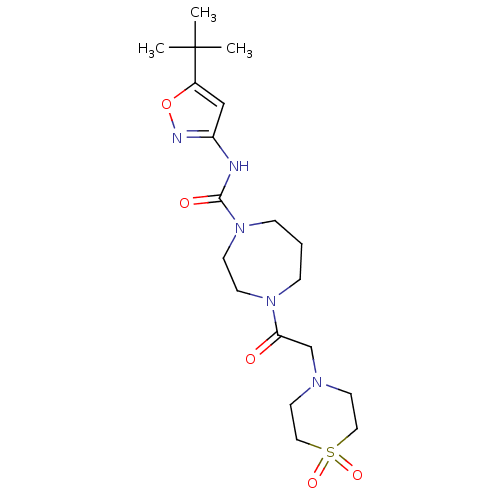
(4-[2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-ace...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)CN2CCS(=O)(=O)CC2)no1 Show InChI InChI=1S/C19H31N5O5S/c1-19(2,3)15-13-16(21-29-15)20-18(26)24-6-4-5-23(7-8-24)17(25)14-22-9-11-30(27,28)12-10-22/h13H,4-12,14H2,1-3H3,(H,20,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using flourescent probe 7-benzyloxyquinoline and 7-benzyloxy-4-(trifluoromethyl)-coumarin) |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50341023
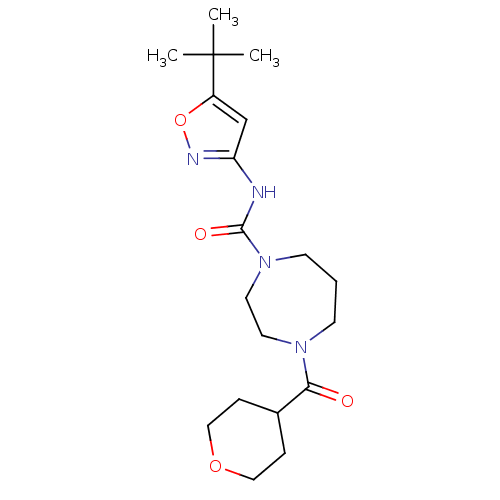
(CHEMBL1762293 | N-(5-tert-butylisoxazol-3-yl)-4-(t...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)C2CCOCC2)no1 Show InChI InChI=1S/C19H30N4O4/c1-19(2,3)15-13-16(21-27-15)20-18(25)23-8-4-7-22(9-10-23)17(24)14-5-11-26-12-6-14/h13-14H,4-12H2,1-3H3,(H,20,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 using flourescent probe 7-methoxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50341023

(CHEMBL1762293 | N-(5-tert-butylisoxazol-3-yl)-4-(t...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)C2CCOCC2)no1 Show InChI InChI=1S/C19H30N4O4/c1-19(2,3)15-13-16(21-27-15)20-18(25)23-8-4-7-22(9-10-23)17(24)14-5-11-26-12-6-14/h13-14H,4-12H2,1-3H3,(H,20,21,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 flourescent probe 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50341023

(CHEMBL1762293 | N-(5-tert-butylisoxazol-3-yl)-4-(t...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)C2CCOCC2)no1 Show InChI InChI=1S/C19H30N4O4/c1-19(2,3)15-13-16(21-27-15)20-18(25)23-8-4-7-22(9-10-23)17(24)14-5-11-26-12-6-14/h13-14H,4-12H2,1-3H3,(H,20,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using flourescent probe 7-benzyloxyquinoline and 7-benzyloxy-4-(trifluoromethyl)-coumarin) |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50341008

(4-[2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-ace...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)CN2CCS(=O)(=O)CC2)no1 Show InChI InChI=1S/C19H31N5O5S/c1-19(2,3)15-13-16(21-29-15)20-18(26)24-6-4-5-23(7-8-24)17(25)14-22-9-11-30(27,28)12-10-22/h13H,4-12,14H2,1-3H3,(H,20,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 using flourescent probe 7-methoxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50341008

(4-[2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-ace...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)CN2CCS(=O)(=O)CC2)no1 Show InChI InChI=1S/C19H31N5O5S/c1-19(2,3)15-13-16(21-29-15)20-18(26)24-6-4-5-23(7-8-24)17(25)14-22-9-11-30(27,28)12-10-22/h13H,4-12,14H2,1-3H3,(H,20,21,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 flourescent probe 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50340991
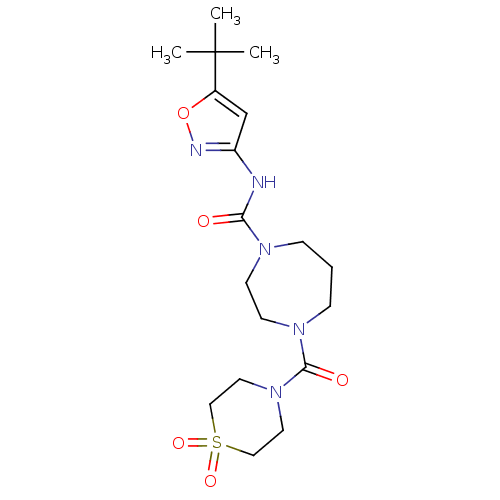
(4-(1,1-Dioxo-1lambda*6*-thiomorpholine-4-carbonyl)...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)N2CCS(=O)(=O)CC2)no1 Show InChI InChI=1S/C18H29N5O5S/c1-18(2,3)14-13-15(20-28-14)19-16(24)21-5-4-6-22(8-7-21)17(25)23-9-11-29(26,27)12-10-23/h13H,4-12H2,1-3H3,(H,19,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using flourescent probe 7-benzyloxyquinoline and 7-benzyloxy-4-(trifluoromethyl)-coumarin) |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50340991

(4-(1,1-Dioxo-1lambda*6*-thiomorpholine-4-carbonyl)...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)N2CCS(=O)(=O)CC2)no1 Show InChI InChI=1S/C18H29N5O5S/c1-18(2,3)14-13-15(20-28-14)19-16(24)21-5-4-6-22(8-7-21)17(25)23-9-11-29(26,27)12-10-23/h13H,4-12H2,1-3H3,(H,19,20,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 flourescent probe 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50340991

(4-(1,1-Dioxo-1lambda*6*-thiomorpholine-4-carbonyl)...)Show SMILES CC(C)(C)c1cc(NC(=O)N2CCCN(CC2)C(=O)N2CCS(=O)(=O)CC2)no1 Show InChI InChI=1S/C18H29N5O5S/c1-18(2,3)14-13-15(20-28-14)19-16(24)21-5-4-6-22(8-7-21)17(25)23-9-11-29(26,27)12-10-23/h13H,4-12H2,1-3H3,(H,19,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 using flourescent probe 7-methoxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 21: 2011-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.017
BindingDB Entry DOI: 10.7270/Q2KK9C2M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50042912

(CHEMBL3354553)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCCN2C(=O)N2CCS(=O)(=O)CC2)no1 |r| Show InChI InChI=1S/C18H28N4O5S/c1-18(2,3)14-12-15(20-27-14)19-16(23)13-6-4-5-7-22(13)17(24)21-8-10-28(25,26)11-9-21/h12-13H,4-11H2,1-3H3,(H,19,20,23)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
Bioorg Med Chem Lett 25: 587-92 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.031
BindingDB Entry DOI: 10.7270/Q2QZ2CM5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50042912

(CHEMBL3354553)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCCN2C(=O)N2CCS(=O)(=O)CC2)no1 |r| Show InChI InChI=1S/C18H28N4O5S/c1-18(2,3)14-12-15(20-27-14)19-16(23)13-6-4-5-7-22(13)17(24)21-8-10-28(25,26)11-9-21/h12-13H,4-11H2,1-3H3,(H,19,20,23)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem Lett 25: 587-92 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.031
BindingDB Entry DOI: 10.7270/Q2QZ2CM5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data