

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50420334 (CHEMBL2086421) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tight binding inhibition of human matriptase expressed in Drosophila melanogaster S2 cells using Boc-QAR-AMC as substrate incubated for 15 mins prior... | ACS Med Chem Lett 3: 530-534 (2012) Article DOI: 10.1021/ml3000534 BindingDB Entry DOI: 10.7270/Q2DN469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein [596-855] (Homo sapiens (Human)) | BDBM236490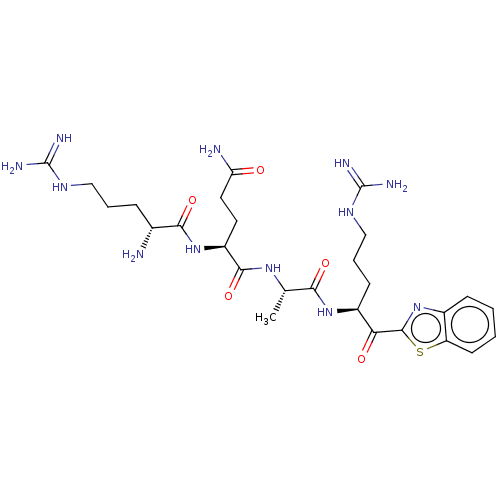 (US9365853, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0110 | -62.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
TBA US Patent | Assay Description Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti... | US Patent US9365853 (2016) BindingDB Entry DOI: 10.7270/Q28W3C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032696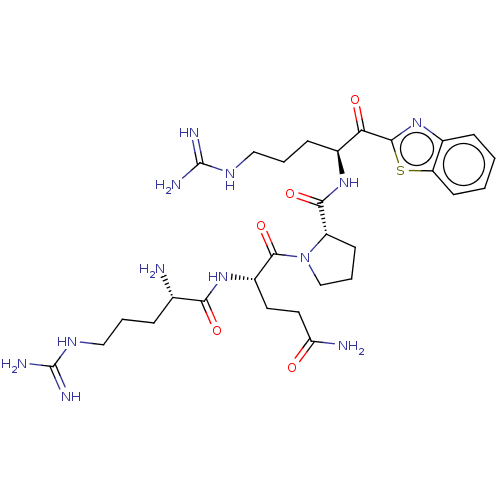 (CHEMBL3354674) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50420336 (CHEMBL2089123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tight binding inhibition of human matriptase expressed in Drosophila melanogaster S2 cells using Boc-QAR-AMC as substrate incubated for 15 mins prior... | ACS Med Chem Lett 3: 530-534 (2012) Article DOI: 10.1021/ml3000534 BindingDB Entry DOI: 10.7270/Q2DN469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein [596-855] (Homo sapiens (Human)) | BDBM236493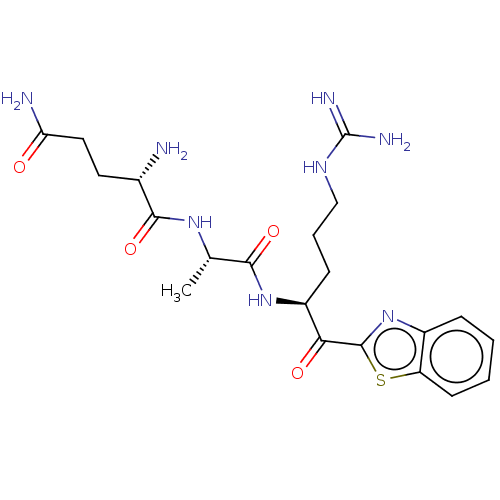 (US10988505, Comparative #2 | US9365853, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0880 | -57.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
TBA US Patent | Assay Description Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti... | US Patent US9365853 (2016) BindingDB Entry DOI: 10.7270/Q28W3C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032702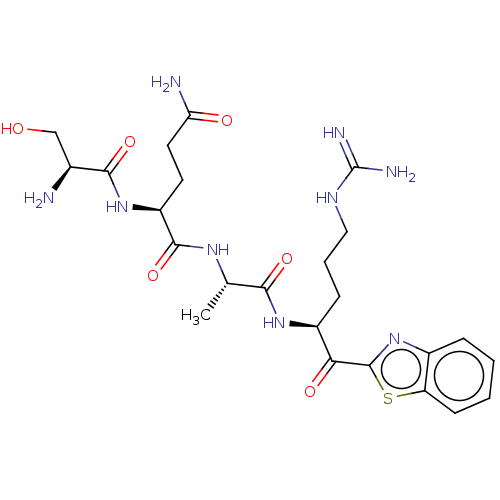 (CHEMBL3352840) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032699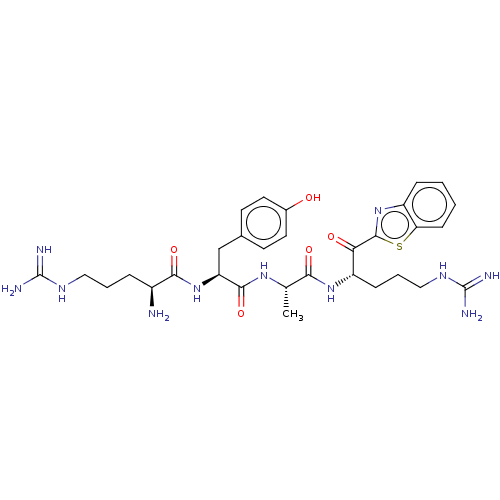 (CHEMBL3354677) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032701 (CHEMBL3354679) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032697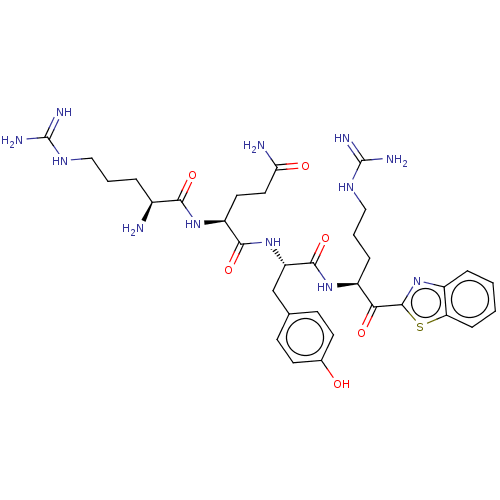 (CHEMBL3354675) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032698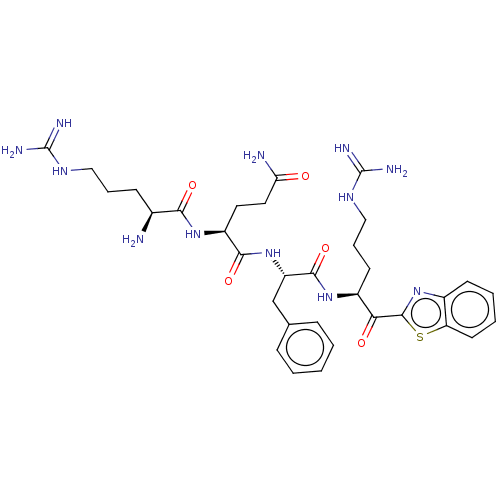 (CHEMBL3354676 | N-0130) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032705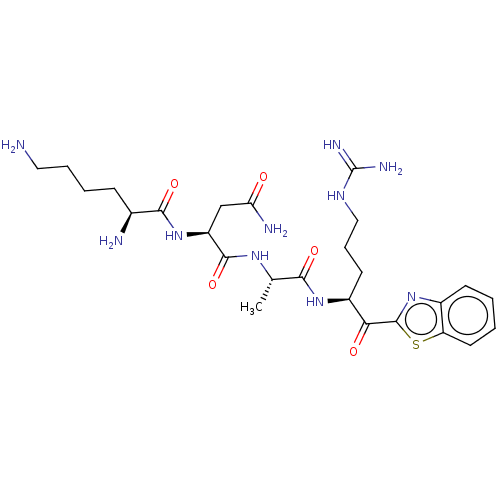 (CHEMBL3354682) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032700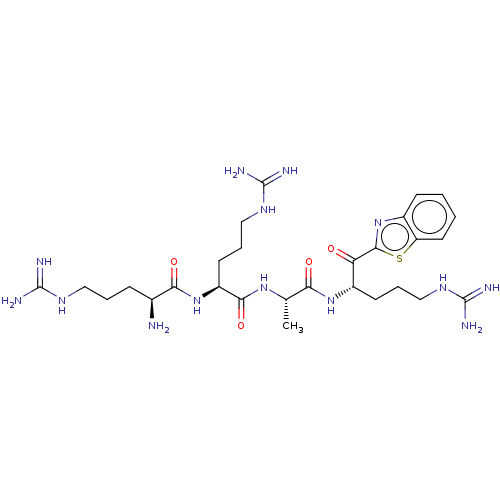 (CHEMBL3354678) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032697 (CHEMBL3354675) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032710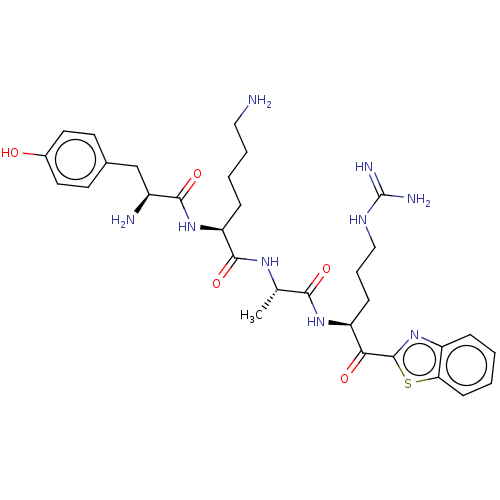 (CHEMBL3354687) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032704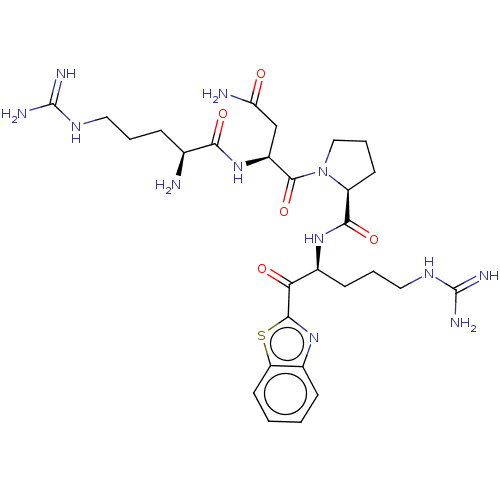 (CHEMBL3354681) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032706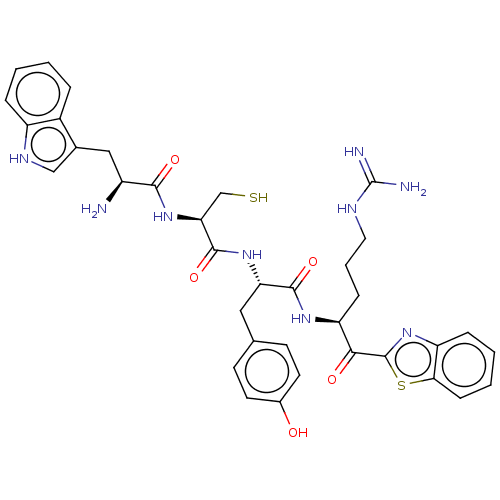 (CHEMBL3354683) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50420334 (CHEMBL2086421) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tight binding inhibition of human hepsin expressed in Drosophila melanogaster S2 cells using Boc-QAR-AMC as substrate incubated for 15 mins prior to ... | ACS Med Chem Lett 3: 530-534 (2012) Article DOI: 10.1021/ml3000534 BindingDB Entry DOI: 10.7270/Q2DN469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein [596-855] (Homo sapiens (Human)) | BDBM236494 (US9365853, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 1.40 | -50.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
TBA US Patent | Assay Description Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti... | US Patent US9365853 (2016) BindingDB Entry DOI: 10.7270/Q28W3C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032709 (CHEMBL3354686) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50420337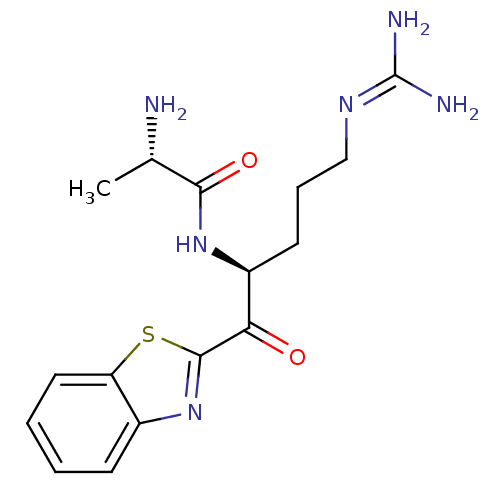 (CHEMBL2089124) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tight binding inhibition of human matriptase expressed in Drosophila melanogaster S2 cells using Boc-QAR-AMC as substrate incubated for 15 mins prior... | ACS Med Chem Lett 3: 530-534 (2012) Article DOI: 10.1021/ml3000534 BindingDB Entry DOI: 10.7270/Q2DN469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032698 (CHEMBL3354676 | N-0130) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032708 (CHEMBL3354685) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032709 (CHEMBL3354686) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032707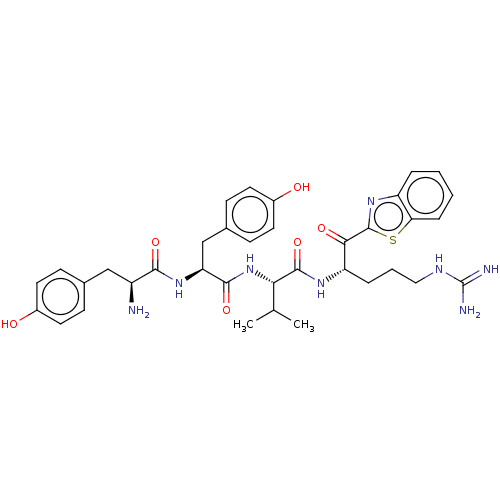 (CHEMBL3354684) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032708 (CHEMBL3354685) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50420334 (CHEMBL2086421) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tight binding inhibition of human matriptase2 expressed in Drosophila melanogaster S2 cells using Boc-QAR-AMC as substrate incubated for 15 mins prio... | ACS Med Chem Lett 3: 530-534 (2012) Article DOI: 10.1021/ml3000534 BindingDB Entry DOI: 10.7270/Q2DN469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50420338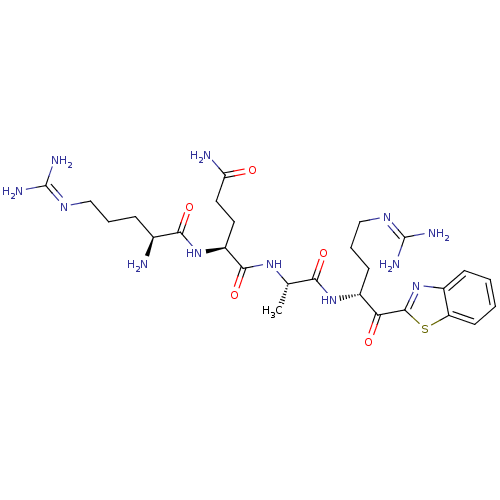 (CHEMBL2089154) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tight binding inhibition of human matriptase expressed in Drosophila melanogaster S2 cells using Boc-QAR-AMC as substrate incubated for 15 mins prior... | ACS Med Chem Lett 3: 530-534 (2012) Article DOI: 10.1021/ml3000534 BindingDB Entry DOI: 10.7270/Q2DN469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein [596-855] (Homo sapiens (Human)) | BDBM236496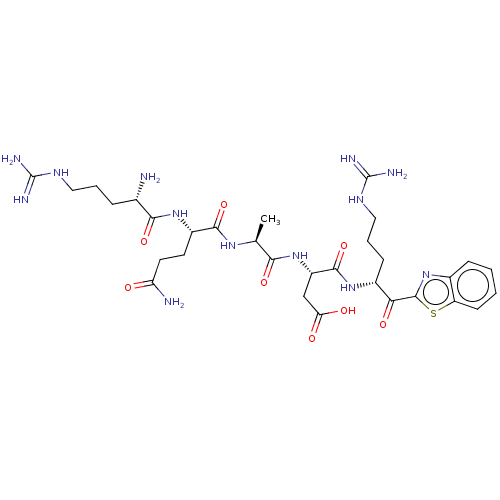 (US9365853, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.60 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
TBA US Patent | Assay Description Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti... | US Patent US9365853 (2016) BindingDB Entry DOI: 10.7270/Q28W3C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032699 (CHEMBL3354677) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032696 (CHEMBL3354674) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032706 (CHEMBL3354683) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 11D (Homo sapiens (Human)) | BDBM50420334 (CHEMBL2086421) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tight binding inhibition of human TMPRSS11D expressed in Drosophila melanogaster S2 cells using Boc-QAR-AMC as substrate incubated for 15 mins prior ... | ACS Med Chem Lett 3: 530-534 (2012) Article DOI: 10.1021/ml3000534 BindingDB Entry DOI: 10.7270/Q2DN469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein [596-855] (Homo sapiens (Human)) | BDBM236492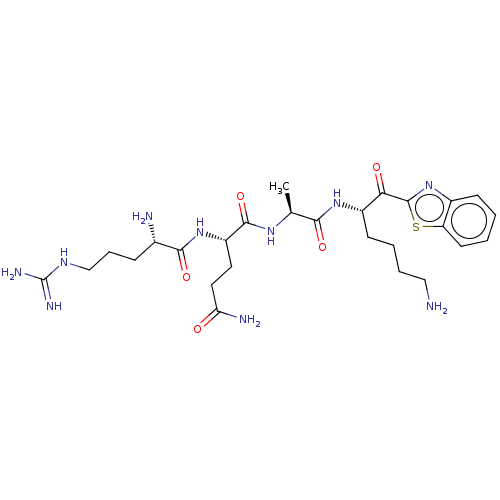 (US9365853, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.5 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
TBA US Patent | Assay Description Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti... | US Patent US9365853 (2016) BindingDB Entry DOI: 10.7270/Q28W3C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50420335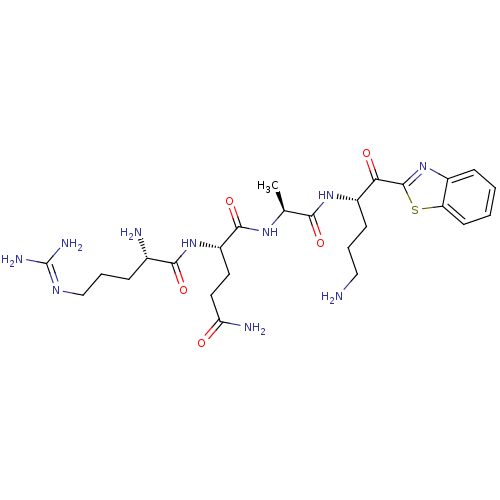 (CHEMBL2089122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tight binding inhibition of human matriptase expressed in Drosophila melanogaster S2 cells using Boc-QAR-AMC as substrate incubated for 15 mins prior... | ACS Med Chem Lett 3: 530-534 (2012) Article DOI: 10.1021/ml3000534 BindingDB Entry DOI: 10.7270/Q2DN469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032701 (CHEMBL3354679) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032703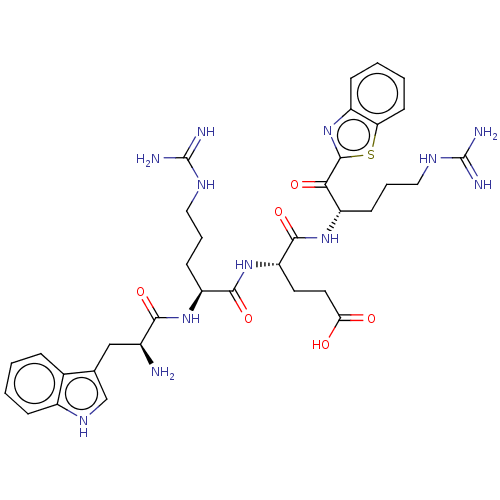 (CHEMBL3354680) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032700 (CHEMBL3354678) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032710 (CHEMBL3354687) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032705 (CHEMBL3354682) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032707 (CHEMBL3354684) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032702 (CHEMBL3352840) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032703 (CHEMBL3354680) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 6 (Homo sapiens (Human)) | BDBM50032704 (CHEMBL3354681) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sherbrooke Curated by ChEMBL | Assay Description Inhibition of human recombinant matriptase-2 using Boc-Gln-Ala-Arg-AMC as substrate by microplate reader analysis | J Med Chem 57: 10198-204 (2014) Article DOI: 10.1021/jm5015633 BindingDB Entry DOI: 10.7270/Q2DJ5H73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50420340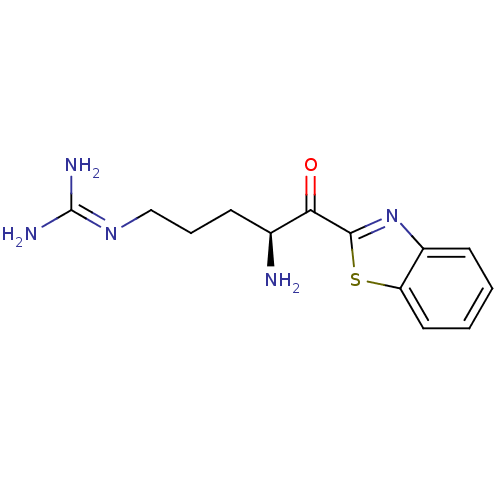 (CHEMBL2089125) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed inhibition of human matriptase expressed in Drosophila melanogaster S2 cells using Boc-QAR-AMC as substrate by fluorimetric analysis | ACS Med Chem Lett 3: 530-534 (2012) Article DOI: 10.1021/ml3000534 BindingDB Entry DOI: 10.7270/Q2DN469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein [596-855] (Homo sapiens (Human)) | BDBM236495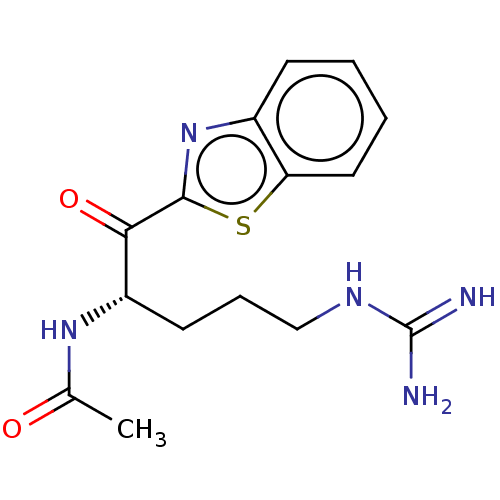 (US9365853, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 457 | -36.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
TBA US Patent | Assay Description Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti... | US Patent US9365853 (2016) BindingDB Entry DOI: 10.7270/Q28W3C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50420334 (CHEMBL2086421) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed inhibition of human thrombin expressed in Drosophila melanogaster S2 cells using Boc-QAR-AMC as substrate by fluorimetric analysis | ACS Med Chem Lett 3: 530-534 (2012) Article DOI: 10.1021/ml3000534 BindingDB Entry DOI: 10.7270/Q2DN469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein [596-855] (Homo sapiens (Human)) | BDBM236491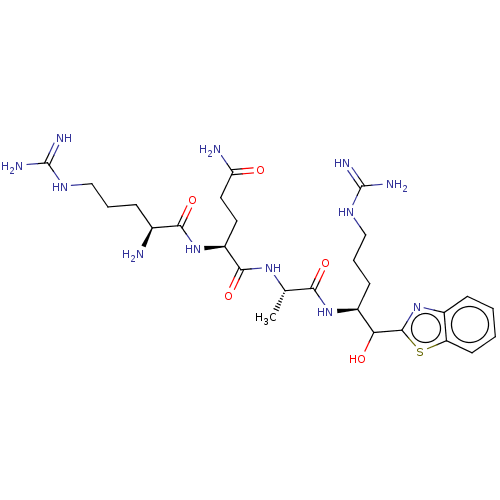 (US9365853, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.12E+3 | -29.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
TBA US Patent | Assay Description Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti... | US Patent US9365853 (2016) BindingDB Entry DOI: 10.7270/Q28W3C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50420339 (CHEMBL2089121) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed inhibition of human matriptase expressed in Drosophila melanogaster S2 cells using Boc-QAR-AMC as substrate by fluorimetric analysis | ACS Med Chem Lett 3: 530-534 (2012) Article DOI: 10.1021/ml3000534 BindingDB Entry DOI: 10.7270/Q2DN469X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM19187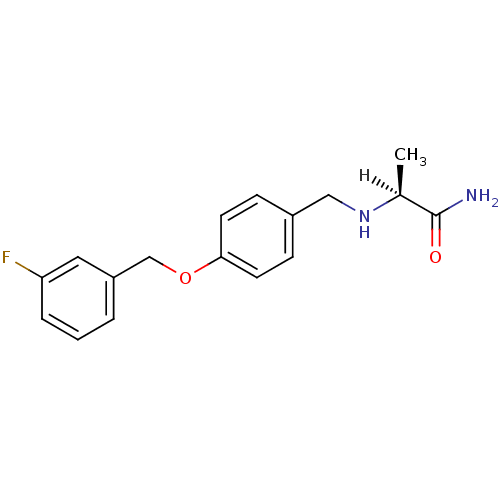 ((2S)-2-[({4-[(3-fluorophenyl)methoxy]phenyl}methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Newron Pharmaceuticals S.P.A. US Patent | Assay Description The enzyme activity was assessed with a radioenzymatic assay using the substrate 14C-phenylethylamine (PEA) specific for MAO-B.The mitochondrial pell... | US Patent US9051240 (2015) BindingDB Entry DOI: 10.7270/Q2KK99J2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM161094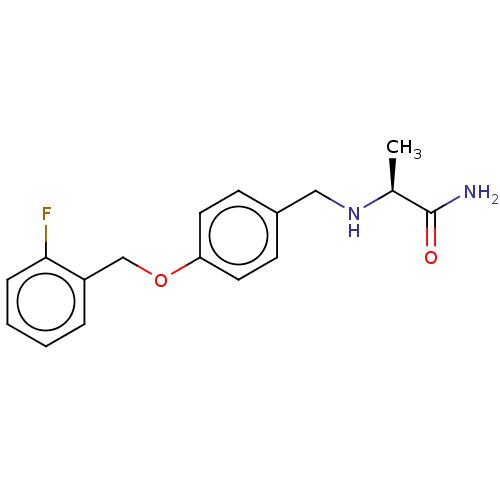 (US9051240, (S)-(+)-2-[4-(2-Fluorobenzyloxy)-benzyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Newron Pharmaceuticals S.P.A. US Patent | Assay Description The enzyme activity was assessed with a radioenzymatic assay using the substrate 14C-phenylethylamine (PEA) specific for MAO-B.The mitochondrial pell... | US Patent US9051240 (2015) BindingDB Entry DOI: 10.7270/Q2KK99J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 66 total ) | Next | Last >> |