 Found 113 hits with Last Name = 'combs' and Initial = 'k'
Found 113 hits with Last Name = 'combs' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50259376
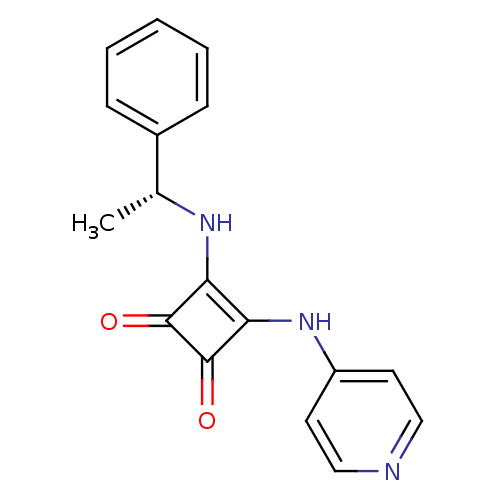
(3-((R)-1-Phenyl-ethylamino)-4-(pyridin-4-ylamino)-...)Show SMILES C[C@@H](Nc1c(Nc2ccncc2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C17H15N3O2/c1-11(12-5-3-2-4-6-12)19-14-15(17(22)16(14)21)20-13-7-9-18-10-8-13/h2-11,19H,1H3,(H,18,20)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50259563
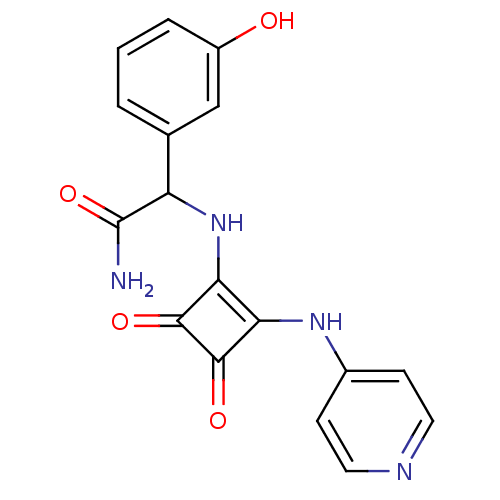
(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show SMILES NC(=O)C(Nc1c(Nc2ccncc2)c(=O)c1=O)c1cccc(O)c1 Show InChI InChI=1S/C17H14N4O4/c18-17(25)12(9-2-1-3-11(22)8-9)21-14-13(15(23)16(14)24)20-10-4-6-19-7-5-10/h1-8,12,21-22H,(H2,18,25)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform gamma-1
(Homo sapiens (Human)) | BDBM50259563

(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show SMILES NC(=O)C(Nc1c(Nc2ccncc2)c(=O)c1=O)c1cccc(O)c1 Show InChI InChI=1S/C17H14N4O4/c18-17(25)12(9-2-1-3-11(22)8-9)21-14-13(15(23)16(14)24)20-10-4-6-19-7-5-10/h1-8,12,21-22H,(H2,18,25)(H,19,20) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CK1-gamma1 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform gamma-1
(Homo sapiens (Human)) | BDBM50259376

(3-((R)-1-Phenyl-ethylamino)-4-(pyridin-4-ylamino)-...)Show SMILES C[C@@H](Nc1c(Nc2ccncc2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C17H15N3O2/c1-11(12-5-3-2-4-6-12)19-14-15(17(22)16(14)21)20-13-7-9-18-10-8-13/h2-11,19H,1H3,(H,18,20)/t11-/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CK1-gamma1 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM50259563

(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show SMILES NC(=O)C(Nc1c(Nc2ccncc2)c(=O)c1=O)c1cccc(O)c1 Show InChI InChI=1S/C17H14N4O4/c18-17(25)12(9-2-1-3-11(22)8-9)21-14-13(15(23)16(14)24)20-10-4-6-19-7-5-10/h1-8,12,21-22H,(H2,18,25)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of RSK1 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259483
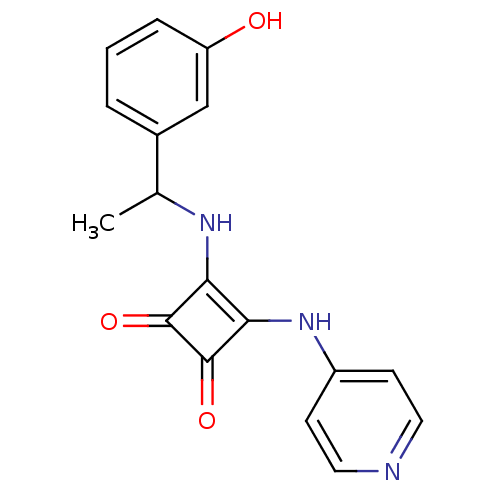
(3-{[1-(3-Hydroxyphenyl)ethyl]amino}-4-(pyridin-4-y...)Show InChI InChI=1S/C17H15N3O3/c1-10(11-3-2-4-13(21)9-11)19-14-15(17(23)16(14)22)20-12-5-7-18-8-6-12/h2-10,19,21H,1H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259563

(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show SMILES NC(=O)C(Nc1c(Nc2ccncc2)c(=O)c1=O)c1cccc(O)c1 Show InChI InChI=1S/C17H14N4O4/c18-17(25)12(9-2-1-3-11(22)8-9)21-14-13(15(23)16(14)24)20-10-4-6-19-7-5-10/h1-8,12,21-22H,(H2,18,25)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform gamma-1
(Homo sapiens (Human)) | BDBM50259532
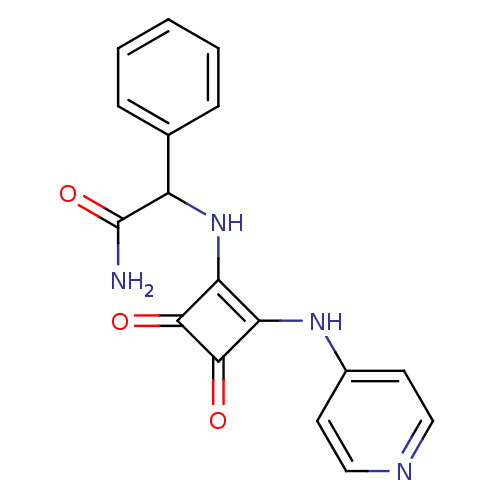
(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show InChI InChI=1S/C17H14N4O3/c18-17(24)12(10-4-2-1-3-5-10)21-14-13(15(22)16(14)23)20-11-6-8-19-9-7-11/h1-9,12,21H,(H2,18,24)(H,19,20) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CK1-gamma1 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50259376

(3-((R)-1-Phenyl-ethylamino)-4-(pyridin-4-ylamino)-...)Show SMILES C[C@@H](Nc1c(Nc2ccncc2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C17H15N3O2/c1-11(12-5-3-2-4-6-12)19-14-15(17(22)16(14)21)20-13-7-9-18-10-8-13/h2-11,19H,1H3,(H,18,20)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50258907

(3-{[(1R)-1-Phenylethyl]amino}-4-{[2-(pyrimidin-4-y...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(Nc3ccncn3)c2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C21H18N6O2/c1-13(14-5-3-2-4-6-14)25-18-19(21(29)20(18)28)26-15-7-10-23-17(11-15)27-16-8-9-22-12-24-16/h2-13,25H,1H3,(H2,22,23,24,26,27)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM50259532

(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show InChI InChI=1S/C17H14N4O3/c18-17(24)12(10-4-2-1-3-5-10)21-14-13(15(22)16(14)23)20-11-6-8-19-9-7-11/h1-9,12,21H,(H2,18,24)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of RSK1 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM50259376

(3-((R)-1-Phenyl-ethylamino)-4-(pyridin-4-ylamino)-...)Show SMILES C[C@@H](Nc1c(Nc2ccncc2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C17H15N3O2/c1-11(12-5-3-2-4-6-12)19-14-15(17(22)16(14)21)20-13-7-9-18-10-8-13/h2-11,19H,1H3,(H,18,20)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of RSK1 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50258950
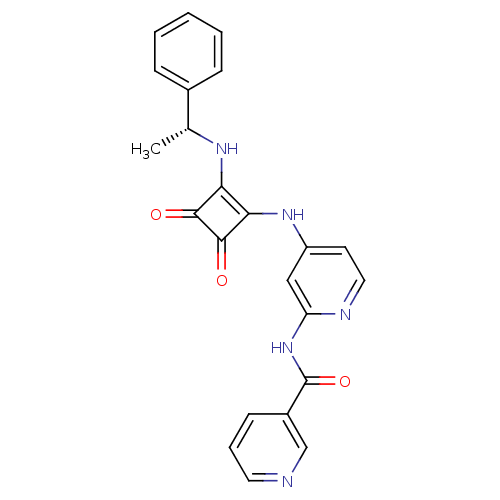
(CHEMBL468322 | N-{4-[(3,4-Dioxo-2-{[(1R)-1-phenyle...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(NC(=O)c3cccnc3)c2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C23H19N5O3/c1-14(15-6-3-2-4-7-15)26-19-20(22(30)21(19)29)27-17-9-11-25-18(12-17)28-23(31)16-8-5-10-24-13-16/h2-14,26H,1H3,(H2,25,27,28,31)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50259376

(3-((R)-1-Phenyl-ethylamino)-4-(pyridin-4-ylamino)-...)Show SMILES C[C@@H](Nc1c(Nc2ccncc2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C17H15N3O2/c1-11(12-5-3-2-4-6-12)19-14-15(17(22)16(14)21)20-13-7-9-18-10-8-13/h2-11,19H,1H3,(H,18,20)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50259563

(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show SMILES NC(=O)C(Nc1c(Nc2ccncc2)c(=O)c1=O)c1cccc(O)c1 Show InChI InChI=1S/C17H14N4O4/c18-17(25)12(9-2-1-3-11(22)8-9)21-14-13(15(23)16(14)24)20-10-4-6-19-7-5-10/h1-8,12,21-22H,(H2,18,25)(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50258908
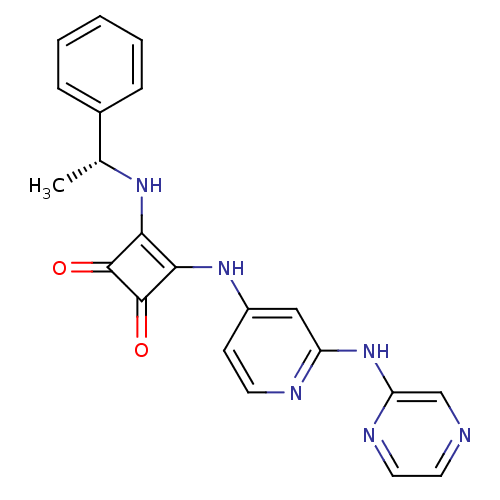
(3-{[(1R)-1-Phenylethyl]amino}-4-{[2-(pyrazin-2-yla...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(Nc3cnccn3)c2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C21H18N6O2/c1-13(14-5-3-2-4-6-14)25-18-19(21(29)20(18)28)26-15-7-8-23-16(11-15)27-17-12-22-9-10-24-17/h2-13,25H,1H3,(H2,23,24,26,27)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50258909
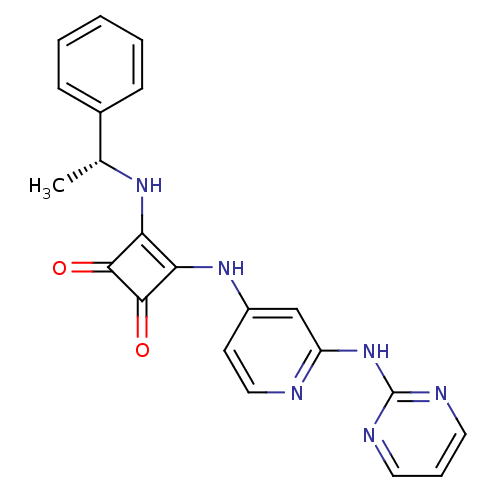
(3-{[(1R)-1-Phenylethyl]amino}-4-{[2-(pyrimidin-2-y...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(Nc3ncccn3)c2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C21H18N6O2/c1-13(14-6-3-2-4-7-14)25-17-18(20(29)19(17)28)26-15-8-11-22-16(12-15)27-21-23-9-5-10-24-21/h2-13,25H,1H3,(H2,22,23,24,26,27)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259379
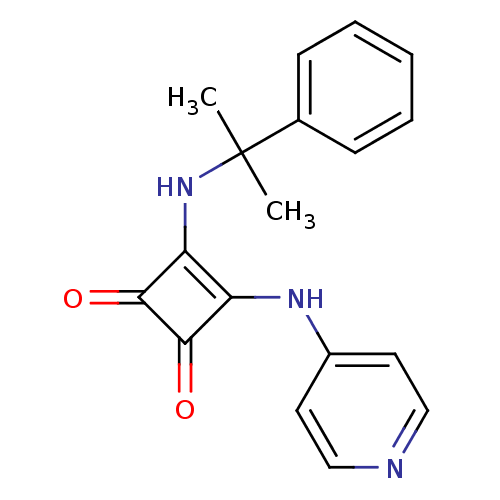
(3-[(1-Methyl-1-phenylethyl)amino]-4-(pyridin-4-yla...)Show InChI InChI=1S/C18H17N3O2/c1-18(2,12-6-4-3-5-7-12)21-15-14(16(22)17(15)23)20-13-8-10-19-11-9-13/h3-11,21H,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50258906
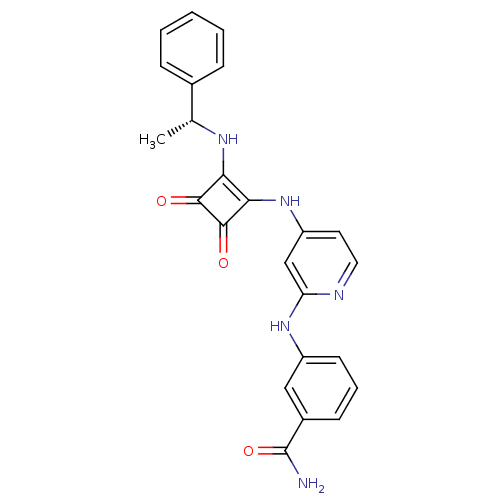
(3-({4-[(3,4-Dioxo-2-{[(1R)-1-phenylethyl]amino}-cy...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(Nc3cccc(c3)C(N)=O)c2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C24H21N5O3/c1-14(15-6-3-2-4-7-15)27-20-21(23(31)22(20)30)29-18-10-11-26-19(13-18)28-17-9-5-8-16(12-17)24(25)32/h2-14,27H,1H3,(H2,25,32)(H2,26,28,29)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259600

(3-{[(1R)-1-Phenylethyl]amino}-4-[(2-phenylpyridin-...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(c2)-c2ccccc2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C23H19N3O2/c1-15(16-8-4-2-5-9-16)25-20-21(23(28)22(20)27)26-18-12-13-24-19(14-18)17-10-6-3-7-11-17/h2-15,25H,1H3,(H,24,26)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50259376

(3-((R)-1-Phenyl-ethylamino)-4-(pyridin-4-ylamino)-...)Show SMILES C[C@@H](Nc1c(Nc2ccncc2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C17H15N3O2/c1-11(12-5-3-2-4-6-12)19-14-15(17(22)16(14)21)20-13-7-9-18-10-8-13/h2-11,19H,1H3,(H,18,20)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50259376

(3-((R)-1-Phenyl-ethylamino)-4-(pyridin-4-ylamino)-...)Show SMILES C[C@@H](Nc1c(Nc2ccncc2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C17H15N3O2/c1-11(12-5-3-2-4-6-12)19-14-15(17(22)16(14)21)20-13-7-9-18-10-8-13/h2-11,19H,1H3,(H,18,20)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259636
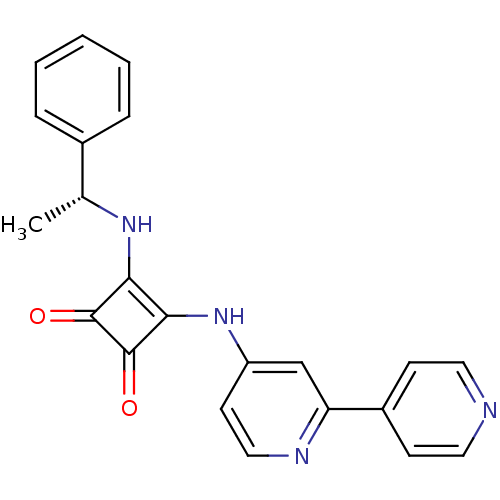
(3-(2,40-Bipyridin-4-ylamino)-4-{[(1R)-1-phenylethy...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(c2)-c2ccncc2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C22H18N4O2/c1-14(15-5-3-2-4-6-15)25-19-20(22(28)21(19)27)26-17-9-12-24-18(13-17)16-7-10-23-11-8-16/h2-14,25H,1H3,(H,24,26)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259635
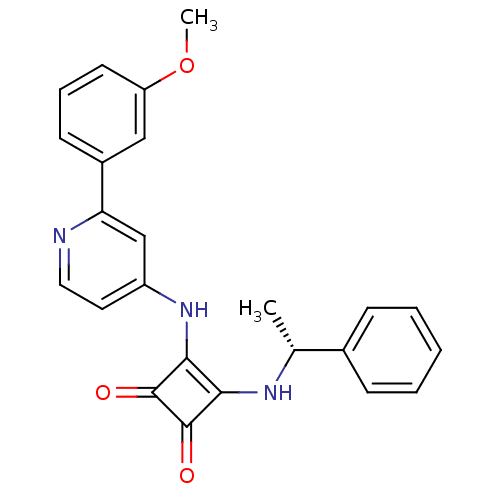
(3-{[2-(3-Methoxyphenyl)pyridin-4-yl]amino}-4-{[(1R...)Show SMILES COc1cccc(c1)-c1cc(Nc2c(N[C@H](C)c3ccccc3)c(=O)c2=O)ccn1 |r| Show InChI InChI=1S/C24H21N3O3/c1-15(16-7-4-3-5-8-16)26-21-22(24(29)23(21)28)27-18-11-12-25-20(14-18)17-9-6-10-19(13-17)30-2/h3-15,26H,1-2H3,(H,25,27)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50258910

(CHEMBL468292 | N-{4-[(3,4-Dioxo-2-{[(1R)-1-phenyle...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(NC(C)=O)c2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C19H18N4O3/c1-11(13-6-4-3-5-7-13)21-16-17(19(26)18(16)25)23-14-8-9-20-15(10-14)22-12(2)24/h3-11,21H,1-2H3,(H2,20,22,23,24)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50259563

(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show SMILES NC(=O)C(Nc1c(Nc2ccncc2)c(=O)c1=O)c1cccc(O)c1 Show InChI InChI=1S/C17H14N4O4/c18-17(25)12(9-2-1-3-11(22)8-9)21-14-13(15(23)16(14)24)20-10-4-6-19-7-5-10/h1-8,12,21-22H,(H2,18,25)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259532

(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show InChI InChI=1S/C17H14N4O3/c18-17(24)12(10-4-2-1-3-5-10)21-14-13(15(22)16(14)23)20-11-6-8-19-9-7-11/h1-9,12,21H,(H2,18,24)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50259376

(3-((R)-1-Phenyl-ethylamino)-4-(pyridin-4-ylamino)-...)Show SMILES C[C@@H](Nc1c(Nc2ccncc2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C17H15N3O2/c1-11(12-5-3-2-4-6-12)19-14-15(17(22)16(14)21)20-13-7-9-18-10-8-13/h2-11,19H,1H3,(H,18,20)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform gamma-1
(Homo sapiens (Human)) | BDBM50259637
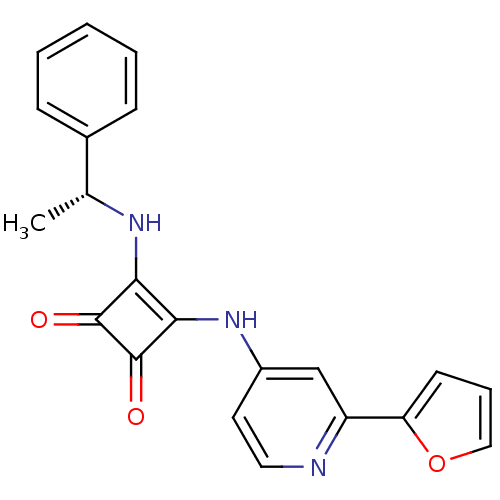
(3-{[2-(2-Furyl)pyridin-4-yl]amino}-4-{[(1R)-1-phen...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(c2)-c2ccco2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C21H17N3O3/c1-13(14-6-3-2-4-7-14)23-18-19(21(26)20(18)25)24-15-9-10-22-16(12-15)17-8-5-11-27-17/h2-13,23H,1H3,(H,22,24)/t13-/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CK1-gamma1 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259531

(3-{[1-(4-hydroxyphenyl)ethyl]amino}-4-(pyridin-4-y...)Show InChI InChI=1S/C17H15N3O3/c1-10(11-2-4-13(21)5-3-11)19-14-15(17(23)16(14)22)20-12-6-8-18-9-7-12/h2-10,19,21H,1H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50259532

(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show InChI InChI=1S/C17H14N4O3/c18-17(24)12(10-4-2-1-3-5-10)21-14-13(15(22)16(14)23)20-11-6-8-19-9-7-11/h1-9,12,21H,(H2,18,24)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259637

(3-{[2-(2-Furyl)pyridin-4-yl]amino}-4-{[(1R)-1-phen...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(c2)-c2ccco2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C21H17N3O3/c1-13(14-6-3-2-4-7-14)23-18-19(21(26)20(18)25)24-15-9-10-22-16(12-15)17-8-5-11-27-17/h2-13,23H,1H3,(H,22,24)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259564

(3-{[(1S)-2-Hydroxy-1-phenylethyl]amino}-4-(pyridin...)Show InChI InChI=1S/C17H15N3O3/c21-10-13(11-4-2-1-3-5-11)20-15-14(16(22)17(15)23)19-12-6-8-18-9-7-12/h1-9,13,20-21H,10H2,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259638

(3-{[2-(3-Thienyl)pyridin-4-yl]amino}-4-{[(1R)-1-ph...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(c2)-c2ccsc2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C21H17N3O2S/c1-13(14-5-3-2-4-6-14)23-18-19(21(26)20(18)25)24-16-7-9-22-17(11-16)15-8-10-27-12-15/h2-13,23H,1H3,(H,22,24)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259528
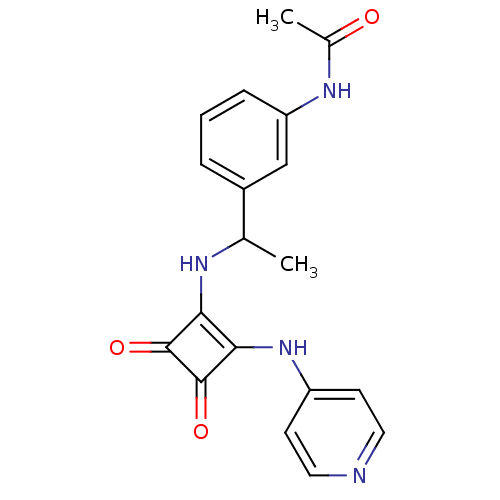
(CHEMBL452699 | N-[3-(1-{[3,4-Dioxo-2-(pyridin-4-yl...)Show SMILES CC(Nc1c(Nc2ccncc2)c(=O)c1=O)c1cccc(NC(C)=O)c1 Show InChI InChI=1S/C19H18N4O3/c1-11(13-4-3-5-15(10-13)22-12(2)24)21-16-17(19(26)18(16)25)23-14-6-8-20-9-7-14/h3-11,21H,1-2H3,(H,20,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259601
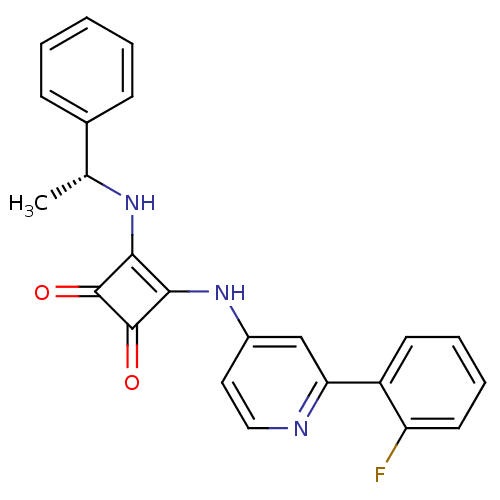
(3-{[2-(2-Fluorophenyl)pyridin-4-yl]amino}-4-{[(1R)...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(c2)-c2ccccc2F)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C23H18FN3O2/c1-14(15-7-3-2-4-8-15)26-20-21(23(29)22(20)28)27-16-11-12-25-19(13-16)17-9-5-6-10-18(17)24/h2-14,26H,1H3,(H,25,27)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50259532

(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show InChI InChI=1S/C17H14N4O3/c18-17(24)12(10-4-2-1-3-5-10)21-14-13(15(22)16(14)23)20-11-6-8-19-9-7-11/h1-9,12,21H,(H2,18,24)(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50259532

(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show InChI InChI=1S/C17H14N4O3/c18-17(24)12(10-4-2-1-3-5-10)21-14-13(15(22)16(14)23)20-11-6-8-19-9-7-11/h1-9,12,21H,(H2,18,24)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50259563

(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show SMILES NC(=O)C(Nc1c(Nc2ccncc2)c(=O)c1=O)c1cccc(O)c1 Show InChI InChI=1S/C17H14N4O4/c18-17(25)12(9-2-1-3-11(22)8-9)21-14-13(15(23)16(14)24)20-10-4-6-19-7-5-10/h1-8,12,21-22H,(H2,18,25)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259602
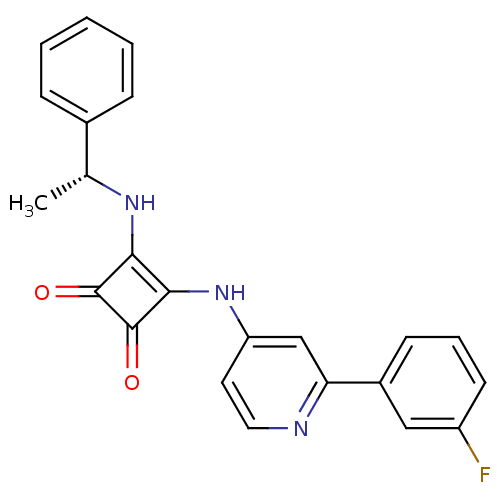
(3-{[2-(3-Fluorophenyl)pyridin-4-yl]amino}-4-{[(1R)...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(c2)-c2cccc(F)c2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C23H18FN3O2/c1-14(15-6-3-2-4-7-15)26-20-21(23(29)22(20)28)27-18-10-11-25-19(13-18)16-8-5-9-17(24)12-16/h2-14,26H,1H3,(H,25,27)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259530

(3-{[1-(4-Fluorophenyl)ethyl]amino}-4-(pyridin-4-yl...)Show InChI InChI=1S/C17H14FN3O2/c1-10(11-2-4-12(18)5-3-11)20-14-15(17(23)16(14)22)21-13-6-8-19-9-7-13/h2-10,20H,1H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50259532

(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show InChI InChI=1S/C17H14N4O3/c18-17(24)12(10-4-2-1-3-5-10)21-14-13(15(22)16(14)23)20-11-6-8-19-9-7-11/h1-9,12,21H,(H2,18,24)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50259637

(3-{[2-(2-Furyl)pyridin-4-yl]amino}-4-{[(1R)-1-phen...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(c2)-c2ccco2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C21H17N3O3/c1-13(14-6-3-2-4-7-14)23-18-19(21(26)20(18)25)24-15-9-10-22-16(12-15)17-8-5-11-27-17/h2-13,23H,1H3,(H,22,24)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259376

(3-((R)-1-Phenyl-ethylamino)-4-(pyridin-4-ylamino)-...)Show SMILES C[C@@H](Nc1c(Nc2ccncc2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C17H15N3O2/c1-11(12-5-3-2-4-6-12)19-14-15(17(22)16(14)21)20-13-7-9-18-10-8-13/h2-11,19H,1H3,(H,18,20)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259639
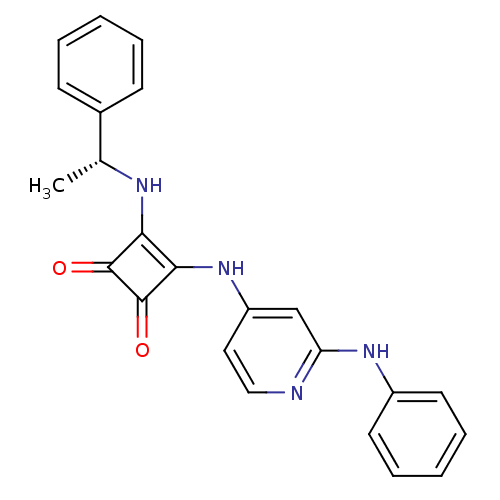
(3-[(2-Anilinopyridin-4-yl)amino]-4-{[(1R)-1-phenyl...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(Nc3ccccc3)c2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C23H20N4O2/c1-15(16-8-4-2-5-9-16)25-20-21(23(29)22(20)28)27-18-12-13-24-19(14-18)26-17-10-6-3-7-11-17/h2-15,25H,1H3,(H2,24,26,27)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50259479

(3-{[1-(2-Fluorophenyl)ethyl]amino}-4-(pyridin-4-yl...)Show InChI InChI=1S/C17H14FN3O2/c1-10(12-4-2-3-5-13(12)18)20-14-15(17(23)16(14)22)21-11-6-8-19-9-7-11/h2-10,20H,1H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MK2 |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50259563

(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show SMILES NC(=O)C(Nc1c(Nc2ccncc2)c(=O)c1=O)c1cccc(O)c1 Show InChI InChI=1S/C17H14N4O4/c18-17(25)12(9-2-1-3-11(22)8-9)21-14-13(15(23)16(14)24)20-10-4-6-19-7-5-10/h1-8,12,21-22H,(H2,18,25)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50259563

(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show SMILES NC(=O)C(Nc1c(Nc2ccncc2)c(=O)c1=O)c1cccc(O)c1 Show InChI InChI=1S/C17H14N4O4/c18-17(25)12(9-2-1-3-11(22)8-9)21-14-13(15(23)16(14)24)20-10-4-6-19-7-5-10/h1-8,12,21-22H,(H2,18,25)(H,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50259532

(2-{[3,4-Dioxo-2-(pyridin-4-ylamino)cyclobut-1-en-1...)Show InChI InChI=1S/C17H14N4O3/c18-17(24)12(10-4-2-1-3-5-10)21-14-13(15(22)16(14)23)20-11-6-8-19-9-7-11/h1-9,12,21H,(H2,18,24)(H,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50259638

(3-{[2-(3-Thienyl)pyridin-4-yl]amino}-4-{[(1R)-1-ph...)Show SMILES C[C@@H](Nc1c(Nc2ccnc(c2)-c2ccsc2)c(=O)c1=O)c1ccccc1 |r| Show InChI InChI=1S/C21H17N3O2S/c1-13(14-5-3-2-4-6-14)23-18-19(21(26)20(18)25)24-16-7-9-22-17(11-16)15-8-10-27-12-15/h2-13,23H,1H3,(H,22,24)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B (unknown origin) |
Bioorg Med Chem 17: 3342-51 (2009)
Article DOI: 10.1016/j.bmc.2009.03.041
BindingDB Entry DOI: 10.7270/Q2WW7HK4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data