

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203905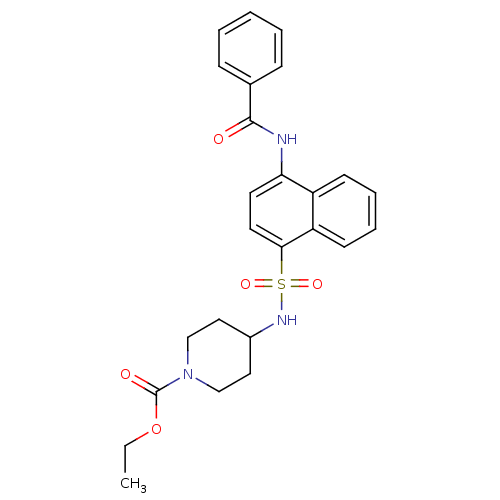 (4-(4-benzoylamino-naphthalene-1-sulfonylamino)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203831 (CHEMBL374939 | N-(4-{[(1-butyrylpiperidin-4-yl)ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203834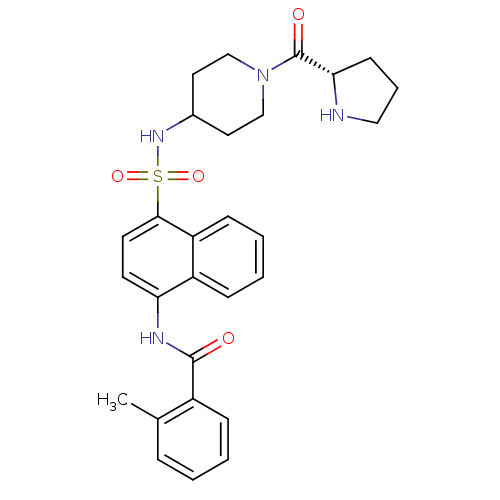 (2-methyl-N-{4-[({1-[(2S)-pyrrolidin-2-ylcarbonyl]p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203832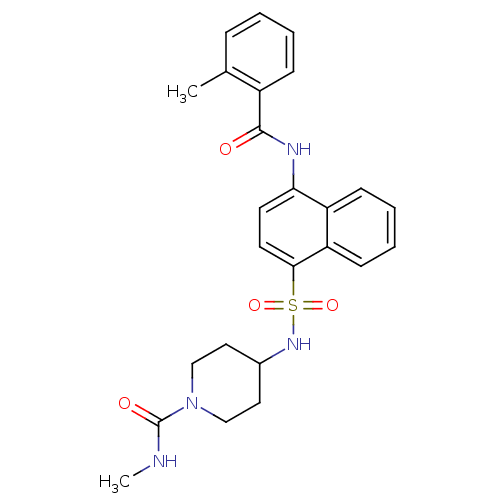 (4-[4-(2-methyl-benzoylamino)-naphthalene-1-sulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203834 (2-methyl-N-{4-[({1-[(2S)-pyrrolidin-2-ylcarbonyl]p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203919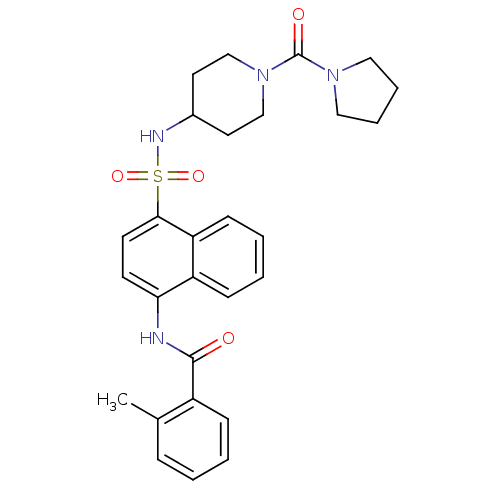 (2-methyl-N-[4-({[1-(pyrrolidin-1-ylcarbonyl)piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26399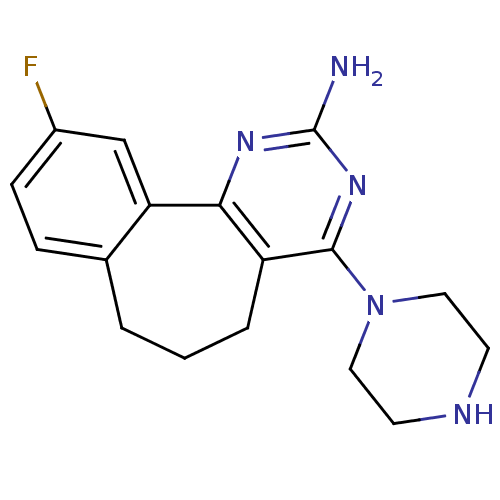 (14-fluoro-6-(piperazin-1-yl)-3,5-diazatricyclo[9.4...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.45 | -50.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203914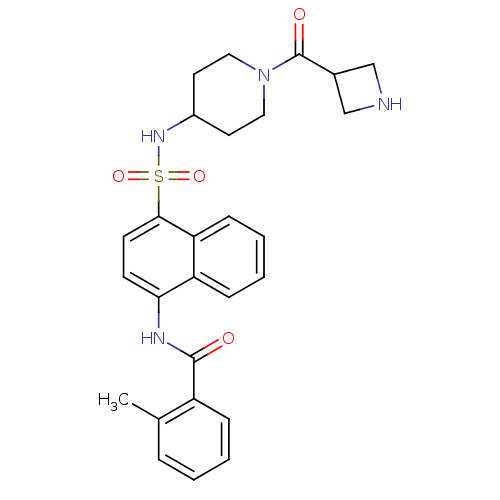 (CHEMBL375854 | N-[4-({[1-(azetidin-3-ylcarbonyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203920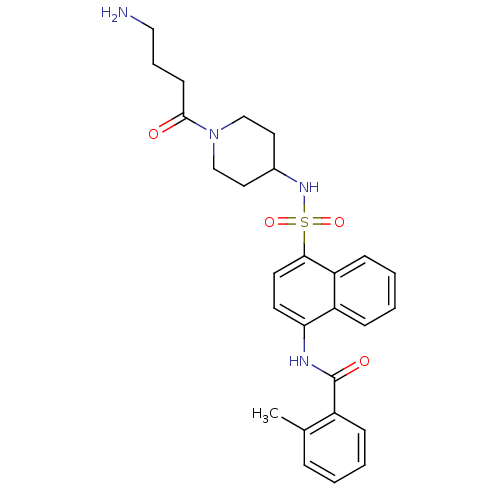 (CHEMBL221867 | N-{4-[1-(4-amino-butyryl)-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203881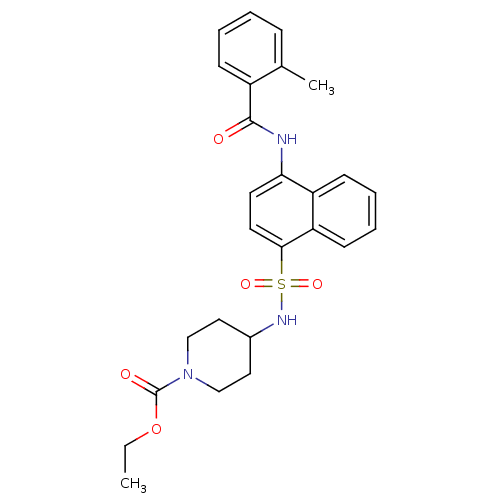 (4-[4-(2-methyl-benzoylamino)-naphthalene-1-sulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203871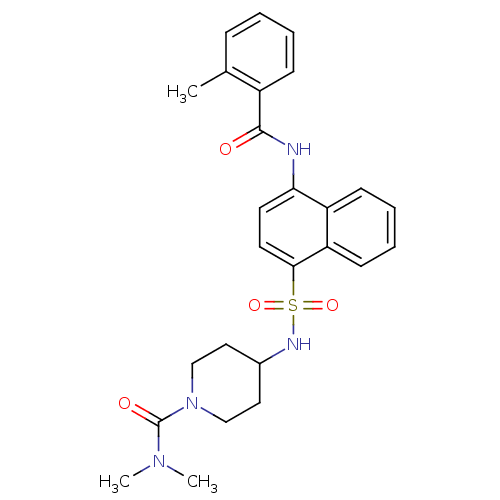 (4-({[4-(benzoylamino)-1-naphthyl]sulfonyl}amino)-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203859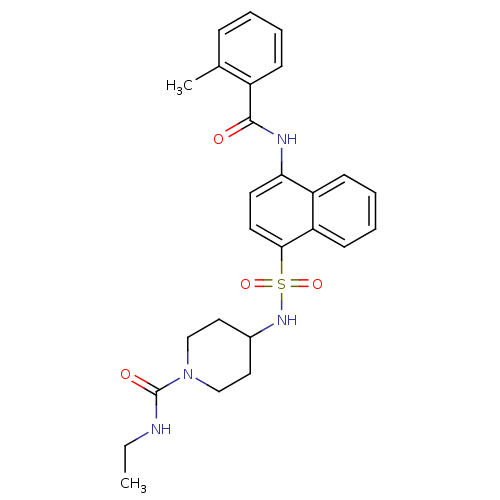 (4-[4-(2-methyl-benzoylamino)-naphthalene-1-sulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203857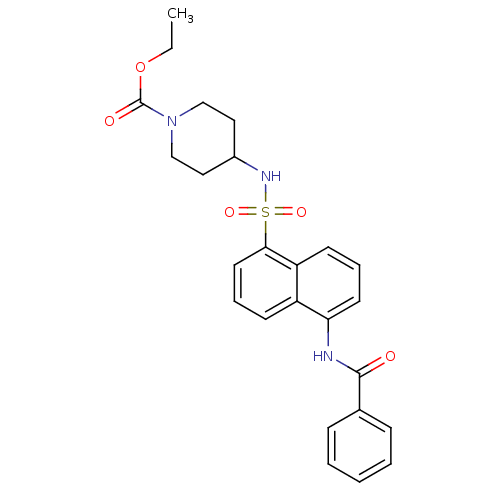 (CHEMBL218037 | ethyl 4-({[5-(benzoylamino)-1-napht...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203900 (2-methyl-N-[4-(1-propionyl-piperidin-4-ylsulfamoyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6571-80 (2008) Article DOI: 10.1021/jm8005959 BindingDB Entry DOI: 10.7270/Q24J0CD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University | Assay Description Inhibition assay using Esherichia coli dihydrofolate reductase (DHFR). | Chem Biol 11: 1423-30 (2004) Article DOI: 10.1016/j.chembiol.2004.08.014 BindingDB Entry DOI: 10.7270/Q2639N6G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203853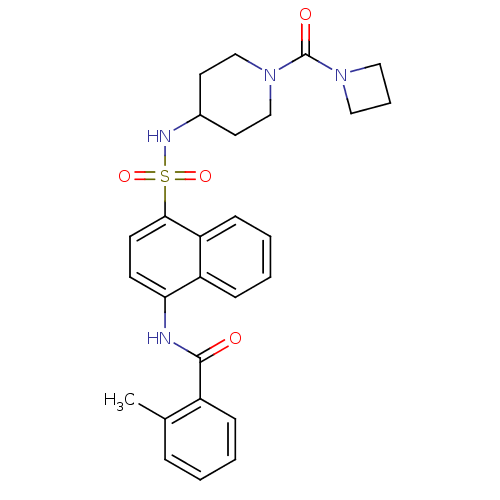 (CHEMBL221130 | N-{4-[1-(azetidine-1-carbonyl)-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM26397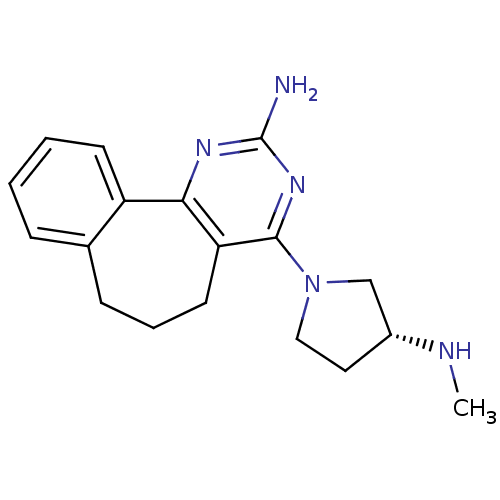 (2,4-diamino-5,6-disubstituted pyrimidine, 11 | 6-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.5 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26398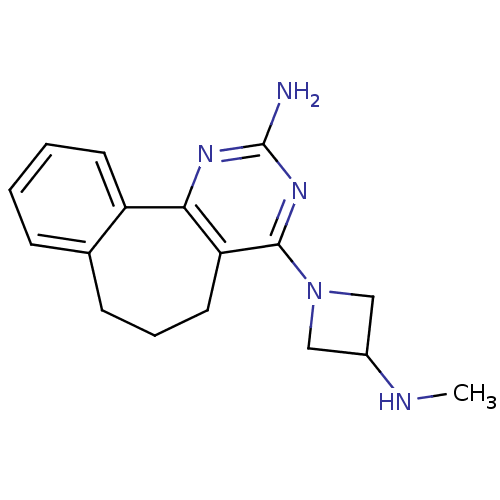 (2,4-diamino-5,6-disubstituted pyrimidine, 12 | 6-[...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26396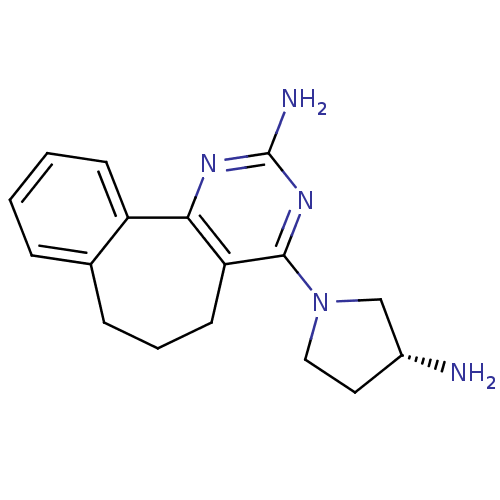 (2,4-diamino-5,6-disubstituted pyrimidine, 10 | 6-[...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80 | -48.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli Dihydrofolate reductase in presence of 100 uM Dihydrofolate reductase | Bioorg Med Chem Lett 13: 2493-6 (2003) BindingDB Entry DOI: 10.7270/Q2CN74G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1/Beta-2/Beta-3 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50227815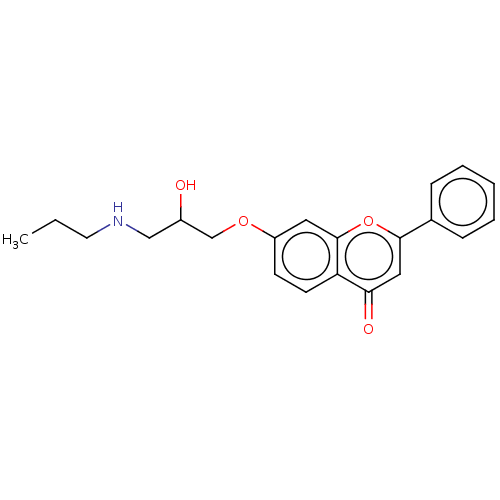 (Flavodilol) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation Curated by ChEMBL | Assay Description Inhibition constant from beta adrenergic receptor binding assay | J Med Chem 32: 183-92 (1989) BindingDB Entry DOI: 10.7270/Q2474D33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26391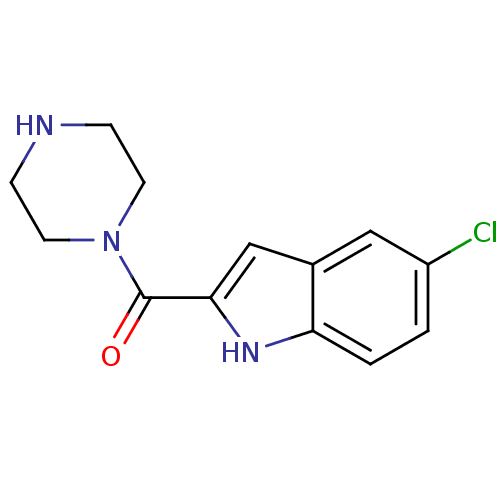 (5-chloro-2-(piperazin-1-ylcarbonyl)-1H-indole | JN...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203849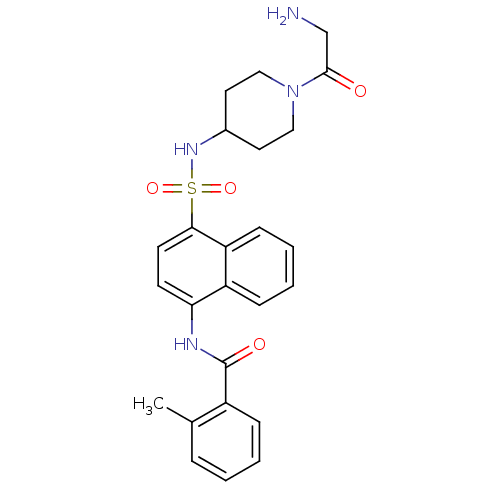 (CHEMBL221904 | N-{4-[1-(2-amino-acetyl)-piperidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203850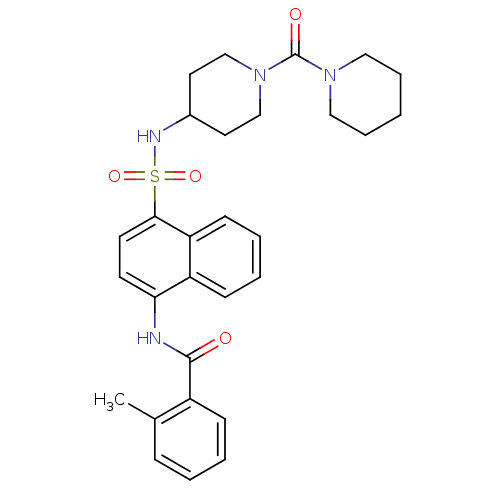 (2-methyl-N-{4-[1-(piperidine-1-carbonyl)-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203882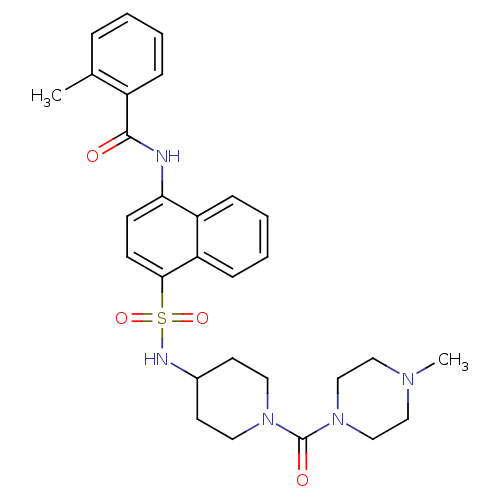 (2-methyl-N-{4-[1-(4-methyl-piperazine-1-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203837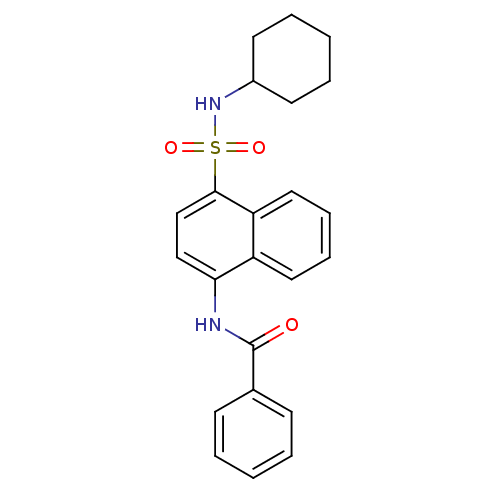 (CHEMBL218503 | N-(4-cyclohexylsulfamoyl-naphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26400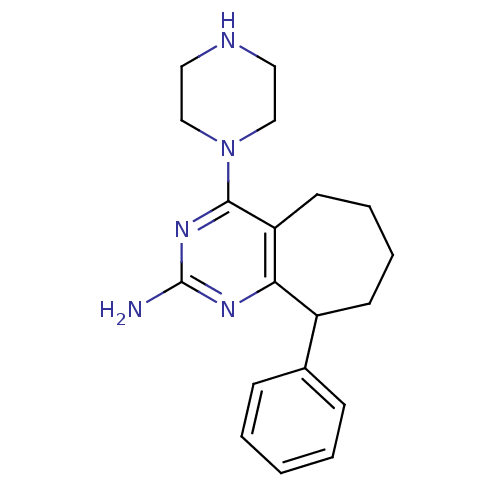 (2,4-diamino-5,6-disubstituted pyrimidine, 14 | 9-p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.5 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM26396 (2,4-diamino-5,6-disubstituted pyrimidine, 10 | 6-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26394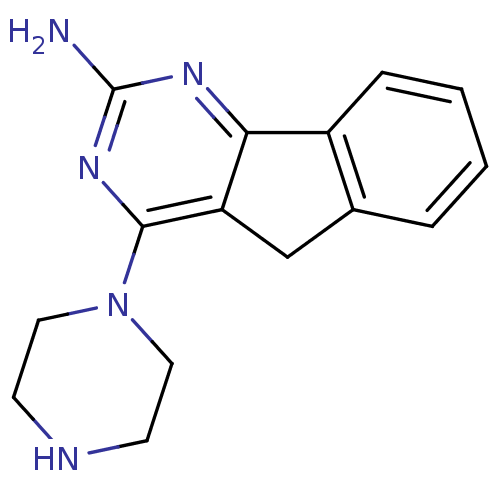 (2,4-diamino-5,6-disubstituted pyrimidine, 8 | 4-(p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM26390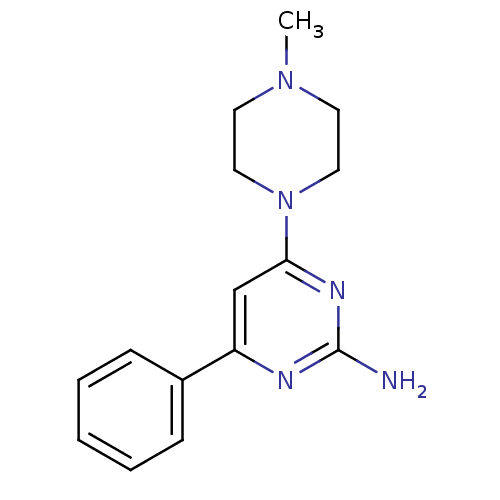 (4-(4-methylpiperazin-1-yl)-6-phenylpyrimidin-2-ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26397 (2,4-diamino-5,6-disubstituted pyrimidine, 11 | 6-[...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.10 | -47.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM26399 (14-fluoro-6-(piperazin-1-yl)-3,5-diazatricyclo[9.4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.10 | -47.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203866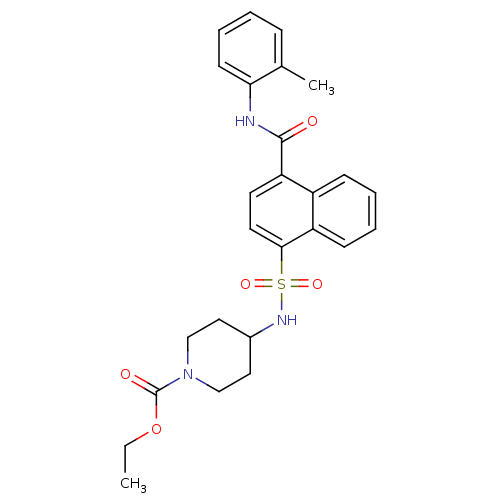 (4-(4-o-tolylcarbamoyl-naphthalene-1-sulfonylamino)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26392 (2,4-diamino-5,6-disubstituted pyrimidine, 6 | 6-(4...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.40 | -47.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203878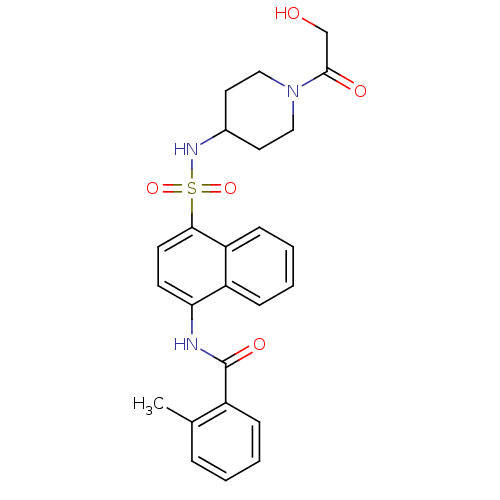 (CHEMBL218036 | N-{4-[1-(2-hydroxy-acetyl)-piperidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203875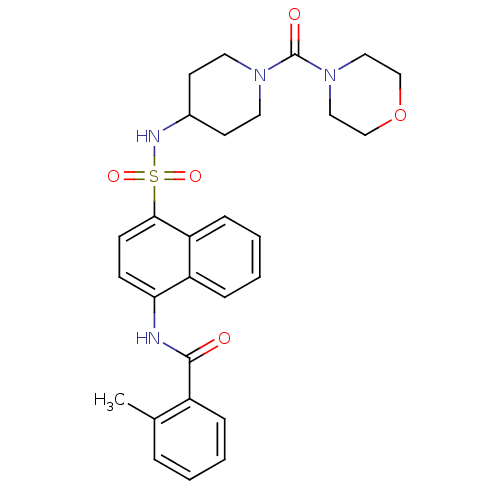 (2-methyl-N-[4-({[1-(morpholin-4-ylcarbonyl)piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203910 (CHEMBL221977 | N-(4-{[(1-acetylpiperidin-4-yl)amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203925 (CHEMBL374979 | N-[4-(1-cyclopropanecarbonyl-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26395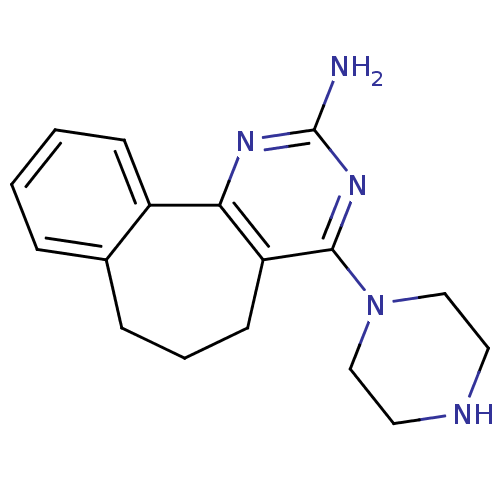 (2,4-diamino-5,6-disubstituted pyrimidine, 9 | 6-(p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7.60 | -46.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203921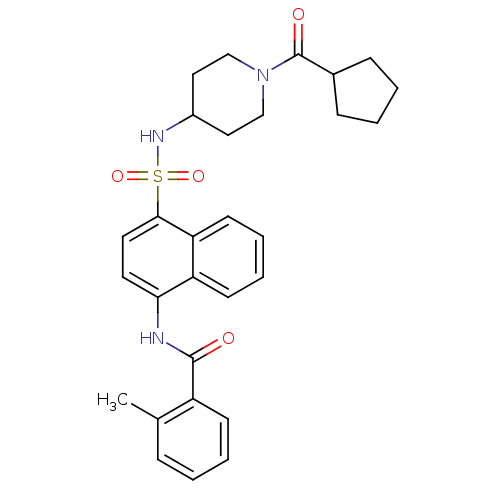 (CHEMBL219433 | N-[4-(1-cyclopentanecarbonyl-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Rattus norvegicus (rat)) | BDBM26393 (2,4-diamino-5,6-disubstituted pyrimidine, 7 | 6-(p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.20 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203917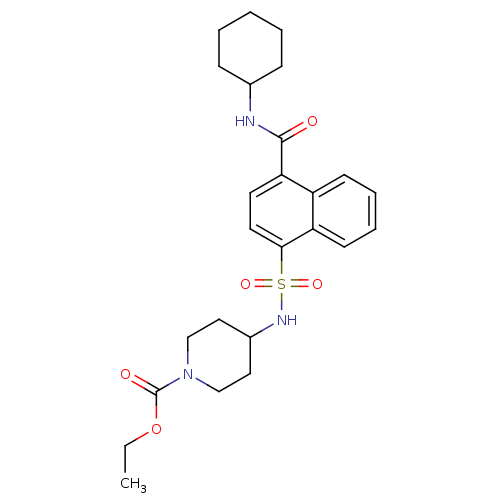 (4-(4-cyclohexylcarbamoyl-naphthalene-1-sulfonylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM26398 (2,4-diamino-5,6-disubstituted pyrimidine, 12 | 6-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203911 (CHEMBL220703 | N-{4-[1-(3-amino-propionyl)-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18049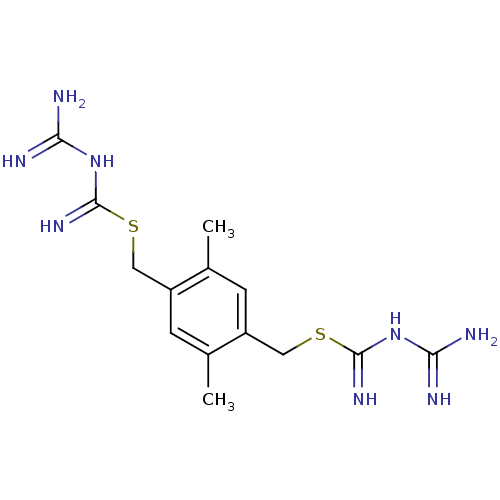 (3-({[(4-{[(carbamimidamidomethanimidoyl)sulfanyl]m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 11.5 | -45.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Prince Edward Island | Assay Description The oxidation of NADPH was monitored at 340 nm using a Molecular Devices SpectraMax Plus 96-well microtiter plate reading spectrophotometer. Plots we... | J Med Chem 49: 6977-86 (2006) Article DOI: 10.1021/jm060570v BindingDB Entry DOI: 10.7270/Q2T43RCM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6571-80 (2008) Article DOI: 10.1021/jm8005959 BindingDB Entry DOI: 10.7270/Q24J0CD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM26394 (2,4-diamino-5,6-disubstituted pyrimidine, 8 | 4-(p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 6547-57 (2008) Article DOI: 10.1021/jm800670r BindingDB Entry DOI: 10.7270/Q2KH0KM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3336 total ) | Next | Last >> |