

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catechol O-methyltransferase (Homo sapiens (Human)) | BDBM50019329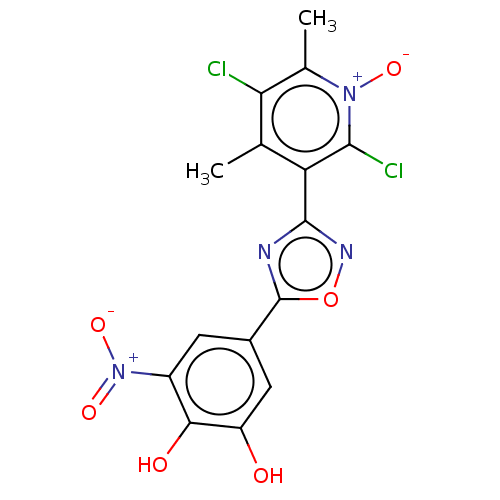 (CHEMBL1089318) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human cloned COMT expressed in Escherichia coli using [3H]-S-adenosylmethionine as substrate after 20 mins by liq... | J Med Chem 57: 5459-63 (2014) Article DOI: 10.1021/jm500475k BindingDB Entry DOI: 10.7270/Q2G73G9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50019329 (CHEMBL1089318) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of catalytic activity of rat COMT expressed in Escherichia coli using [3H]-S-adenosylmethionine as substrate after 20 mins by liquid scint... | J Med Chem 57: 5459-63 (2014) Article DOI: 10.1021/jm500475k BindingDB Entry DOI: 10.7270/Q2G73G9B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Catechol O-methyltransferase (Mus musculus) | BDBM50019329 (CHEMBL1089318) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of catalytic activity of mouse COMT expressed in Escherichia coli using [3H]-S-adenosylmethionine as substrate after 20 mins by liquid sci... | J Med Chem 57: 5459-63 (2014) Article DOI: 10.1021/jm500475k BindingDB Entry DOI: 10.7270/Q2G73G9B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Catechol O-methyltransferase (Homo sapiens (Human)) | BDBM50019331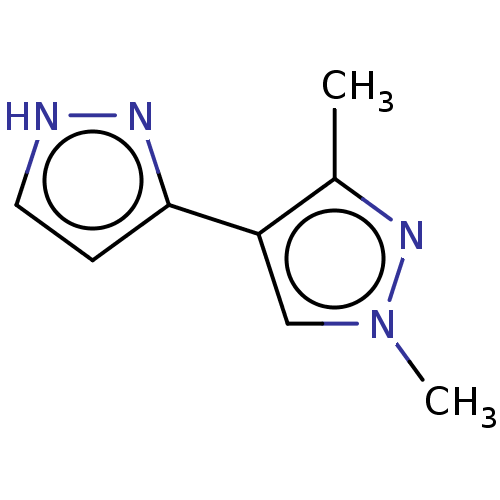 (CHEMBL3289430) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human cloned COMT expressed in Escherichia coli using [3H]-S-adenosylmethionine as substrate after 20 mins by liq... | J Med Chem 57: 5459-63 (2014) Article DOI: 10.1021/jm500475k BindingDB Entry DOI: 10.7270/Q2G73G9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Homo sapiens (Human)) | BDBM50019333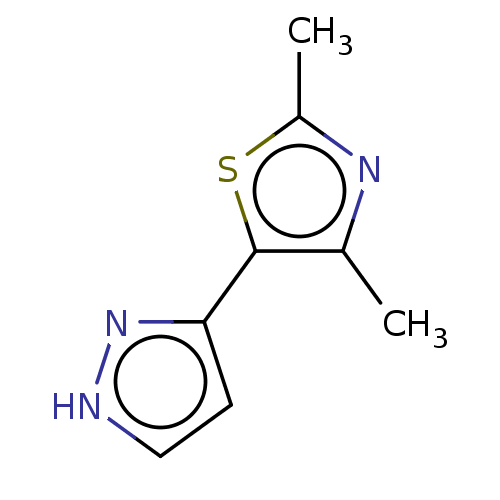 (CHEMBL3289432) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human cloned COMT expressed in Escherichia coli using [3H]-S-adenosylmethionine as substrate after 20 mins by liq... | J Med Chem 57: 5459-63 (2014) Article DOI: 10.1021/jm500475k BindingDB Entry DOI: 10.7270/Q2G73G9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Homo sapiens (Human)) | BDBM50019332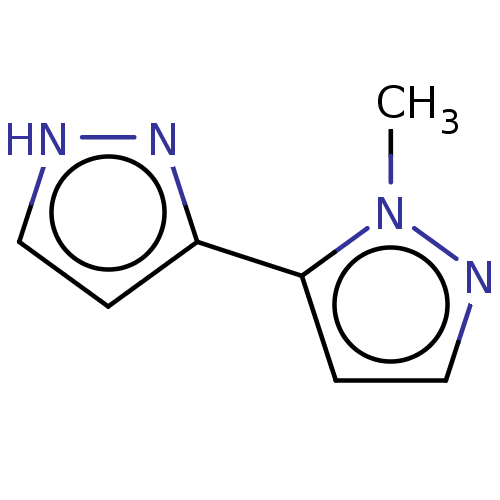 (CHEMBL3289431) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human cloned COMT expressed in Escherichia coli using [3H]-S-adenosylmethionine as substrate after 20 mins by liq... | J Med Chem 57: 5459-63 (2014) Article DOI: 10.1021/jm500475k BindingDB Entry DOI: 10.7270/Q2G73G9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catechol O-methyltransferase (Mus musculus) | BDBM50019331 (CHEMBL3289430) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of catalytic activity of mouse COMT expressed in Escherichia coli using [3H]-S-adenosylmethionine as substrate after 20 mins by liquid sci... | J Med Chem 57: 5459-63 (2014) Article DOI: 10.1021/jm500475k BindingDB Entry DOI: 10.7270/Q2G73G9B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Catechol O-methyltransferase (Rattus norvegicus (Rat)) | BDBM50019331 (CHEMBL3289430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of catalytic activity of rat COMT expressed in Escherichia coli using [3H]-S-adenosylmethionine as substrate after 20 mins by liquid scint... | J Med Chem 57: 5459-63 (2014) Article DOI: 10.1021/jm500475k BindingDB Entry DOI: 10.7270/Q2G73G9B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Catechol O-methyltransferase (Homo sapiens (Human)) | BDBM50019330 (CHEMBL3289429) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of catalytic activity of human cloned COMT expressed in Escherichia coli using [3H]-S-adenosylmethionine as substrate after 20 mins by liq... | J Med Chem 57: 5459-63 (2014) Article DOI: 10.1021/jm500475k BindingDB Entry DOI: 10.7270/Q2G73G9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251697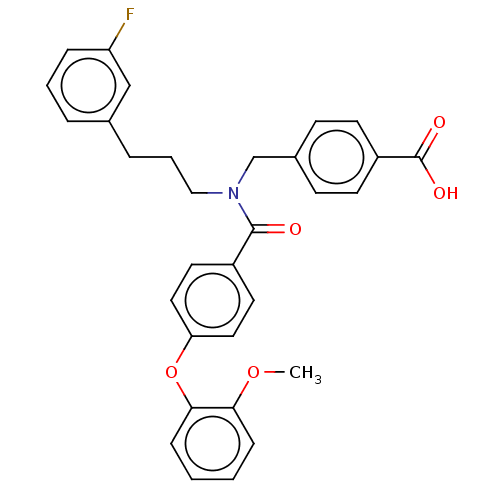 (US10100018, Example 33 | US9464060, 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251697 (US10100018, Example 33 | US9464060, 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 5 (Homo sapiens (Human)) | BDBM251697 (US10100018, Example 33 | US9464060, 33) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA5 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251681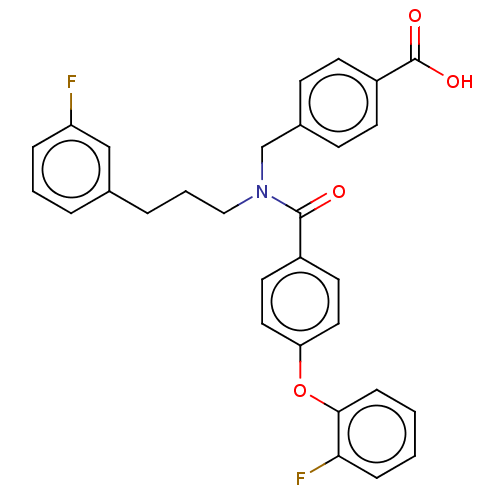 (US10100018, Example 17 | US9464060, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 5 (Homo sapiens (Human)) | BDBM251696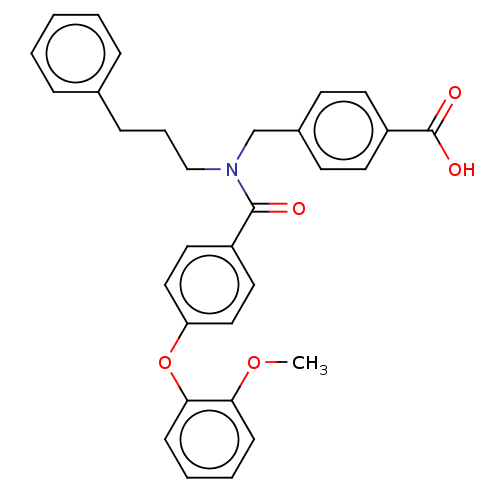 (US10100018, Example 32 | US9464060, 32) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA5 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251696 (US10100018, Example 32 | US9464060, 32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251681 (US10100018, Example 17 | US9464060, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251697 (US10100018, Example 33 | US9464060, 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251682 (US10100018, Example 18 | US9464060, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 5 (Homo sapiens (Human)) | BDBM251680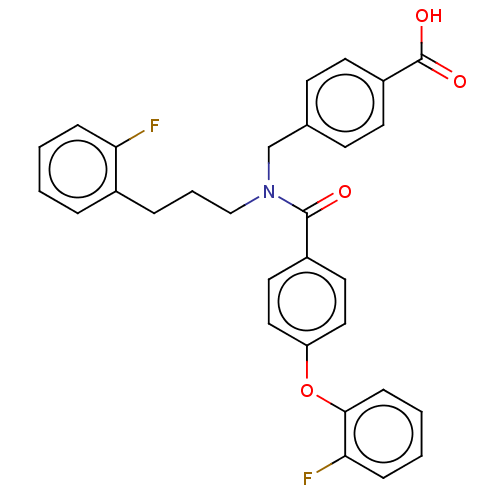 (US10100018, Example 16 | US9464060, 16) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA5 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251682 (US10100018, Example 18 | US9464060, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251680 (US10100018, Example 16 | US9464060, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251681 (US10100018, Example 17 | US9464060, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 5 (Homo sapiens (Human)) | BDBM251681 (US10100018, Example 17 | US9464060, 17) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA5 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251678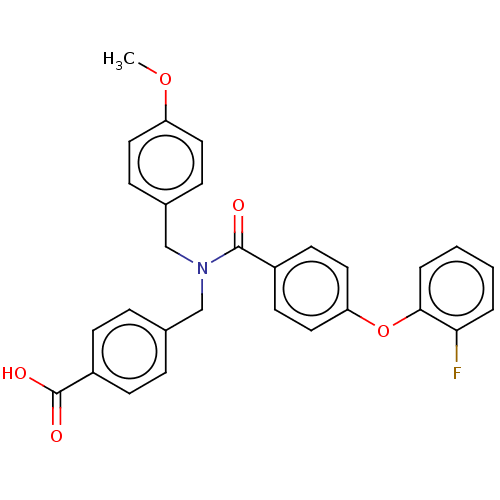 (US10100018, Example 14 | US9464060, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251696 (US10100018, Example 32 | US9464060, 32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 5 (Homo sapiens (Human)) | BDBM251678 (US10100018, Example 14 | US9464060, 14) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA5 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 5 (Homo sapiens (Human)) | BDBM251682 (US10100018, Example 18 | US9464060, 18) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA5 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50243822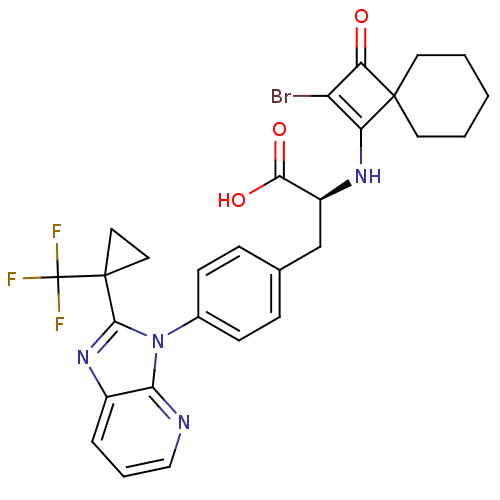 ((S)-2-(2-Bromo-3-oxo-spiro[3.5]non-1-en-1-ylamino)...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Curated by ChEMBL | Assay Description Inhibition of integrin alpha4beta1 receptor in human whole blood by flow cytometry | Bioorg Med Chem Lett 18: 4146-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.075 BindingDB Entry DOI: 10.7270/Q2JW8DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251696 (US10100018, Example 32 | US9464060, 32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50243737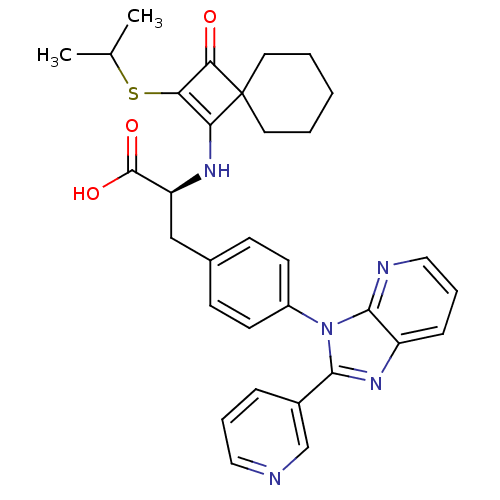 ((S)-2-(2-Isopropylsulfanyl-3-oxo-spiro[3.5]non-1-e...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Curated by ChEMBL | Assay Description Inhibition of integrin alpha4beta1 receptor in human whole blood by flow cytometry | Bioorg Med Chem Lett 18: 4146-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.075 BindingDB Entry DOI: 10.7270/Q2JW8DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251678 (US10100018, Example 14 | US9464060, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251682 (US10100018, Example 18 | US9464060, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251678 (US10100018, Example 14 | US9464060, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50243738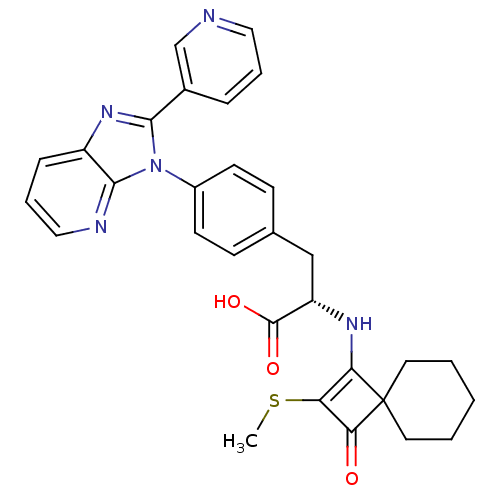 ((S)-2-(2-Methylsulfanyl-3-oxo-spiro[3.5]non-1-en-1...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Curated by ChEMBL | Assay Description Inhibition of integrin alpha4beta1 receptor in human whole blood by flow cytometry | Bioorg Med Chem Lett 18: 4146-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.075 BindingDB Entry DOI: 10.7270/Q2JW8DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 5 (Homo sapiens (Human)) | BDBM251677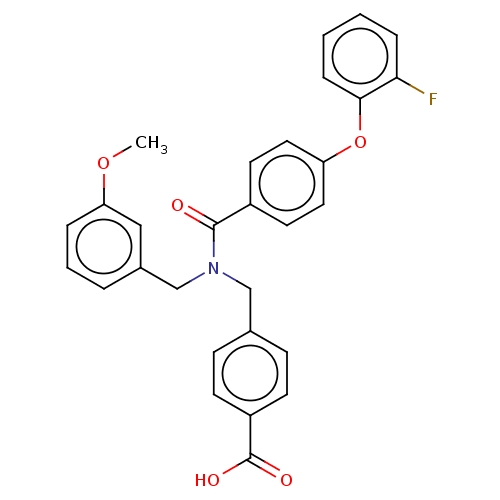 (US10100018, Example 13 | US9464060, 13) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA5 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50243758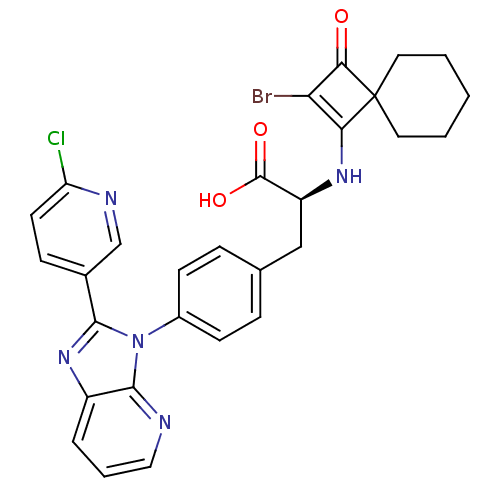 ((S)-2-(2-Bromo-3-oxo-spiro[3.5]non-1-en-1-ylamino)...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Curated by ChEMBL | Assay Description Inhibition of integrin alpha4beta1 receptor in human whole blood by flow cytometry | Bioorg Med Chem Lett 18: 4146-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.075 BindingDB Entry DOI: 10.7270/Q2JW8DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50243759 ((S)-2-(2-Bromo-3-oxo-spiro[3.5]non-1-en-1-ylamino)...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Curated by ChEMBL | Assay Description Inhibition of integrin alpha4beta1 receptor in human whole blood by flow cytometry | Bioorg Med Chem Lett 18: 4146-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.075 BindingDB Entry DOI: 10.7270/Q2JW8DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251677 (US10100018, Example 13 | US9464060, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50243736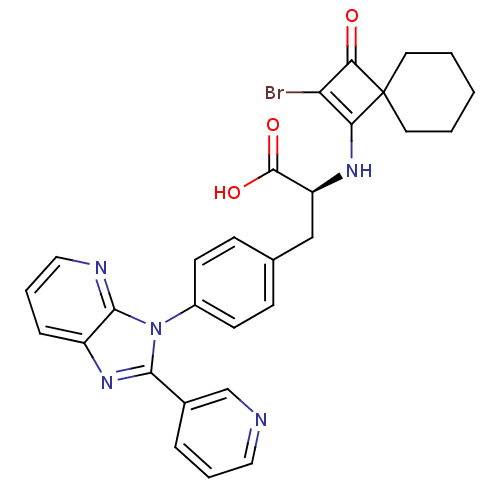 ((S)-2-(2-Bromo-3-oxo-spiro[3.5]non-1-en-1-ylamino)...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Curated by ChEMBL | Assay Description Inhibition of integrin alpha4beta1 receptor in human whole blood by flow cytometry | Bioorg Med Chem Lett 18: 4146-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.075 BindingDB Entry DOI: 10.7270/Q2JW8DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4 (Homo sapiens (Human)) | BDBM50243823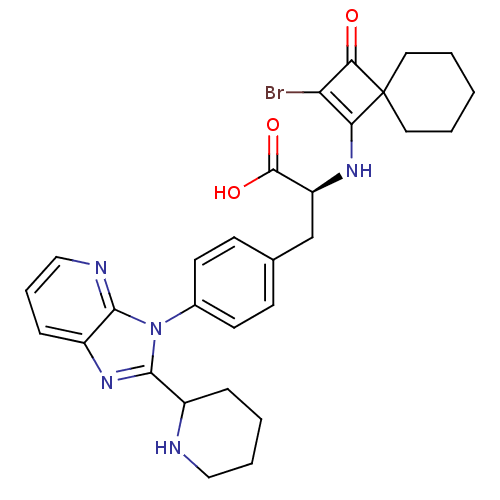 ((S)-2-(2-Bromo-3-oxo-spiro[3.5]non-1-en-1-ylamino)...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Curated by ChEMBL | Assay Description Inhibition of integrin alpha4beta1 receptor in human whole blood by flow cytometry | Bioorg Med Chem Lett 18: 4146-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.075 BindingDB Entry DOI: 10.7270/Q2JW8DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50219014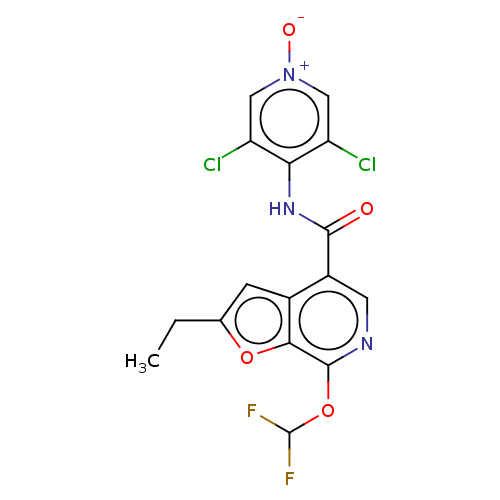 (CHEMBL149559) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R& D Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 4 from U937 cells | Bioorg Med Chem Lett 12: 509-12 (2002) BindingDB Entry DOI: 10.7270/Q2T43W9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 5 (Homo sapiens (Human)) | BDBM251729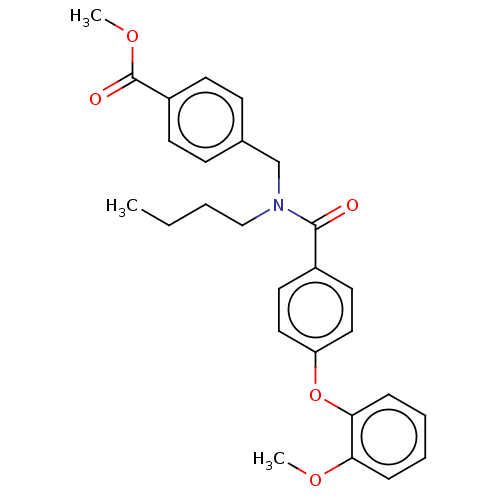 (US10100018, Example 65 | US9464060, 65) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA5 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251677 (US10100018, Example 13 | US9464060, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251695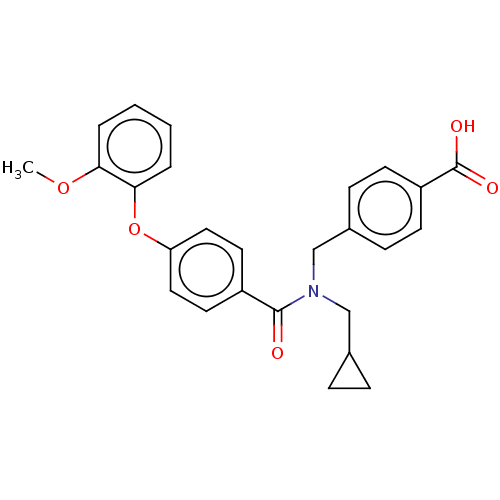 (US10100018, Example 31 | US9464060, 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251695 (US10100018, Example 31 | US9464060, 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251729 (US10100018, Example 65 | US9464060, 65) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251725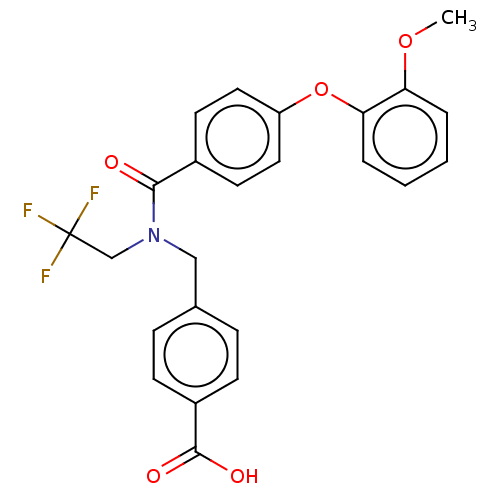 (US10100018, Example 61 | US9464060, 61) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251677 (US10100018, Example 13 | US9464060, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium i... | US Patent US10100018 (2018) BindingDB Entry DOI: 10.7270/Q2PR7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM251725 (US10100018, Example 61 | US9464060, 61) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA1 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 5 (Homo sapiens (Human)) | BDBM251695 (US10100018, Example 31 | US9464060, 31) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description Engagement of the LPA5 receptor by its ligand, oleoyl-L-α-lysophosphatidic acid (LPA), leads to the release of intracellular stores of calcium into ... | US Patent US9464060 (2016) BindingDB Entry DOI: 10.7270/Q23R0RT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1278 total ) | Next | Last >> |