 Found 725 hits with Last Name = 'dehaven-hudkins' and Initial = 'dl'
Found 725 hits with Last Name = 'dehaven-hudkins' and Initial = 'dl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50450592
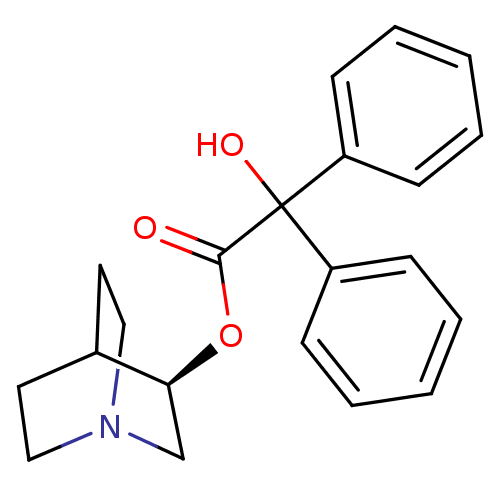
(CHEMBL558910)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(8.38,-12.97,;7.3,-14.07,;8.63,-14.83,;8.64,-16.37,;9.97,-14.06,;11.3,-14.82,;11.3,-16.36,;12.63,-17.13,;13.96,-16.36,;13.96,-14.82,;12.63,-14.05,;13.05,-15.29,;12,-15.64,;5.97,-14.84,;4.63,-14.07,;3.3,-14.84,;3.31,-16.38,;4.65,-17.15,;5.98,-16.37,;7.29,-12.53,;8.62,-11.76,;8.62,-10.22,;7.28,-9.45,;5.95,-10.23,;5.96,-11.77,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H](-)-quinuclidinyl benzilate(QNB) from muscarinic (M2) receptor in rat heart homogenates |
J Med Chem 34: 2984-9 (1991)
BindingDB Entry DOI: 10.7270/Q27H1K5M |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50450592

(CHEMBL558910)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(8.38,-12.97,;7.3,-14.07,;8.63,-14.83,;8.64,-16.37,;9.97,-14.06,;11.3,-14.82,;11.3,-16.36,;12.63,-17.13,;13.96,-16.36,;13.96,-14.82,;12.63,-14.05,;13.05,-15.29,;12,-15.64,;5.97,-14.84,;4.63,-14.07,;3.3,-14.84,;3.31,-16.38,;4.65,-17.15,;5.98,-16.37,;7.29,-12.53,;8.62,-11.76,;8.62,-10.22,;7.28,-9.45,;5.95,-10.23,;5.96,-11.77,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory
Curated by ChEMBL
| Assay Description
Ability to displace [3H](-)-quinuclidinyl bezilate(QNB) from M2 receptor in rat heart homogenate |
J Med Chem 36: 848-54 (1993)
BindingDB Entry DOI: 10.7270/Q2S46SM0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50450592

(CHEMBL558910)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(8.38,-12.97,;7.3,-14.07,;8.63,-14.83,;8.64,-16.37,;9.97,-14.06,;11.3,-14.82,;11.3,-16.36,;12.63,-17.13,;13.96,-16.36,;13.96,-14.82,;12.63,-14.05,;13.05,-15.29,;12,-15.64,;5.97,-14.84,;4.63,-14.07,;3.3,-14.84,;3.31,-16.38,;4.65,-17.15,;5.98,-16.37,;7.29,-12.53,;8.62,-11.76,;8.62,-10.22,;7.28,-9.45,;5.95,-10.23,;5.96,-11.77,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB (quinuclidinyl benzylate) from muscarinic M2 receptor of rat heart homogenates. |
Bioorg Med Chem Lett 7: 979-984 (1997)
Article DOI: 10.1016/S0960-894X(97)00143-1
BindingDB Entry DOI: 10.7270/Q2N29XFM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50007344
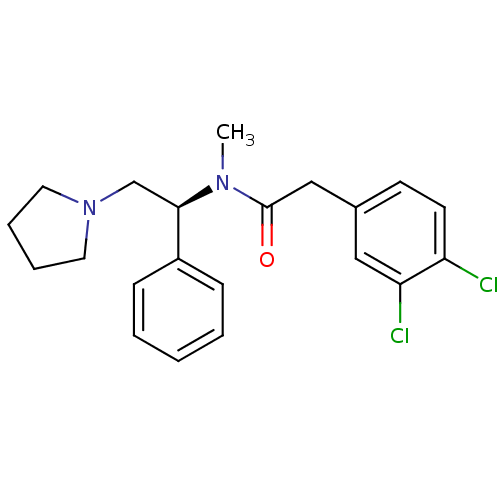
((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligand |
Bioorg Med Chem Lett 15: 2647-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.020
BindingDB Entry DOI: 10.7270/Q2PR7VH4 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50007377
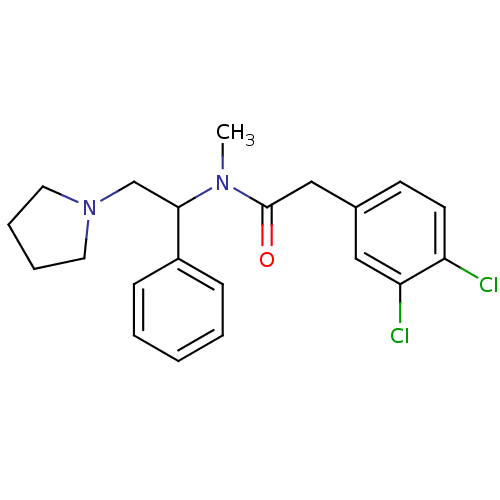
(2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2-pyr...)Show SMILES CN(C(CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity for human Kappa opioid receptor |
Bioorg Med Chem Lett 14: 5693-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.041
BindingDB Entry DOI: 10.7270/Q27D2TMP |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50007344

((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human kappa opioid receptor was determined by using [3H]-diprenorphine as radioligand |
Bioorg Med Chem Lett 15: 1279-82 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.038
BindingDB Entry DOI: 10.7270/Q2QF8SDJ |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50038707
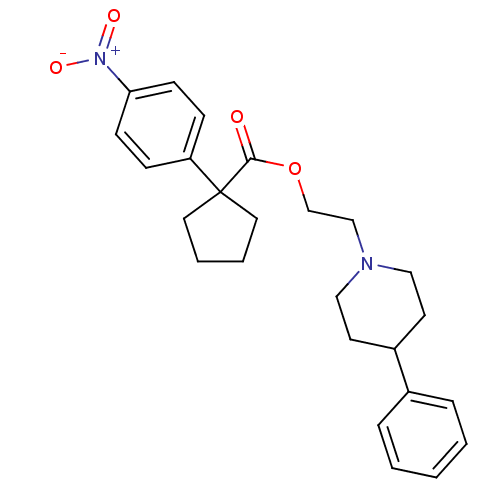
(1-(4-Nitro-phenyl)-cyclopentanecarboxylic acid 2-(...)Show SMILES [O-][N+](=O)c1ccc(cc1)C1(CCCC1)C(=O)OCCN1CCC(CC1)c1ccccc1 Show InChI InChI=1S/C25H30N2O4/c28-24(25(14-4-5-15-25)22-8-10-23(11-9-22)27(29)30)31-19-18-26-16-12-21(13-17-26)20-6-2-1-3-7-20/h1-3,6-11,21H,4-5,12-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Affinity at sigma-1 site by inhibition of [3H](+)-pentazocine (PENT) binding in guinea pig brain |
J Med Chem 37: 1964-70 (1994)
BindingDB Entry DOI: 10.7270/Q2QZ2BMR |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50038707

(1-(4-Nitro-phenyl)-cyclopentanecarboxylic acid 2-(...)Show SMILES [O-][N+](=O)c1ccc(cc1)C1(CCCC1)C(=O)OCCN1CCC(CC1)c1ccccc1 Show InChI InChI=1S/C25H30N2O4/c28-24(25(14-4-5-15-25)22-8-10-23(11-9-22)27(29)30)31-19-18-26-16-12-21(13-17-26)20-6-2-1-3-7-20/h1-3,6-11,21H,4-5,12-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Affinity at sigma-1 site by inhibition of [3H](+)-pentazocine (PENT) binding in guinea pig brain |
J Med Chem 37: 1964-70 (1994)
BindingDB Entry DOI: 10.7270/Q2QZ2BMR |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50007344

((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093965
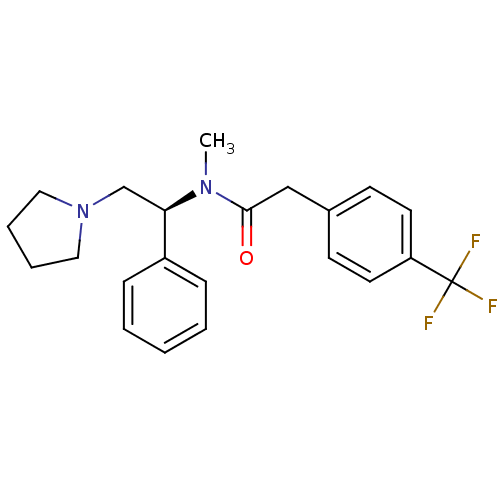
(CHEMBL86324 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-17-9-11-19(12-10-17)22(23,24)25)20(16-27-13-5-6-14-27)18-7-3-2-4-8-18/h2-4,7-12,20H,5-6,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093965

(CHEMBL86324 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-17-9-11-19(12-10-17)22(23,24)25)20(16-27-13-5-6-14-27)18-7-3-2-4-8-18/h2-4,7-12,20H,5-6,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093964
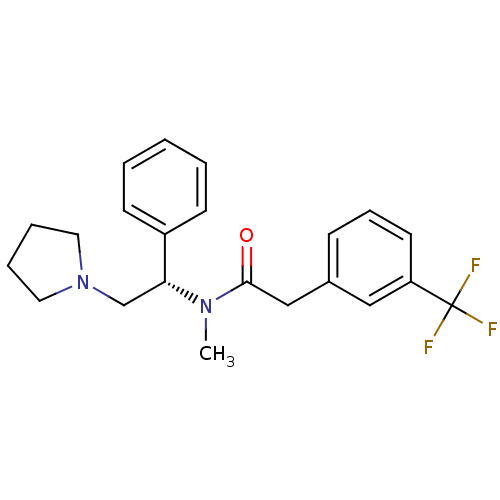
(CHEMBL313484 | N-Methyl-N-((S)-1-phenyl-2-pyrrolid...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-17-8-7-11-19(14-17)22(23,24)25)20(16-27-12-5-6-13-27)18-9-3-2-4-10-18/h2-4,7-11,14,20H,5-6,12-13,15-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093969
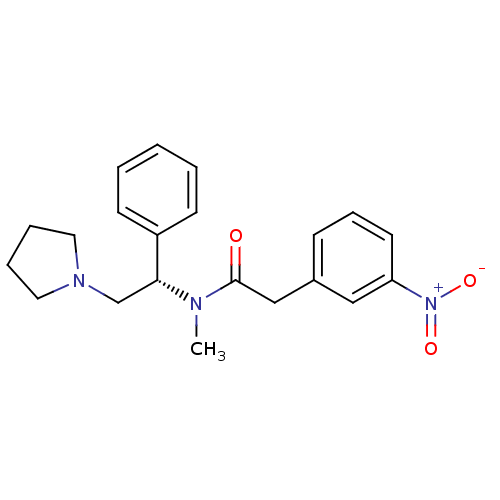
(CHEMBL82919 | N-Methyl-2-(3-nitro-phenyl)-N-((S)-1...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H25N3O3/c1-22(21(25)15-17-8-7-11-19(14-17)24(26)27)20(16-23-12-5-6-13-23)18-9-3-2-4-10-18/h2-4,7-11,14,20H,5-6,12-13,15-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093958
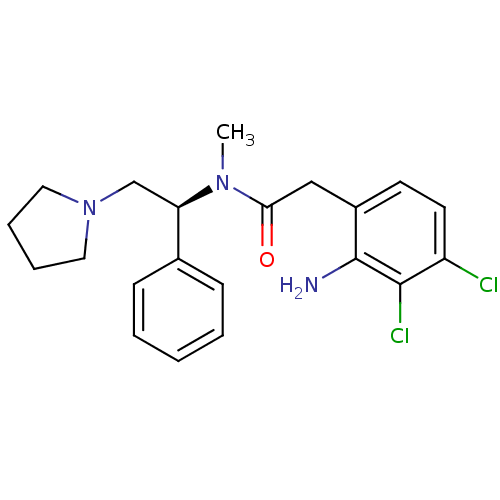
(2-(2-Amino-3,4-dichloro-phenyl)-N-methyl-N-((S)-1-...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1N Show InChI InChI=1S/C21H25Cl2N3O/c1-25(19(27)13-16-9-10-17(22)20(23)21(16)24)18(14-26-11-5-6-12-26)15-7-3-2-4-8-15/h2-4,7-10,18H,5-6,11-14,24H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093966
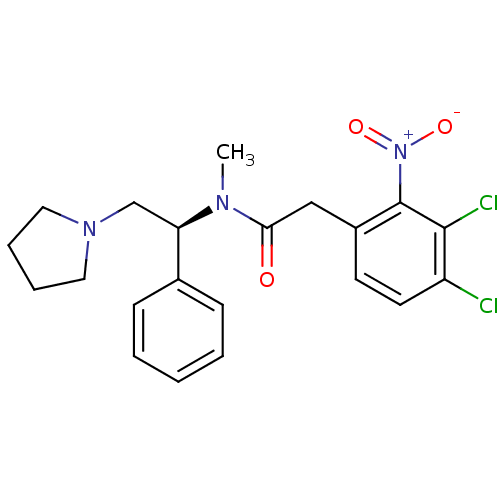
(2-(3,4-Dichloro-2-nitro-phenyl)-N-methyl-N-((S)-1-...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1[N+]([O-])=O Show InChI InChI=1S/C21H23Cl2N3O3/c1-24(18(14-25-11-5-6-12-25)15-7-3-2-4-8-15)19(27)13-16-9-10-17(22)20(23)21(16)26(28)29/h2-4,7-10,18H,5-6,11-14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093970
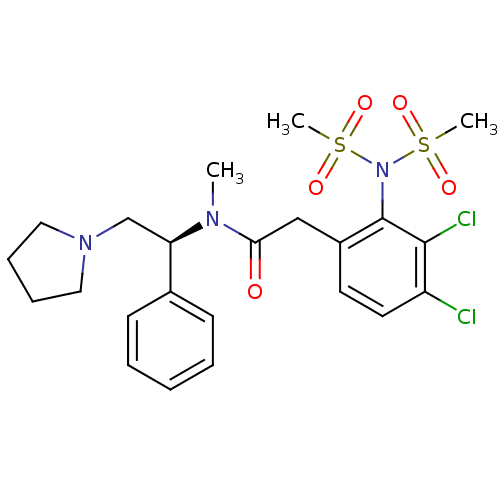
(2-(3,4-Dichloro-2-dimethanesulfonylamino-phenyl)-N...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1N(S(C)(=O)=O)S(C)(=O)=O Show InChI InChI=1S/C23H29Cl2N3O5S2/c1-26(20(16-27-13-7-8-14-27)17-9-5-4-6-10-17)21(29)15-18-11-12-19(24)22(25)23(18)28(34(2,30)31)35(3,32)33/h4-6,9-12,20H,7-8,13-16H2,1-3H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176375
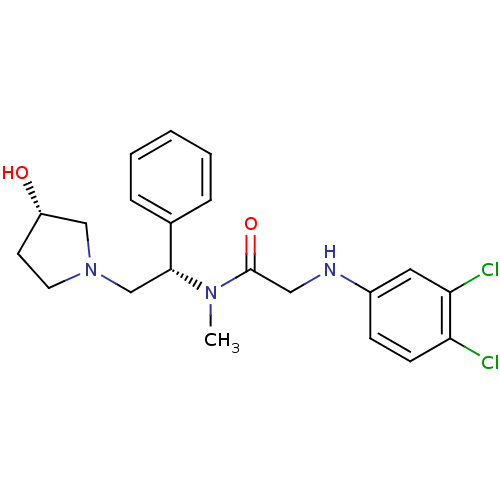
(2-(3,4-dichlorophenylamino)-N-((S)-2-((S)-3-hydrox...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C21H25Cl2N3O2/c1-25(21(28)12-24-16-7-8-18(22)19(23)11-16)20(15-5-3-2-4-6-15)14-26-10-9-17(27)13-26/h2-8,11,17,20,24,27H,9-10,12-14H2,1H3/t17-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory
Curated by ChEMBL
| Assay Description
Ability to displace [3H]N-methylscopolamine (NMS) from M3 receptor in rat submaxillary gland homogenate |
J Med Chem 36: 848-54 (1993)
BindingDB Entry DOI: 10.7270/Q2S46SM0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093962
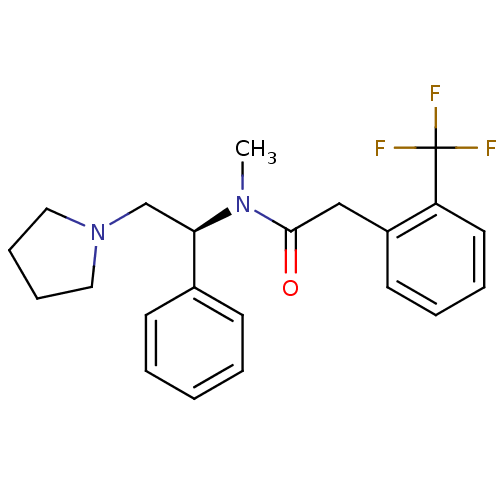
(CHEMBL87306 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccccc1C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-18-11-5-6-12-19(18)22(23,24)25)20(16-27-13-7-8-14-27)17-9-3-2-4-10-17/h2-6,9-12,20H,7-8,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50450592

(CHEMBL558910)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(8.38,-12.97,;7.3,-14.07,;8.63,-14.83,;8.64,-16.37,;9.97,-14.06,;11.3,-14.82,;11.3,-16.36,;12.63,-17.13,;13.96,-16.36,;13.96,-14.82,;12.63,-14.05,;13.05,-15.29,;12,-15.64,;5.97,-14.84,;4.63,-14.07,;3.3,-14.84,;3.31,-16.38,;4.65,-17.15,;5.98,-16.37,;7.29,-12.53,;8.62,-11.76,;8.62,-10.22,;7.28,-9.45,;5.95,-10.23,;5.96,-11.77,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]pirenzepine from muscarinic M1 receptor of rat cortex homogenates. |
Bioorg Med Chem Lett 7: 979-984 (1997)
Article DOI: 10.1016/S0960-894X(97)00143-1
BindingDB Entry DOI: 10.7270/Q2N29XFM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50450592

(CHEMBL558910)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(8.38,-12.97,;7.3,-14.07,;8.63,-14.83,;8.64,-16.37,;9.97,-14.06,;11.3,-14.82,;11.3,-16.36,;12.63,-17.13,;13.96,-16.36,;13.96,-14.82,;12.63,-14.05,;13.05,-15.29,;12,-15.64,;5.97,-14.84,;4.63,-14.07,;3.3,-14.84,;3.31,-16.38,;4.65,-17.15,;5.98,-16.37,;7.29,-12.53,;8.62,-11.76,;8.62,-10.22,;7.28,-9.45,;5.95,-10.23,;5.96,-11.77,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory
Curated by ChEMBL
| Assay Description
Ability to displace [3H]pirenzepine (PZ) from M1 receptor in rat cortex homogenate |
J Med Chem 36: 848-54 (1993)
BindingDB Entry DOI: 10.7270/Q2S46SM0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50450592

(CHEMBL558910)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(8.38,-12.97,;7.3,-14.07,;8.63,-14.83,;8.64,-16.37,;9.97,-14.06,;11.3,-14.82,;11.3,-16.36,;12.63,-17.13,;13.96,-16.36,;13.96,-14.82,;12.63,-14.05,;13.05,-15.29,;12,-15.64,;5.97,-14.84,;4.63,-14.07,;3.3,-14.84,;3.31,-16.38,;4.65,-17.15,;5.98,-16.37,;7.29,-12.53,;8.62,-11.76,;8.62,-10.22,;7.28,-9.45,;5.95,-10.23,;5.96,-11.77,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]pirenzepine from muscarinic acetylcholine receptor M1 in rat cortex homogenates |
J Med Chem 34: 2984-9 (1991)
BindingDB Entry DOI: 10.7270/Q27H1K5M |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50450592

(CHEMBL558910)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(8.38,-12.97,;7.3,-14.07,;8.63,-14.83,;8.64,-16.37,;9.97,-14.06,;11.3,-14.82,;11.3,-16.36,;12.63,-17.13,;13.96,-16.36,;13.96,-14.82,;12.63,-14.05,;13.05,-15.29,;12,-15.64,;5.97,-14.84,;4.63,-14.07,;3.3,-14.84,;3.31,-16.38,;4.65,-17.15,;5.98,-16.37,;7.29,-12.53,;8.62,-11.76,;8.62,-10.22,;7.28,-9.45,;5.95,-10.23,;5.96,-11.77,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory
Curated by ChEMBL
| Assay Description
Ability to displace [3H]N-methylscopolamine (NMS) from M3 receptor in rat submaxillary gland homogenate |
J Med Chem 36: 848-54 (1993)
BindingDB Entry DOI: 10.7270/Q2S46SM0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176369
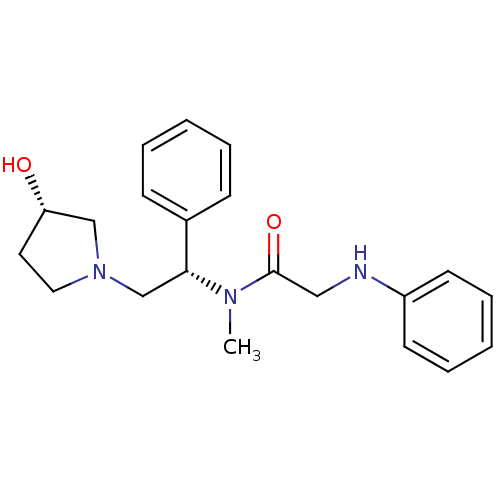
(CHEMBL201884 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1 Show InChI InChI=1S/C21H27N3O2/c1-23(21(26)14-22-18-10-6-3-7-11-18)20(17-8-4-2-5-9-17)16-24-13-12-19(25)15-24/h2-11,19-20,22,25H,12-16H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50155498

((S)-3-(3,4-Dichloro-phenyl)-1-[(S)-2-((S)-3-hydrox...)Show SMILES O[C@H]1CCN(C[C@@H](N2CC=CC[C@@H](c3ccc(Cl)c(Cl)c3)C2=O)c2ccccc2)C1 |c:9| Show InChI InChI=1S/C24H26Cl2N2O2/c25-21-10-9-18(14-22(21)26)20-8-4-5-12-28(24(20)30)23(17-6-2-1-3-7-17)16-27-13-11-19(29)15-27/h1-7,9-10,14,19-20,23,29H,8,11-13,15-16H2/t19-,20-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity for human Kappa opioid receptor |
Bioorg Med Chem Lett 14: 5693-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.041
BindingDB Entry DOI: 10.7270/Q27D2TMP |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176370
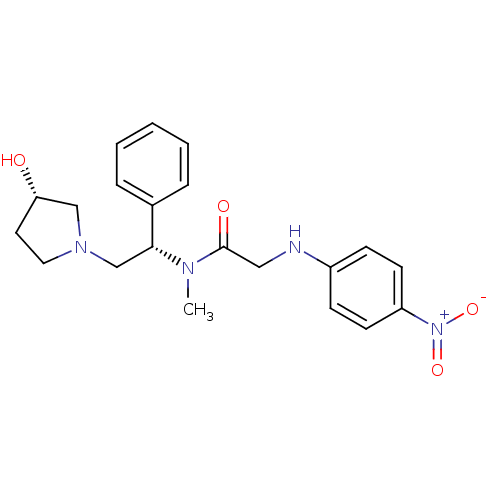
(CHEMBL201572 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H26N4O4/c1-23(21(27)13-22-17-7-9-18(10-8-17)25(28)29)20(16-5-3-2-4-6-16)15-24-12-11-19(26)14-24/h2-10,19-20,22,26H,11-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H]pirenzepine from muscarinic acetylcholine receptor M1 in rat cortex homogenates |
J Med Chem 34: 2984-9 (1991)
BindingDB Entry DOI: 10.7270/Q27H1K5M |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory
Curated by ChEMBL
| Assay Description
Ability to displace [3H]pirenzepine (PZ) from M1 receptor in rat cortex homogenate |
J Med Chem 36: 848-54 (1993)
BindingDB Entry DOI: 10.7270/Q2S46SM0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50456344

(CHEMBL2112942)Show SMILES OC(CC=CI)(C(=O)OC1CN2CCC1CC2)c1ccccc1 |(8.21,-10.55,;8.2,-9.01,;9.52,-9.78,;11.01,-9.37,;12.1,-10.45,;12.09,-12,;8.2,-7.46,;6.88,-6.69,;9.55,-6.69,;10.32,-5.34,;10.34,-3.8,;11.68,-3.03,;13,-3.83,;12.99,-5.35,;11.65,-6.11,;11,-5.18,;10.91,-4.29,;6.85,-9.78,;5.53,-8.97,;4.19,-9.75,;4.18,-11.27,;5.53,-12.06,;6.85,-11.3,)| Show InChI InChI=1S/C18H22INO3/c19-10-4-9-18(22,15-5-2-1-3-6-15)17(21)23-16-13-20-11-7-14(16)8-12-20/h1-6,10,14,16,22H,7-9,11-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory
Curated by ChEMBL
| Assay Description
Ability to displace [3H]N-methylscopolamine (NMS) from M3 receptor in rat submaxillary gland homogenate |
J Med Chem 36: 848-54 (1993)
BindingDB Entry DOI: 10.7270/Q2S46SM0 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50038717
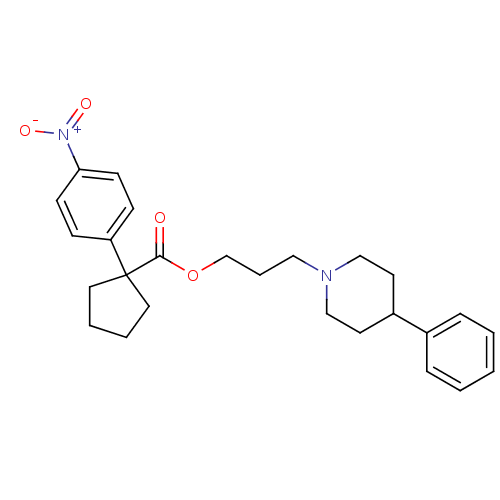
(1-(4-Nitro-phenyl)-cyclopentanecarboxylic acid 3-(...)Show SMILES [O-][N+](=O)c1ccc(cc1)C1(CCCC1)C(=O)OCCCN1CCC(CC1)c1ccccc1 Show InChI InChI=1S/C26H32N2O4/c29-25(26(15-4-5-16-26)23-9-11-24(12-10-23)28(30)31)32-20-6-17-27-18-13-22(14-19-27)21-7-2-1-3-8-21/h1-3,7-12,22H,4-6,13-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Affinity at sigma-1 site by inhibition of [3H](+)-pentazocine (PENT) binding in guinea pig brain |
J Med Chem 37: 1964-70 (1994)
BindingDB Entry DOI: 10.7270/Q2QZ2BMR |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50205684
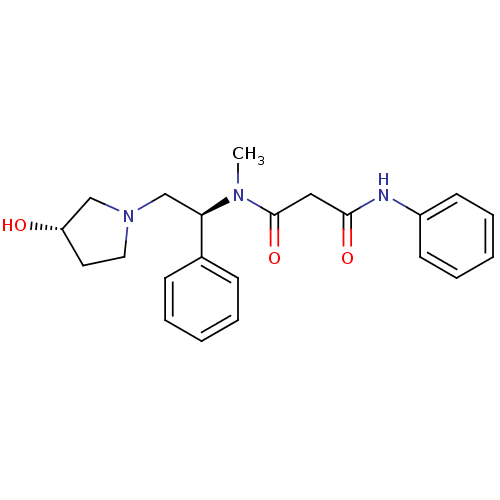
(CHEMBL230288 | N1-((S)-2-((S)-3-hydroxypyrrolidin-...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CC(=O)Nc1ccccc1 Show InChI InChI=1S/C22H27N3O3/c1-24(22(28)14-21(27)23-18-10-6-3-7-11-18)20(17-8-4-2-5-9-17)16-25-13-12-19(26)15-25/h2-11,19-20,26H,12-16H2,1H3,(H,23,27)/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 1951-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.053
BindingDB Entry DOI: 10.7270/Q2Q52P9X |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160834
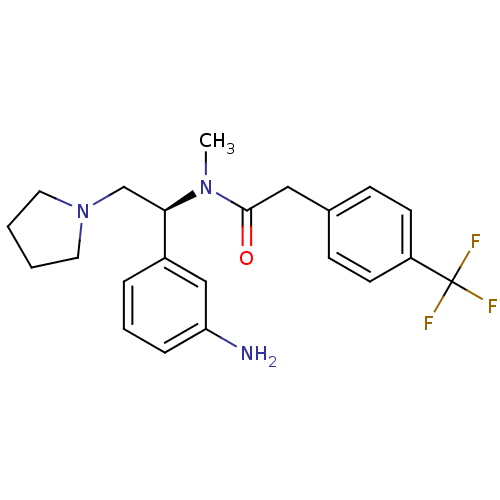
(CHEMBL183248 | N-[(S)-1-(3-Amino-phenyl)-2-pyrroli...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(N)c1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H26F3N3O/c1-27(21(29)13-16-7-9-18(10-8-16)22(23,24)25)20(15-28-11-2-3-12-28)17-5-4-6-19(26)14-17/h4-10,14,20H,2-3,11-13,15,26H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50039026
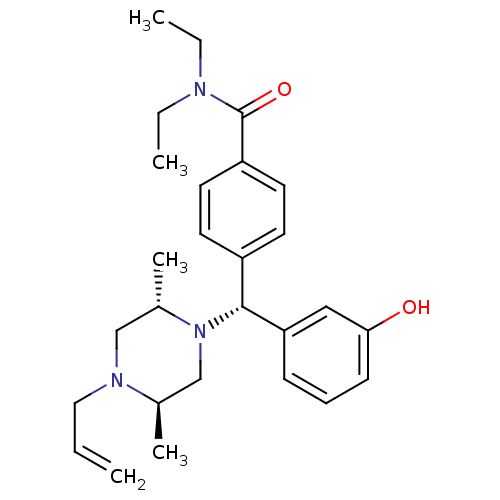
(4-((R)-((2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1C[C@@H](C)N(CC=C)C[C@@H]1C)c1cccc(O)c1 Show InChI InChI=1S/C27H37N3O2/c1-6-16-29-18-21(5)30(19-20(29)4)26(24-10-9-11-25(31)17-24)22-12-14-23(15-13-22)27(32)28(7-2)8-3/h6,9-15,17,20-21,26,31H,1,7-8,16,18-19H2,2-5H3/t20-,21+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor in CHO cells |
J Med Chem 51: 5893-6 (2008)
Article DOI: 10.1021/jm8008986
BindingDB Entry DOI: 10.7270/Q26W9C0D |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176374

(CHEMBL382932 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN(CC(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C22H29N3O2/c1-23(19-11-7-4-8-12-19)17-22(27)24(2)21(18-9-5-3-6-10-18)16-25-14-13-20(26)15-25/h3-12,20-21,26H,13-17H2,1-2H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50155494
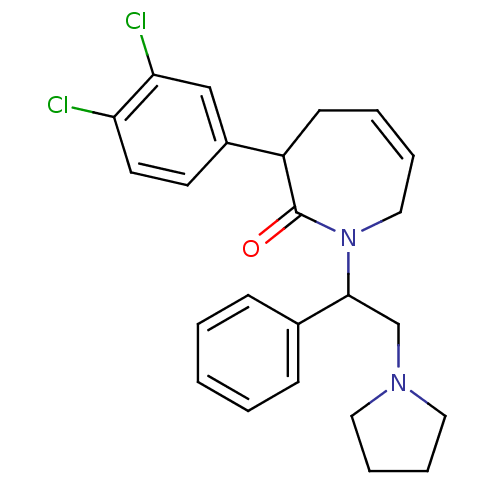
(3-(3,4-Dichloro-phenyl)-1-(1-phenyl-2-pyrrolidin-1...)Show SMILES Clc1ccc(cc1Cl)C1CC=CCN(C(CN2CCCC2)c2ccccc2)C1=O |c:11| Show InChI InChI=1S/C24H26Cl2N2O/c25-21-12-11-19(16-22(21)26)20-10-4-5-15-28(24(20)29)23(17-27-13-6-7-14-27)18-8-2-1-3-9-18/h1-5,8-9,11-12,16,20,23H,6-7,10,13-15,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity for human Kappa opioid receptor |
Bioorg Med Chem Lett 14: 5693-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.041
BindingDB Entry DOI: 10.7270/Q27D2TMP |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160840
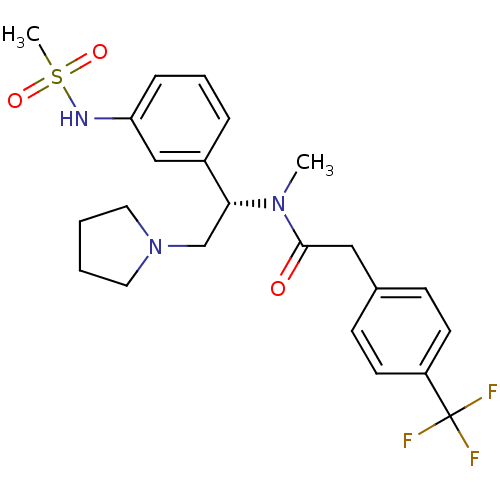
(CHEMBL365486 | N-[(S)-1-(3-Methanesulfonylamino-ph...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NS(C)(=O)=O)c1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H28F3N3O3S/c1-28(22(30)14-17-8-10-19(11-9-17)23(24,25)26)21(16-29-12-3-4-13-29)18-6-5-7-20(15-18)27-33(2,31)32/h5-11,15,21,27H,3-4,12-14,16H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176373
(2-(2-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccccc1C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-20-10-6-5-9-18(20)13-23)21(17-7-3-2-4-8-17)16-26-12-11-19(27)15-26/h2-10,19,21,24,27H,11-12,14-16H2,1H3/t19-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176365
(CHEMBL201905 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H26F3N3O2/c1-27(21(30)13-26-18-9-7-17(8-10-18)22(23,24)25)20(16-5-3-2-4-6-16)15-28-12-11-19(29)14-28/h2-10,19-20,26,29H,11-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50166621
(CHEMBL191987 | N-[(S)-2-((S)-3-Hydroxy-pyrrolidin-...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)Cc1ccc(NS(C)(=O)=O)cc1 Show InChI InChI=1S/C22H29N3O4S/c1-24(22(27)14-17-8-10-19(11-9-17)23-30(2,28)29)21(18-6-4-3-5-7-18)16-25-13-12-20(26)15-25/h3-11,20-21,23,26H,12-16H2,1-2H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Opioid receptor kappa using [3H]-diprenorphine as radio ligand |
Bioorg Med Chem Lett 15: 2647-52 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.020
BindingDB Entry DOI: 10.7270/Q2PR7VH4 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86965
((S)-5-amino-2-((S)-2-(((3R,4R)-4-(3-hydroxyphenyl)...)Show SMILES CC1CN(CC(Cc2ccccc2)C(=O)NC(CCCN)C(O)=O)CCC1(C)c1cccc(O)c1 Show InChI InChI=1S/C28H39N3O4/c1-20-18-31(15-13-28(20,2)23-10-6-11-24(32)17-23)19-22(16-21-8-4-3-5-9-21)26(33)30-25(27(34)35)12-7-14-29/h3-6,8-11,17,20,22,25,32H,7,12-16,18-19,29H2,1-2H3,(H,30,33)(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 2006-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.106
BindingDB Entry DOI: 10.7270/Q2B856Q8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093968
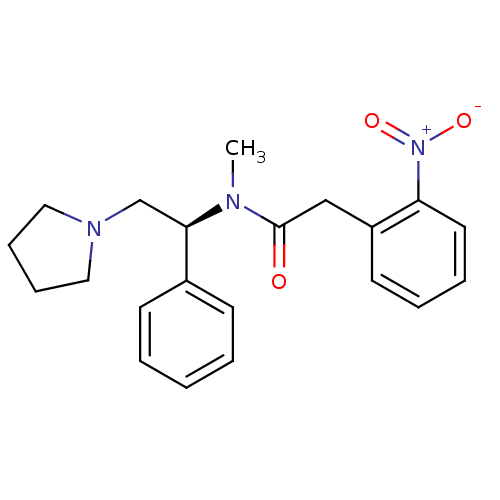
(CHEMBL87207 | N-Methyl-2-(2-nitro-phenyl)-N-((S)-1...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccccc1[N+]([O-])=O Show InChI InChI=1S/C21H25N3O3/c1-22(21(25)15-18-11-5-6-12-19(18)24(26)27)20(16-23-13-7-8-14-23)17-9-3-2-4-10-17/h2-6,9-12,20H,7-8,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50252953
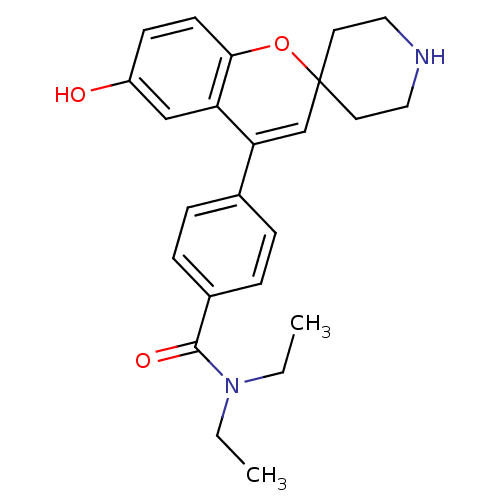
(CHEMBL494479 | N,N-Diethyl-4-(6-hydroxyspiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccc(O)cc12 |t:14| Show InChI InChI=1S/C24H28N2O3/c1-3-26(4-2)23(28)18-7-5-17(6-8-18)21-16-24(11-13-25-14-12-24)29-22-10-9-19(27)15-20(21)22/h5-10,15-16,25,27H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor in CHO cells |
J Med Chem 51: 5893-6 (2008)
Article DOI: 10.1021/jm8008986
BindingDB Entry DOI: 10.7270/Q26W9C0D |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160832
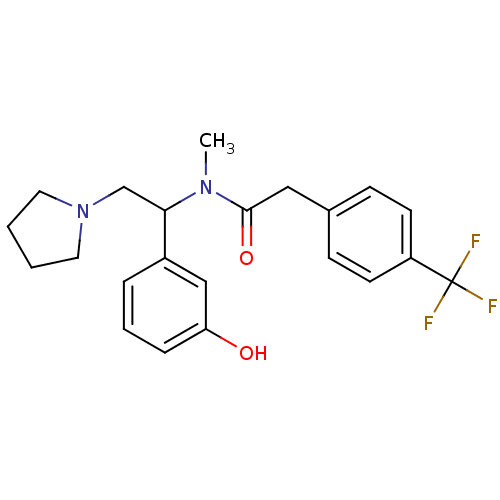
(CHEMBL181279 | N-[1-(3-Hydroxy-phenyl)-2-pyrrolidi...)Show SMILES CN(C(CN1CCCC1)c1cccc(O)c1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O2/c1-26(21(29)13-16-7-9-18(10-8-16)22(23,24)25)20(15-27-11-2-3-12-27)17-5-4-6-19(28)14-17/h4-10,14,20,28H,2-3,11-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86964
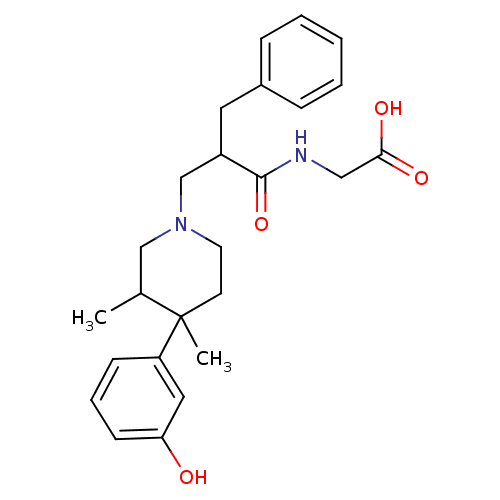
(2-((S)-2-benzyl-3-((3R,4R)-4-(3-hydroxyphenyl)-3,4...)Show SMILES CC1CN(CC(Cc2ccccc2)C(=O)NCC(O)=O)CCC1(C)c1cccc(O)c1 Show InChI InChI=1S/C25H32N2O4/c1-18-16-27(12-11-25(18,2)21-9-6-10-22(28)14-21)17-20(24(31)26-15-23(29)30)13-19-7-4-3-5-8-19/h3-10,14,18,20,28H,11-13,15-17H2,1-2H3,(H,26,31)(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 2006-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.106
BindingDB Entry DOI: 10.7270/Q2B856Q8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50160842
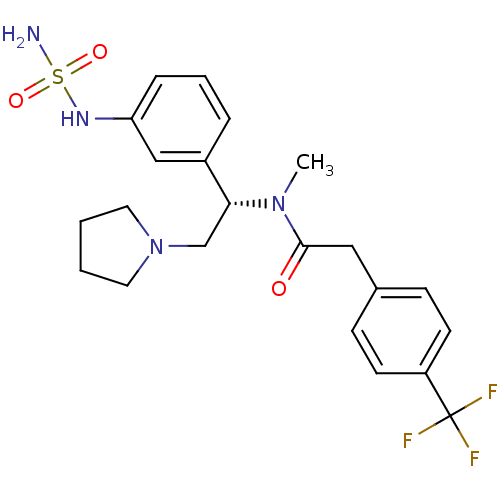
(CHEMBL362248 | N-[(S)-1-(3-aminosulfonylamino-phen...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NS(N)(=O)=O)c1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H27F3N4O3S/c1-28(21(30)13-16-7-9-18(10-8-16)22(23,24)25)20(15-29-11-2-3-12-29)17-5-4-6-19(14-17)27-33(26,31)32/h4-10,14,20,27H,2-3,11-13,15H2,1H3,(H2,26,31,32)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of bound [3H]-diprenorphine from membranes expressing cloned human kappa opioid receptor |
Bioorg Med Chem Lett 15: 1091-5 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.018
BindingDB Entry DOI: 10.7270/Q2JW8DCG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50176367
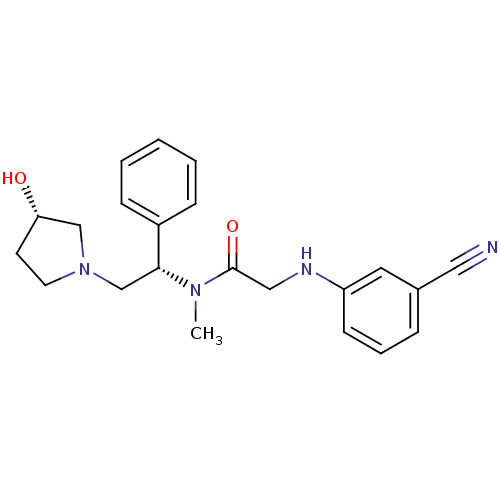
(2-(3-cyanophenylamino)-N-((S)-2-((S)-3-hydroxypyrr...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CNc1cccc(c1)C#N Show InChI InChI=1S/C22H26N4O2/c1-25(22(28)14-24-19-9-5-6-17(12-19)13-23)21(18-7-3-2-4-8-18)16-26-11-10-20(27)15-26/h2-9,12,20-21,24,27H,10-11,14-16H2,1H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 16: 645-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.034
BindingDB Entry DOI: 10.7270/Q26W99N3 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50038718
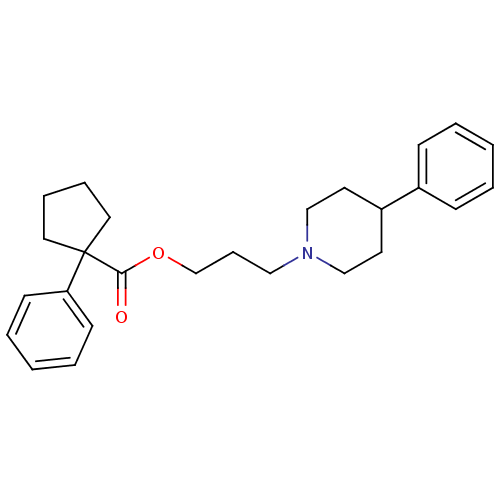
(1-Phenyl-cyclopentanecarboxylic acid 3-(4-phenyl-p...)Show SMILES O=C(OCCCN1CCC(CC1)c1ccccc1)C1(CCCC1)c1ccccc1 Show InChI InChI=1S/C26H33NO2/c28-25(26(16-7-8-17-26)24-12-5-2-6-13-24)29-21-9-18-27-19-14-23(15-20-27)22-10-3-1-4-11-22/h1-6,10-13,23H,7-9,14-21H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Affinity at sigma-1 site by inhibition of [3H](+)-pentazocine (PENT) binding in guinea pig brain |
J Med Chem 37: 1964-70 (1994)
BindingDB Entry DOI: 10.7270/Q2QZ2BMR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86968
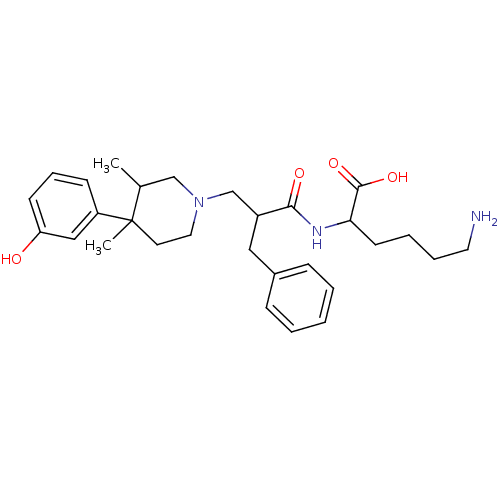
((S)-6-amino-2-((S)-2-(((3R,4R)-4-(3-hydroxyphenyl)...)Show SMILES CC1CN(CC(Cc2ccccc2)C(=O)NC(CCCCN)C(O)=O)CCC1(C)c1cccc(O)c1 Show InChI InChI=1S/C29H41N3O4/c1-21-19-32(16-14-29(21,2)24-11-8-12-25(33)18-24)20-23(17-22-9-4-3-5-10-22)27(34)31-26(28(35)36)13-6-7-15-30/h3-5,8-12,18,21,23,26,33H,6-7,13-17,19-20,30H2,1-2H3,(H,31,34)(H,35,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by PDSP Ki Database
| |
Bioorg Med Chem Lett 18: 2006-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.106
BindingDB Entry DOI: 10.7270/Q2B856Q8 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50038715
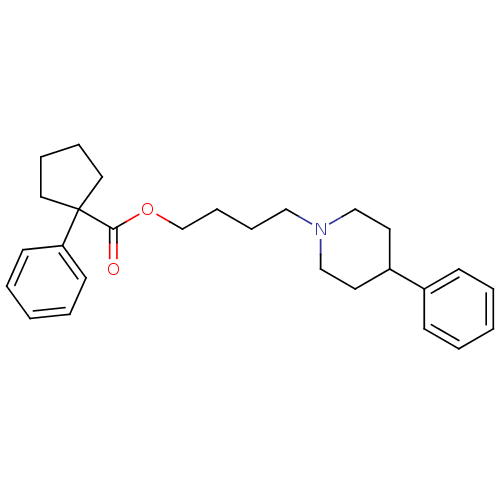
(1-Phenyl-cyclopentanecarboxylic acid 4-(4-phenyl-p...)Show SMILES O=C(OCCCCN1CCC(CC1)c1ccccc1)C1(CCCC1)c1ccccc1 Show InChI InChI=1S/C27H35NO2/c29-26(27(17-7-8-18-27)25-13-5-2-6-14-25)30-22-10-9-19-28-20-15-24(16-21-28)23-11-3-1-4-12-23/h1-6,11-14,24H,7-10,15-22H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research
Curated by ChEMBL
| Assay Description
Affinity at sigma-1 site by inhibition of [3H](+)-pentazocine (PENT) binding in guinea pig brain |
J Med Chem 37: 1964-70 (1994)
BindingDB Entry DOI: 10.7270/Q2QZ2BMR |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50205686
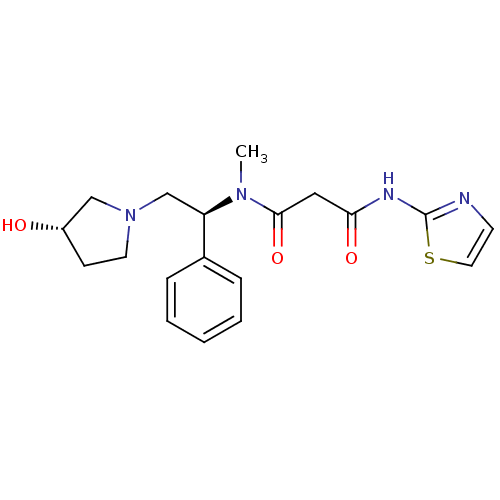
(CHEMBL230607 | N1-((S)-2-((S)-3-hydroxypyrrolidin-...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)CC(=O)Nc1nccs1 Show InChI InChI=1S/C19H24N4O3S/c1-22(18(26)11-17(25)21-19-20-8-10-27-19)16(14-5-3-2-4-6-14)13-23-9-7-15(24)12-23/h2-6,8,10,15-16,24H,7,9,11-13H2,1H3,(H,20,21,25)/t15-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 1951-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.053
BindingDB Entry DOI: 10.7270/Q2Q52P9X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data