 Found 1355 hits with Last Name = 'desjarlais' and Initial = 'rl'
Found 1355 hits with Last Name = 'desjarlais' and Initial = 'rl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598740
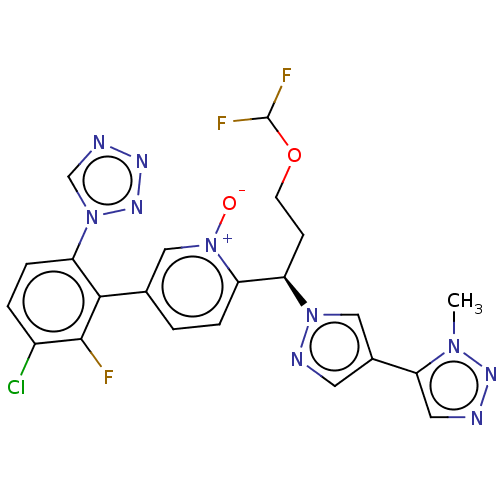
(CHEMBL5175227)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598738
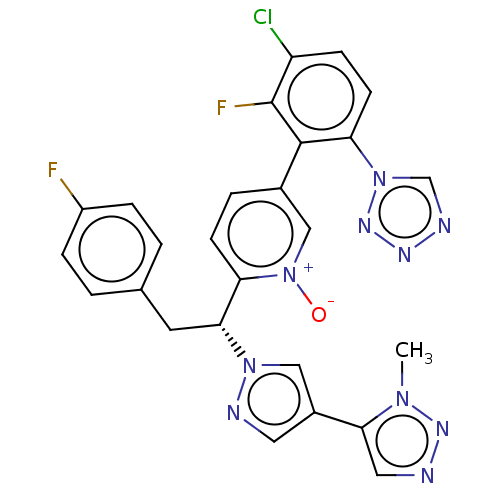
(CHEMBL5204065)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccc(F)cc1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598739

(CHEMBL5188215)Show SMILES COCC[C@H](c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1)n1cc(cn1)-c1cnnn1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598724

(CHEMBL5170592)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598737
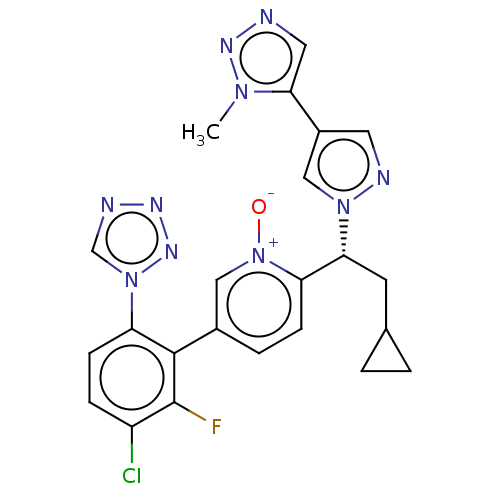
(CHEMBL5205631)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598741

(CHEMBL5204894)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccc(F)cc1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50290872
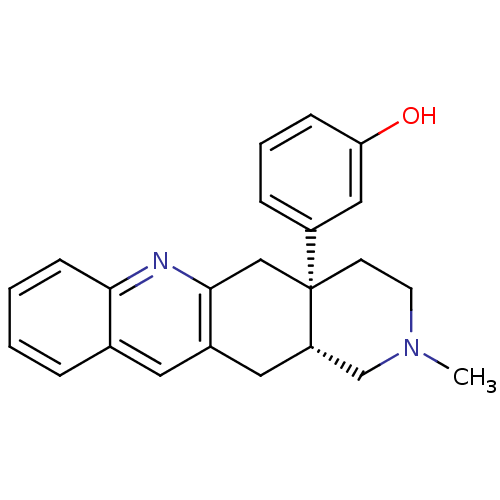
((-)-rel-3-((4aR,12aS)-2-methyl-1,2,3,4,4a,5,12,12a...)Show SMILES CN1CC[C@@]2(Cc3nc4ccccc4cc3C[C@H]2C1)c1cccc(O)c1 |r| Show InChI InChI=1S/C23H24N2O/c1-25-10-9-23(18-6-4-7-20(26)13-18)14-22-17(12-19(23)15-25)11-16-5-2-3-8-21(16)24-22/h2-8,11,13,19,26H,9-10,12,14-15H2,1H3/t19-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [3H]DADLE binding to delta-opioid receptor of mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598725

(CHEMBL5185397)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnc([nH]1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1cc(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598743
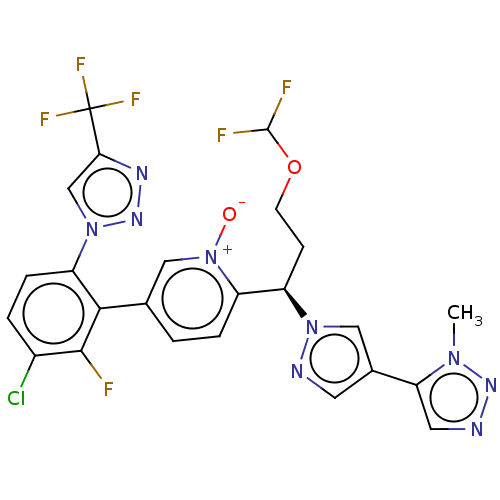
(CHEMBL5178223)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(nn1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598734
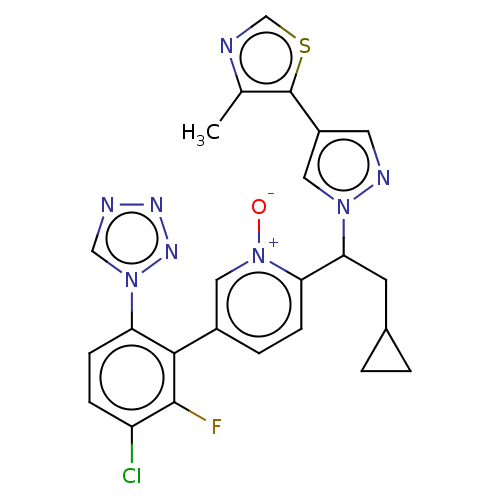
(CHEMBL5197480)Show SMILES Cc1ncsc1-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598745
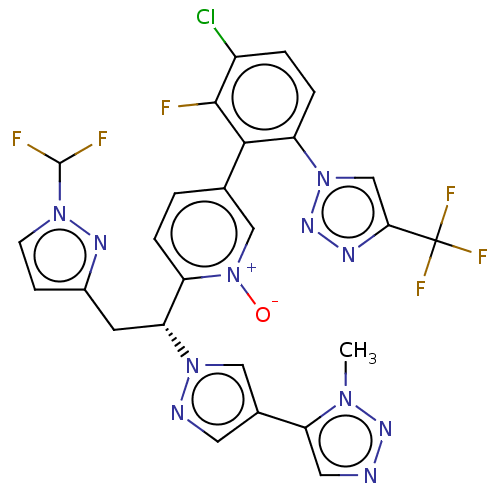
(CHEMBL5198823)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccn(n1)C(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(nn1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598736

(CHEMBL5208095)Show SMILES Cn1cncc1-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598744
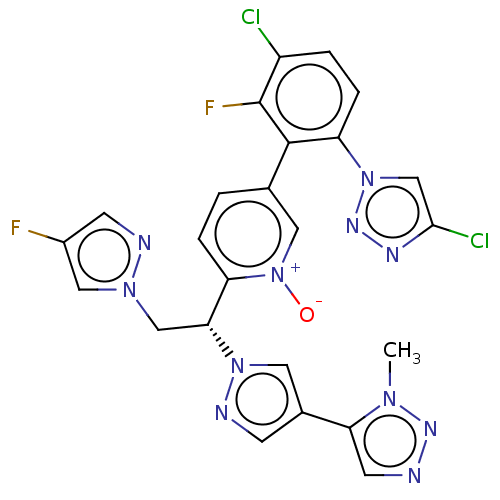
(CHEMBL5190323)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cn1cc(F)cn1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50450528

(CHEMBL2051964)Show SMILES Cc1ccc(O)c(c1)C(=O)NC[C@]1(C)C[C@H](O)CC(C)(C)C1 |r| Show InChI InChI=1S/C18H27NO3/c1-12-5-6-15(21)14(7-12)16(22)19-11-18(4)9-13(20)8-17(2,3)10-18/h5-7,13,20-21H,8-11H2,1-4H3,(H,19,22)/t13-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [3H]DADLE binding to delta-opioid receptor of mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066647

(CHEMBL113724 | {(S)-3-Methyl-1-[3-oxo-1-(4-phenoxy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C31H35N3O7S/c1-22(2)19-28(33-31(37)40-21-23-9-5-3-6-10-23)30(36)32-27-17-18-34(20-29(27)35)42(38,39)26-15-13-25(14-16-26)41-24-11-7-4-8-12-24/h3-16,22,27-28H,17-21H2,1-2H3,(H,32,36)(H,33,37)/t27?,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037121
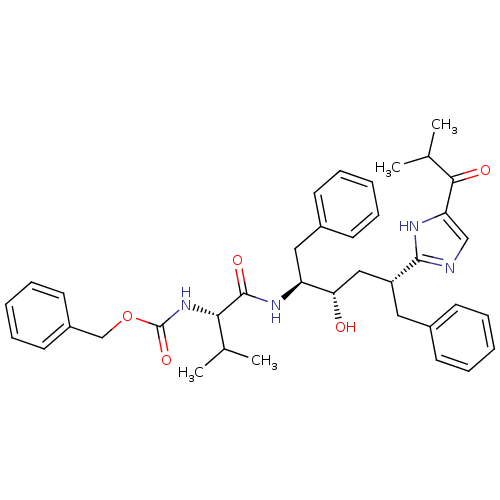
(2-[(1R,3S,4S)-1-BENZYL-4-[N-(BENZYLOXYCARBONYL)-L-...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(=O)C(C)C Show InChI InChI=1S/C38H46N4O5/c1-25(2)34(42-38(46)47-24-29-18-12-7-13-19-29)37(45)41-31(21-28-16-10-6-11-17-28)33(43)22-30(20-27-14-8-5-9-15-27)36-39-23-32(40-36)35(44)26(3)4/h5-19,23,25-26,30-31,33-34,43H,20-22,24H2,1-4H3,(H,39,40)(H,41,45)(H,42,46)/t30-,31+,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against recombinant HIV-1 protease |
J Med Chem 37: 3100-7 (1994)
BindingDB Entry DOI: 10.7270/Q2T72GG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598732
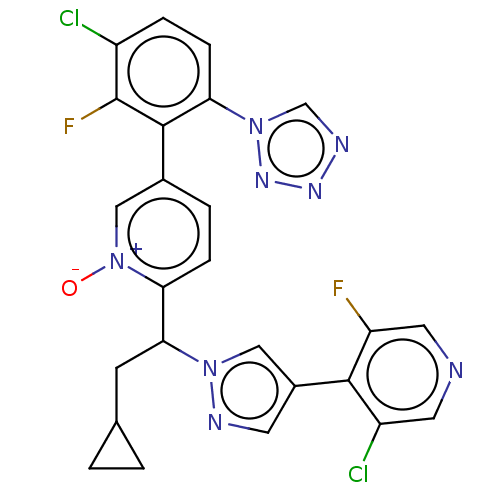
(CHEMBL5192284)Show SMILES [O-][n+]1cc(ccc1C(CC1CC1)n1cc(cn1)-c1c(F)cncc1Cl)-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037124
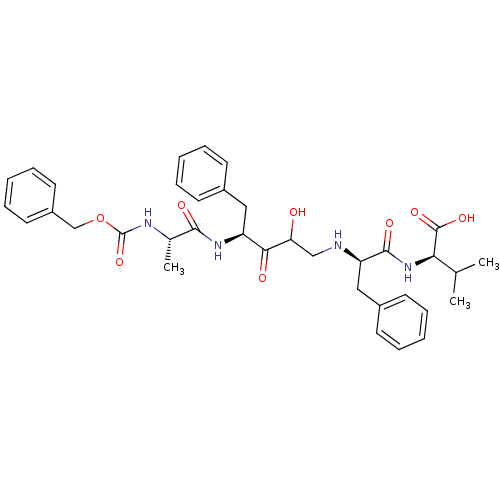
((R)-2-{(R)-2-[(S)-4-((S)-2-Benzyloxycarbonylamino-...)Show SMILES CC(C)[C@@H](NC(=O)[C@@H](Cc1ccccc1)NCC(O)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)OCc1ccccc1)C(O)=O Show InChI InChI=1S/C36H44N4O8/c1-23(2)31(35(45)46)40-34(44)29(20-26-15-9-5-10-16-26)37-21-30(41)32(42)28(19-25-13-7-4-8-14-25)39-33(43)24(3)38-36(47)48-22-27-17-11-6-12-18-27/h4-18,23-24,28-31,37,41H,19-22H2,1-3H3,(H,38,47)(H,39,43)(H,40,44)(H,45,46)/t24-,28-,29+,30?,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against recombinant HIV-1 protease |
J Med Chem 37: 3100-7 (1994)
BindingDB Entry DOI: 10.7270/Q2T72GG9 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408519
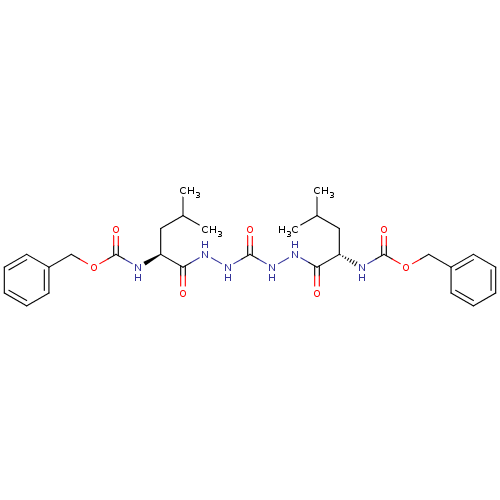
(CHEMBL115357)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C29H40N6O7/c1-19(2)15-23(30-28(39)41-17-21-11-7-5-8-12-21)25(36)32-34-27(38)35-33-26(37)24(16-20(3)4)31-29(40)42-18-22-13-9-6-10-14-22/h5-14,19-20,23-24H,15-18H2,1-4H3,(H,30,39)(H,31,40)(H,32,36)(H,33,37)(H2,34,35,38)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408522
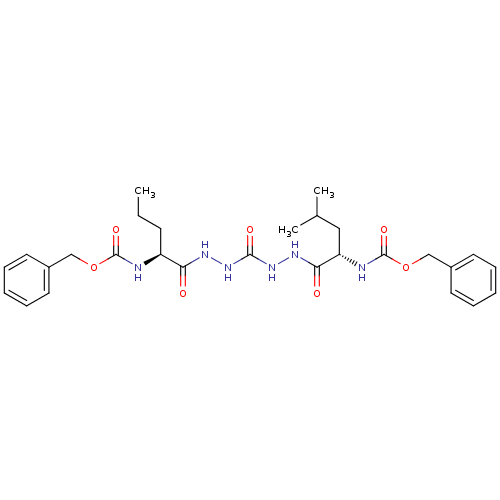
(CHEMBL126820)Show SMILES CCC[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C28H38N6O7/c1-4-11-22(29-27(38)40-17-20-12-7-5-8-13-20)24(35)31-33-26(37)34-32-25(36)23(16-19(2)3)30-28(39)41-18-21-14-9-6-10-15-21/h5-10,12-15,19,22-23H,4,11,16-18H2,1-3H3,(H,29,38)(H,30,39)(H,31,35)(H,32,36)(H2,33,34,37)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50061061
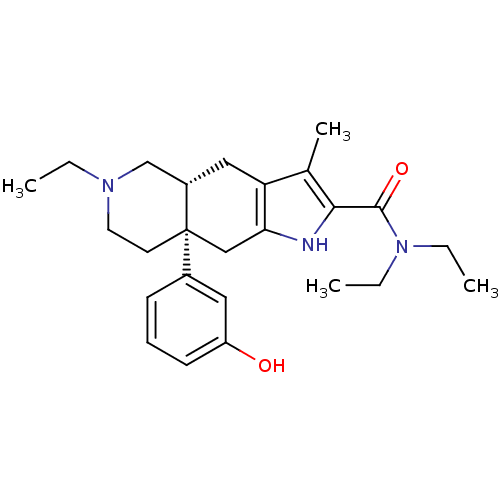
((4aR,8aS)-6-Ethyl-8a-(3-hydroxy-phenyl)-3-methyl-4...)Show SMILES CCN(CC)C(=O)c1[nH]c2C[C@]3(CCN(CC)C[C@@H]3Cc2c1C)c1cccc(O)c1 Show InChI InChI=1S/C25H35N3O2/c1-5-27-12-11-25(18-9-8-10-20(29)13-18)15-22-21(14-19(25)16-27)17(4)23(26-22)24(30)28(6-2)7-3/h8-10,13,19,26,29H,5-7,11-12,14-16H2,1-4H3/t19-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [3H]DADLE binding to delta-opioid receptor of mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408520
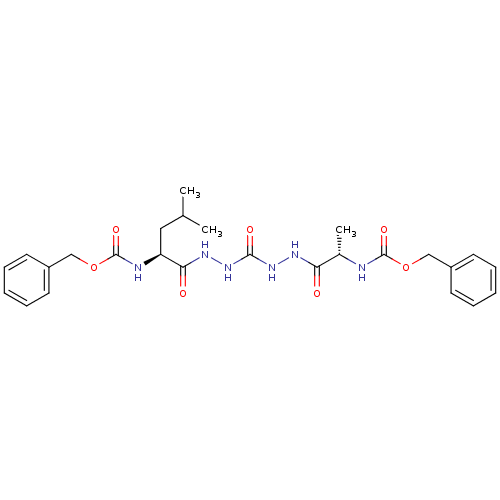
(CHEMBL126352)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)[C@H](C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C26H34N6O7/c1-17(2)14-21(28-26(37)39-16-20-12-8-5-9-13-20)23(34)30-32-24(35)31-29-22(33)18(3)27-25(36)38-15-19-10-6-4-7-11-19/h4-13,17-18,21H,14-16H2,1-3H3,(H,27,36)(H,28,37)(H,29,33)(H,30,34)(H2,31,32,35)/t18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598742
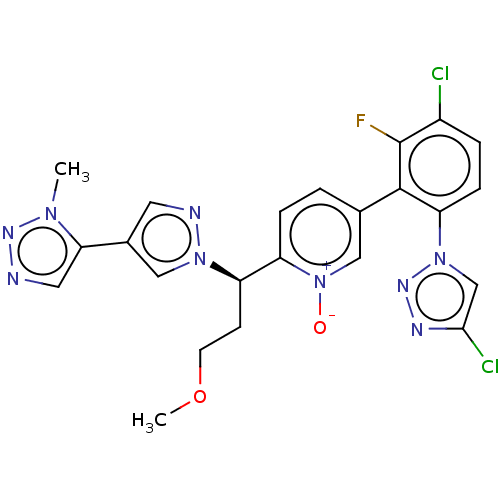
(CHEMBL5182855)Show SMILES COCC[C@H](c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1)n1cc(cn1)-c1cnnn1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598729
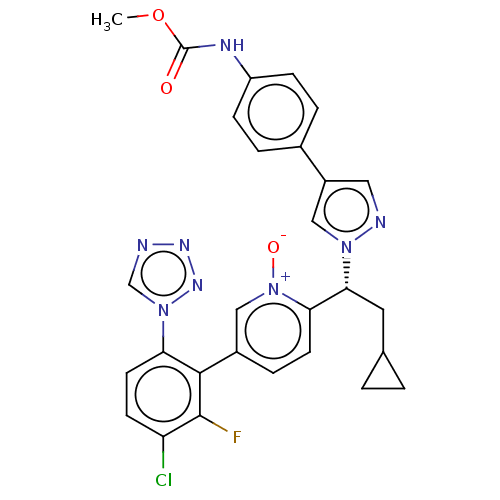
(CHEMBL5195600)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor
(MOUSE) | BDBM50290869
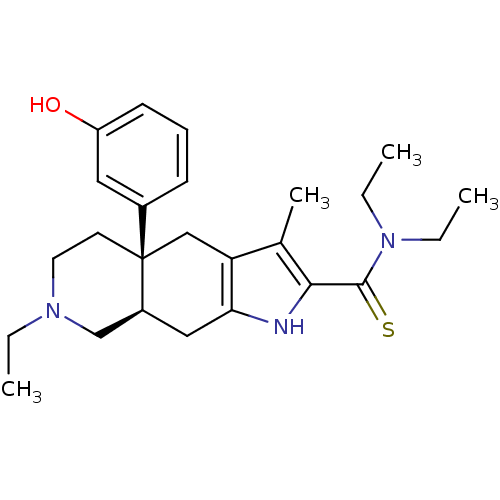
((4aR,8aR)-7-Ethyl-4a-(3-hydroxy-phenyl)-3-methyl-4...)Show SMILES CCN(CC)C(=S)c1[nH]c2C[C@H]3CN(CC)CC[C@@]3(Cc2c1C)c1cccc(O)c1 Show InChI InChI=1S/C25H35N3OS/c1-5-27-12-11-25(18-9-8-10-20(29)13-18)15-21-17(4)23(24(30)28(6-2)7-3)26-22(21)14-19(25)16-27/h8-10,13,19,26,29H,5-7,11-12,14-16H2,1-4H3/t19-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [3H]DADLE binding to delta-opioid receptor of mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598727
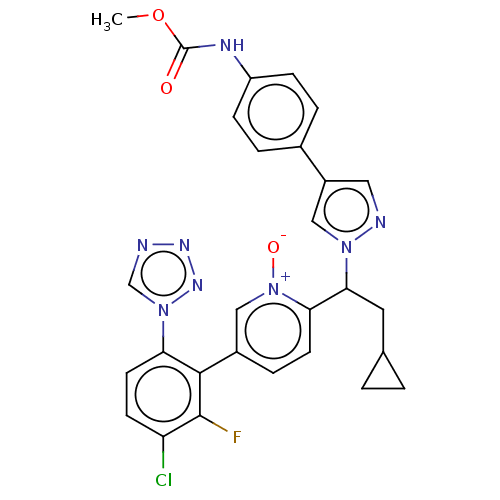
(CHEMBL5198338)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598735
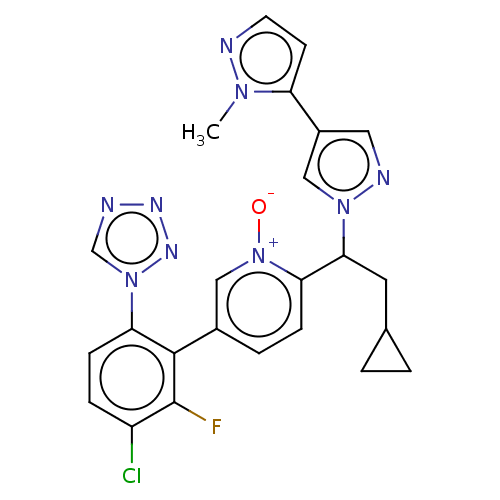
(CHEMBL5193267)Show SMILES Cn1nccc1-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor
(MOUSE) | BDBM50061061

((4aR,8aS)-6-Ethyl-8a-(3-hydroxy-phenyl)-3-methyl-4...)Show SMILES CCN(CC)C(=O)c1[nH]c2C[C@]3(CCN(CC)C[C@@H]3Cc2c1C)c1cccc(O)c1 Show InChI InChI=1S/C25H35N3O2/c1-5-27-12-11-25(18-9-8-10-20(29)13-18)15-22-21(14-19(25)16-27)17(4)23(26-22)24(30)28(6-2)7-3/h8-10,13,19,26,29H,5-7,11-12,14-16H2,1-4H3/t19-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [3H]DADLE binding to delta-opioid receptor of mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50061061

((4aR,8aS)-6-Ethyl-8a-(3-hydroxy-phenyl)-3-methyl-4...)Show SMILES CCN(CC)C(=O)c1[nH]c2C[C@]3(CCN(CC)C[C@@H]3Cc2c1C)c1cccc(O)c1 Show InChI InChI=1S/C25H35N3O2/c1-5-27-12-11-25(18-9-8-10-20(29)13-18)15-22-21(14-19(25)16-27)17(4)23(26-22)24(30)28(6-2)7-3/h8-10,13,19,26,29H,5-7,11-12,14-16H2,1-4H3/t19-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [3H]DADLE binding to delta-opioid receptor of mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50061063
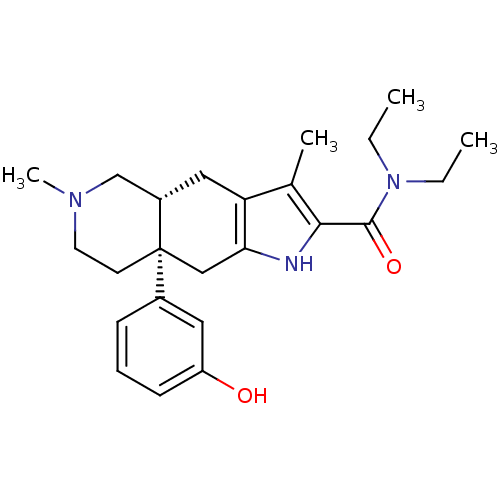
((4aR,8aS)-8a-(3-Hydroxy-phenyl)-3,6-dimethyl-4,4a,...)Show SMILES CCN(CC)C(=O)c1[nH]c2C[C@]3(CCN(C)C[C@@H]3Cc2c1C)c1cccc(O)c1 Show InChI InChI=1S/C24H33N3O2/c1-5-27(6-2)23(29)22-16(3)20-13-18-15-26(4)11-10-24(18,14-21(20)25-22)17-8-7-9-19(28)12-17/h7-9,12,18,25,28H,5-6,10-11,13-15H2,1-4H3/t18-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [3H]DADLE binding to delta-opioid receptor of mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11123
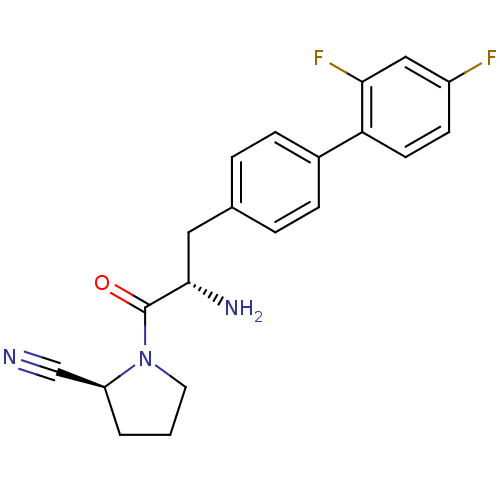
((2S)-1-[(2S)-2-amino-3-[4-(2,4-difluorophenyl)phen...)Show SMILES N[C@@H](Cc1ccc(cc1)-c1ccc(F)cc1F)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C20H19F2N3O/c21-15-7-8-17(18(22)11-15)14-5-3-13(4-6-14)10-19(24)20(26)25-9-1-2-16(25)12-23/h3-8,11,16,19H,1-2,9-10,24H2/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... |
Bioorg Med Chem Lett 16: 123-8 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.037
BindingDB Entry DOI: 10.7270/Q2S180QJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19807
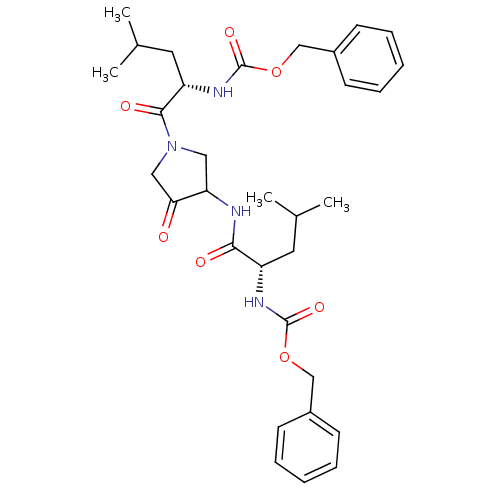
(CHEMBL100563 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C32H42N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-27H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Delta-type opioid receptor
(MOUSE) | BDBM50290866
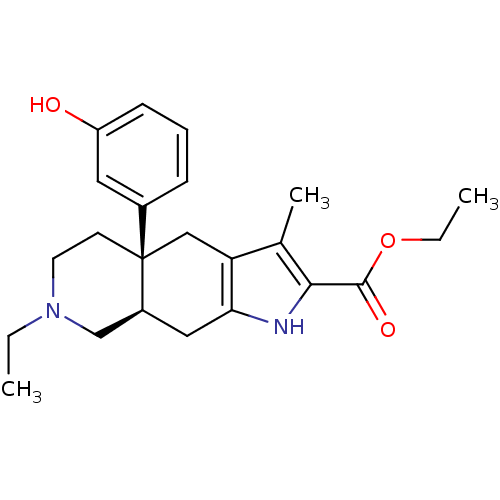
((4aR,8aR)-7-Ethyl-4a-(3-hydroxy-phenyl)-3-methyl-4...)Show SMILES CCOC(=O)c1[nH]c2C[C@H]3CN(CC)CC[C@@]3(Cc2c1C)c1cccc(O)c1 Show InChI InChI=1S/C23H30N2O3/c1-4-25-10-9-23(16-7-6-8-18(26)11-16)13-19-15(3)21(22(27)28-5-2)24-20(19)12-17(23)14-25/h6-8,11,17,24,26H,4-5,9-10,12-14H2,1-3H3/t17-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [3H]DADLE binding to delta-opioid receptor of mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598730

(CHEMBL5204289)Show SMILES Cc1nc(N)ccc1-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor
(MOUSE) | BDBM50290868
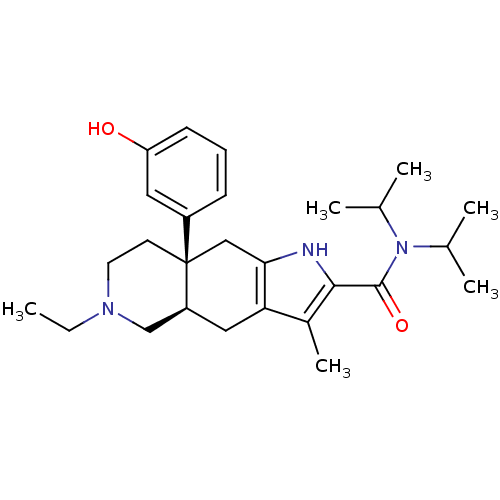
((4aR,8aS)-6-Ethyl-8a-(3-hydroxy-phenyl)-3-methyl-4...)Show SMILES CCN1CC[C@@]2(Cc3[nH]c(C(=O)N(C(C)C)C(C)C)c(C)c3C[C@H]2C1)c1cccc(O)c1 Show InChI InChI=1S/C27H39N3O2/c1-7-29-12-11-27(20-9-8-10-22(31)13-20)15-24-23(14-21(27)16-29)19(6)25(28-24)26(32)30(17(2)3)18(4)5/h8-10,13,17-18,21,28,31H,7,11-12,14-16H2,1-6H3/t21-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [3H]DADLE binding to delta-opioid receptor of mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19810
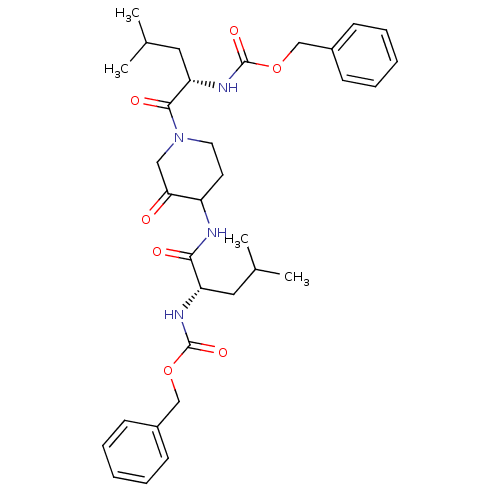
(CHEMBL118449 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Delta-type opioid receptor
(MOUSE) | BDBM50290870

((4aR,8aR)-4a-(3-Hydroxy-phenyl)-3,7-dimethyl-4,4a,...)Show SMILES CCN(CC)C(=O)c1[nH]c2C[C@H]3CN(C)CC[C@@]3(Cc2c1C)c1cccc(O)c1 Show InChI InChI=1S/C24H33N3O2/c1-5-27(6-2)23(29)22-16(3)20-14-24(17-8-7-9-19(28)12-17)10-11-26(4)15-18(24)13-21(20)25-22/h7-9,12,18,25,28H,5-6,10-11,13-15H2,1-4H3/t18-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [3H]DADLE binding to delta-opioid receptor of mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11121
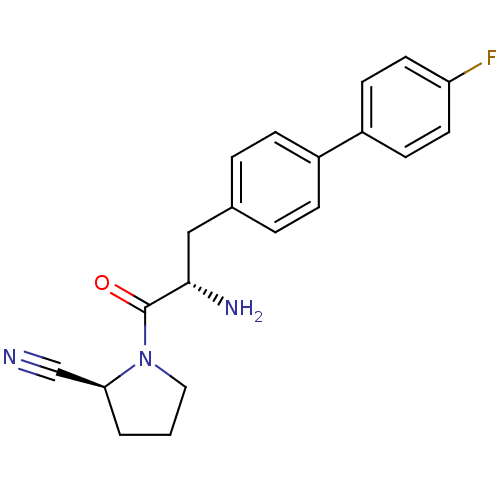
((2S)-1-[(2S)-2-amino-3-[4-(4-fluorophenyl)phenyl]p...)Show SMILES N[C@@H](Cc1ccc(cc1)-c1ccc(F)cc1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C20H20FN3O/c21-17-9-7-16(8-10-17)15-5-3-14(4-6-15)12-19(23)20(25)24-11-1-2-18(24)13-22/h3-10,18-19H,1-2,11-12,23H2/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... |
Bioorg Med Chem Lett 16: 123-8 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.037
BindingDB Entry DOI: 10.7270/Q2S180QJ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598731
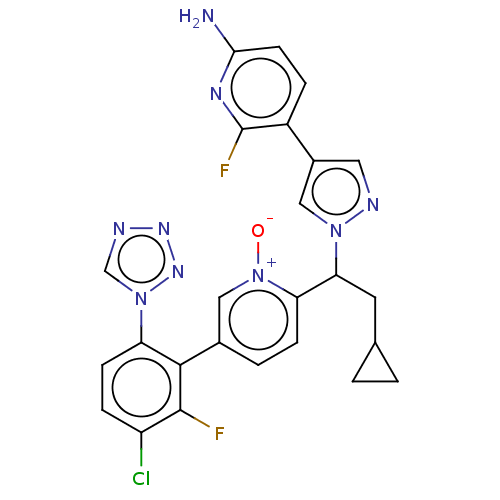
(CHEMBL5198972)Show SMILES Nc1ccc(-c2cnn(c2)C(CC2CC2)c2ccc(c[n+]2[O-])-c2c(F)c(Cl)ccc2-n2cnnn2)c(F)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408521
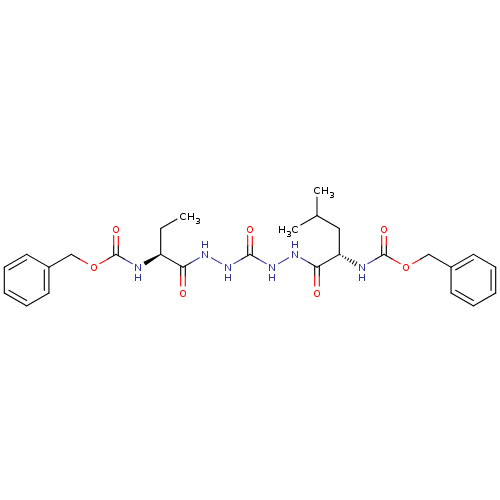
(CHEMBL129773)Show SMILES CC[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C27H36N6O7/c1-4-21(28-26(37)39-16-19-11-7-5-8-12-19)23(34)30-32-25(36)33-31-24(35)22(15-18(2)3)29-27(38)40-17-20-13-9-6-10-14-20/h5-14,18,21-22H,4,15-17H2,1-3H3,(H,28,37)(H,29,38)(H,30,34)(H,31,35)(H2,32,33,36)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066648
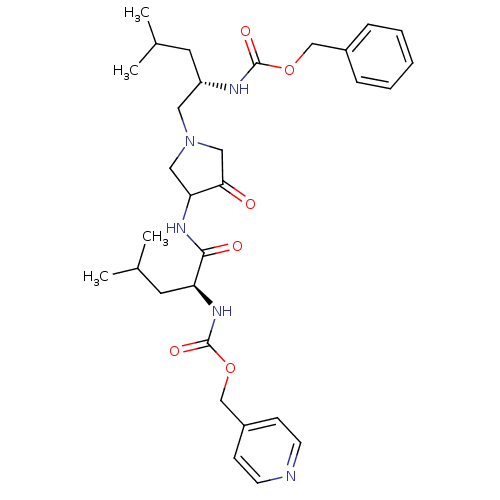
(((S)-3-Methyl-1-{3-[(S)-4-methyl-2-(pyridin-4-ylme...)Show SMILES CC(C)C[C@@H](CN1CC(NC(=O)[C@H](CC(C)C)NC(=O)OCc2ccncc2)C(=O)C1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H43N5O6/c1-21(2)14-25(33-30(39)41-19-23-8-6-5-7-9-23)16-36-17-27(28(37)18-36)34-29(38)26(15-22(3)4)35-31(40)42-20-24-10-12-32-13-11-24/h5-13,21-22,25-27H,14-20H2,1-4H3,(H,33,39)(H,34,38)(H,35,40)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11122
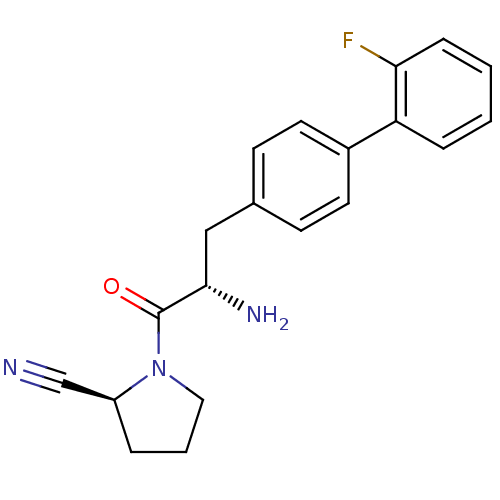
((2S)-1-[(2S)-2-amino-3-[4-(2-fluorophenyl)phenyl]p...)Show SMILES N[C@@H](Cc1ccc(cc1)-c1ccccc1F)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C20H20FN3O/c21-18-6-2-1-5-17(18)15-9-7-14(8-10-15)12-19(23)20(25)24-11-3-4-16(24)13-22/h1-2,5-10,16,19H,3-4,11-12,23H2/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | -47.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... |
Bioorg Med Chem Lett 16: 123-8 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.037
BindingDB Entry DOI: 10.7270/Q2S180QJ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598733
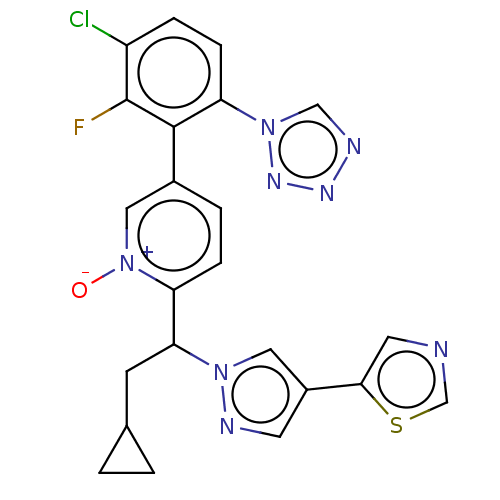
(CHEMBL5188316)Show SMILES [O-][n+]1cc(ccc1C(CC1CC1)n1cc(cn1)-c1cncs1)-c1c(F)c(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor
(MOUSE) | BDBM50290871

((4aR,8aS)-6-Ethyl-8a-(3-hydroxy-phenyl)-3-methyl-4...)Show SMILES CCN1CC[C@@]2(Cc3[nH]c(C(=O)N(C)C)c(C)c3C[C@H]2C1)c1cccc(O)c1 Show InChI InChI=1S/C23H31N3O2/c1-5-26-10-9-23(16-7-6-8-18(27)11-16)13-20-19(12-17(23)14-26)15(2)21(24-20)22(28)25(3)4/h6-8,11,17,24,27H,5,9-10,12-14H2,1-4H3/t17-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [3H]DADLE binding to delta-opioid receptor of mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598726
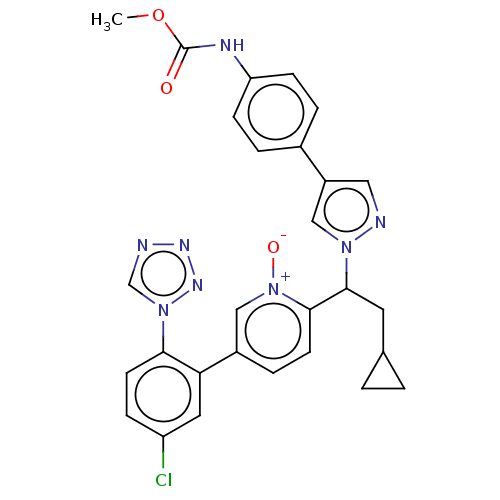
(CHEMBL5171252)Show SMILES COC(=O)Nc1ccc(cc1)-c1cnn(c1)C(CC1CC1)c1ccc(c[n+]1[O-])-c1cc(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50450528

(CHEMBL2051964)Show SMILES Cc1ccc(O)c(c1)C(=O)NC[C@]1(C)C[C@H](O)CC(C)(C)C1 |r| Show InChI InChI=1S/C18H27NO3/c1-12-5-6-15(21)14(7-12)16(22)19-11-18(4)9-13(20)8-17(2,3)10-18/h5-7,13,20-21H,8-11H2,1-4H3,(H,19,22)/t13-,18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Kappa opioid receptor using [3H]U-69593 radioligand in mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50182163
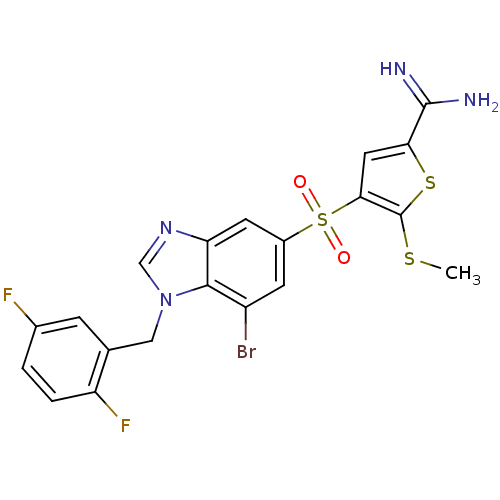
(4-[7-bromo-1-(2,5-difluoro-benzyl)-1H-benzoimidazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cc(Br)c2n(Cc3cc(F)ccc3F)cnc2c1)C(N)=N Show InChI InChI=1S/C20H15BrF2N4O2S3/c1-30-20-17(7-16(31-20)19(24)25)32(28,29)12-5-13(21)18-15(6-12)26-9-27(18)8-10-4-11(22)2-3-14(10)23/h2-7,9H,8H2,1H3,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of C1S |
Bioorg Med Chem Lett 16: 2200-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.036
BindingDB Entry DOI: 10.7270/Q2251HSV |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50079596
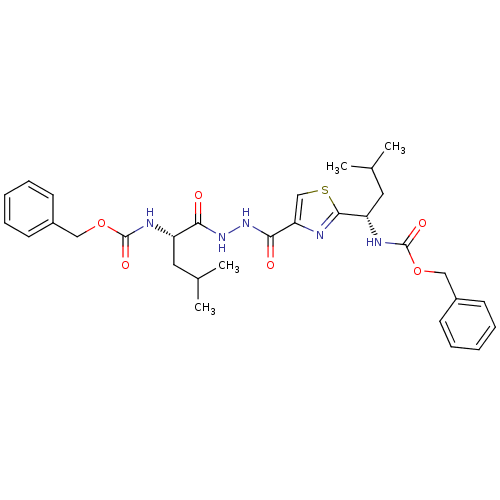
(((S)-1-{N'-[2-((S)-1-Benzyloxycarbonylamino-3-meth...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)c1csc(n1)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H39N5O6S/c1-20(2)15-24(33-30(39)41-17-22-11-7-5-8-12-22)27(37)35-36-28(38)26-19-43-29(32-26)25(16-21(3)4)34-31(40)42-18-23-13-9-6-10-14-23/h5-14,19-21,24-25H,15-18H2,1-4H3,(H,33,39)(H,34,40)(H,35,37)(H,36,38)/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human osteoclast cathepsin K |
Bioorg Med Chem Lett 9: 1907-10 (1999)
BindingDB Entry DOI: 10.7270/Q2M61JGJ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408515
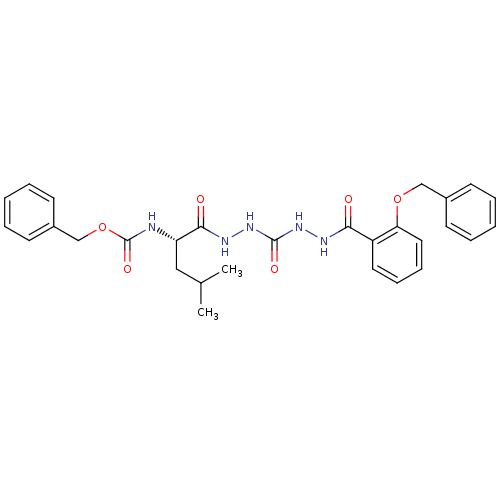
(CHEMBL338770)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)c1ccccc1OCc1ccccc1 Show InChI InChI=1S/C29H33N5O6/c1-20(2)17-24(30-29(38)40-19-22-13-7-4-8-14-22)27(36)32-34-28(37)33-31-26(35)23-15-9-10-16-25(23)39-18-21-11-5-3-6-12-21/h3-16,20,24H,17-19H2,1-2H3,(H,30,38)(H,31,35)(H,32,36)(H2,33,34,37)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11118
((2S)-1-[(2S)-2-amino-3-(4-phenylphenyl)propanoyl]p...)Show SMILES N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C20H21N3O/c21-14-18-7-4-12-23(18)20(24)19(22)13-15-8-10-17(11-9-15)16-5-2-1-3-6-16/h1-3,5-6,8-11,18-19H,4,7,12-13,22H2/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... |
Bioorg Med Chem Lett 16: 123-8 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.037
BindingDB Entry DOI: 10.7270/Q2S180QJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data