 Found 182 hits with Last Name = 'dicapua' and Initial = 'f'
Found 182 hits with Last Name = 'dicapua' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase 4D |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Phosphodiesterase 4B |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase type 4A (PDE4A). |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50062387
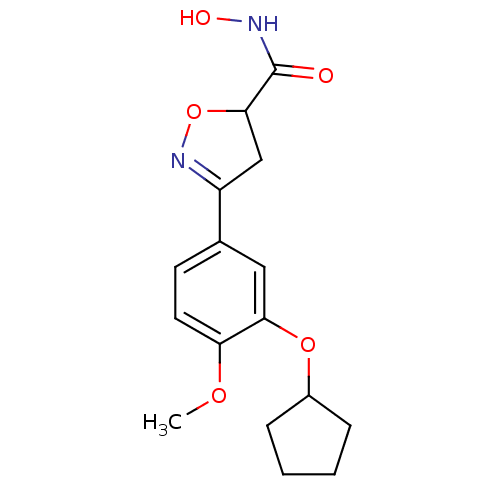
(3-(3-Cyclopentyloxy-4-methoxy-phenyl)-4,5-dihydro-...)Show SMILES COc1ccc(cc1OC1CCCC1)C1=NOC(C1)C(=O)NO |t:16| Show InChI InChI=1S/C16H20N2O5/c1-21-13-7-6-10(8-14(13)22-11-4-2-3-5-11)12-9-15(23-18-12)16(19)17-20/h6-8,11,15,20H,2-5,9H2,1H3,(H,17,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Phosphodiesterase 4B |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50062387

(3-(3-Cyclopentyloxy-4-methoxy-phenyl)-4,5-dihydro-...)Show SMILES COc1ccc(cc1OC1CCCC1)C1=NOC(C1)C(=O)NO |t:16| Show InChI InChI=1S/C16H20N2O5/c1-21-13-7-6-10(8-14(13)22-11-4-2-3-5-11)12-9-15(23-18-12)16(19)17-20/h6-8,11,15,20H,2-5,9H2,1H3,(H,17,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase type 4A (PDE4A). |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50062387

(3-(3-Cyclopentyloxy-4-methoxy-phenyl)-4,5-dihydro-...)Show SMILES COc1ccc(cc1OC1CCCC1)C1=NOC(C1)C(=O)NO |t:16| Show InChI InChI=1S/C16H20N2O5/c1-21-13-7-6-10(8-14(13)22-11-4-2-3-5-11)12-9-15(23-18-12)16(19)17-20/h6-8,11,15,20H,2-5,9H2,1H3,(H,17,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase 4D |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50105466

((S)-6-Amino-2-[(R)-2-[(4-benzenesulfonyl-piperazin...)Show SMILES CC(C)(C)OC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)N1CCN(CC1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C32H44N6O6S/c1-32(2,3)44-30(40)27(15-9-10-16-33)35-29(39)28(21-23-22-34-26-14-8-7-13-25(23)26)36-31(41)37-17-19-38(20-18-37)45(42,43)24-11-5-4-6-12-24/h4-8,11-14,22,27-28,34H,9-10,15-21,33H2,1-3H3,(H,35,39)(H,36,41)/t27-,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells |
Bioorg Med Chem Lett 11: 2731-4 (2001)
BindingDB Entry DOI: 10.7270/Q2JW8D5S |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50105472
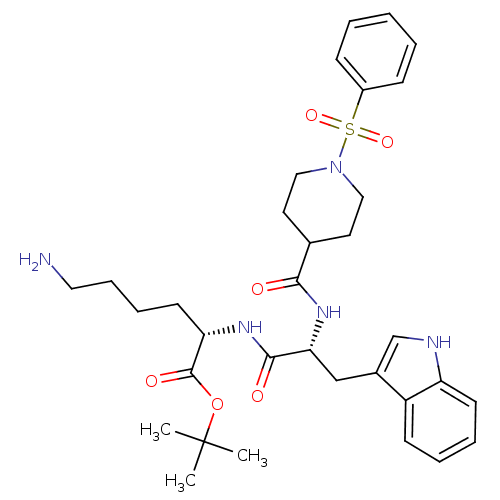
((S)-6-Amino-2-[(R)-2-[(1-benzenesulfonyl-piperidin...)Show SMILES CC(C)(C)OC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)C1CCN(CC1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C33H45N5O6S/c1-33(2,3)44-32(41)28(15-9-10-18-34)36-31(40)29(21-24-22-35-27-14-8-7-13-26(24)27)37-30(39)23-16-19-38(20-17-23)45(42,43)25-11-5-4-6-12-25/h4-8,11-14,22-23,28-29,35H,9-10,15-21,34H2,1-3H3,(H,36,40)(H,37,39)/t28-,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells |
Bioorg Med Chem Lett 11: 2731-4 (2001)
BindingDB Entry DOI: 10.7270/Q2JW8D5S |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase 4C |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50361851
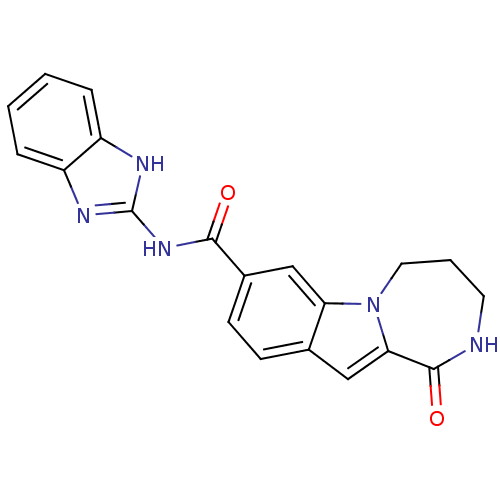
(CHEMBL1938803 | US9150577, 55)Show SMILES O=C(Nc1nc2ccccc2[nH]1)c1ccc2cc3C(=O)NCCCn3c2c1 Show InChI InChI=1S/C20H17N5O2/c26-18(24-20-22-14-4-1-2-5-15(14)23-20)13-7-6-12-10-17-19(27)21-8-3-9-25(17)16(12)11-13/h1-2,4-7,10-11H,3,8-9H2,(H,21,27)(H2,22,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 phosphorylation by luminescence assay |
Bioorg Med Chem Lett 22: 733-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.030
BindingDB Entry DOI: 10.7270/Q2XW4K7N |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50105465
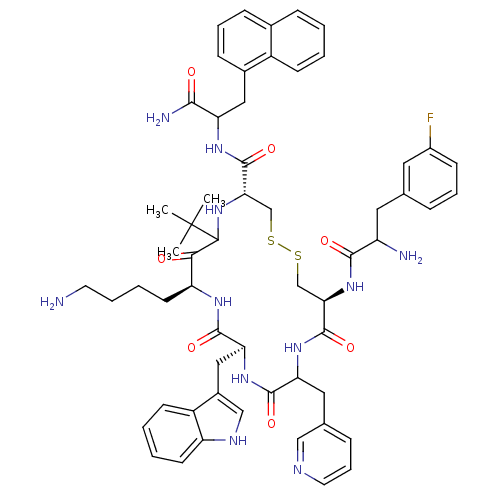
((4R,8S,11S,17S)-8-(4-Amino-butyl)-17-(2-amino-3-na...)Show SMILES CC(C)(C)C1N[C@@H](CSSC[C@@H](NC(=O)C(N)Cc2cccc(F)c2)C(=O)NC(Cc2cccnc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C1=O)C(=O)NC(Cc1cccc2ccccc12)C(N)=O Show InChI InChI=1S/C58H70FN11O7S2/c1-58(2,3)51-50(71)44(22-8-9-23-60)66-55(75)47(29-38-31-64-43-21-7-6-20-41(38)43)69-54(74)46(27-35-14-12-24-63-30-35)68-57(77)49(70-53(73)42(61)26-34-13-10-18-39(59)25-34)33-79-78-32-48(65-51)56(76)67-45(52(62)72)28-37-17-11-16-36-15-4-5-19-40(36)37/h4-7,10-21,24-25,30-31,42,44-49,51,64-65H,8-9,22-23,26-29,32-33,60-61H2,1-3H3,(H2,62,72)(H,66,75)(H,67,76)(H,68,77)(H,69,74)(H,70,73)/t42?,44-,45?,46?,47-,48-,49+,51?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells |
Bioorg Med Chem Lett 11: 2731-4 (2001)
BindingDB Entry DOI: 10.7270/Q2JW8D5S |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50105470

((S)-6-Amino-2-((R)-3-(1H-indol-3-yl)-2-{[4-(4-meth...)Show SMILES Cc1ccc(cc1)C(=O)N1CCN(CC1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C Show InChI InChI=1S/C34H46N6O5/c1-23-12-14-24(15-13-23)31(42)39-17-19-40(20-18-39)33(44)38-29(21-25-22-36-27-10-6-5-9-26(25)27)30(41)37-28(11-7-8-16-35)32(43)45-34(2,3)4/h5-6,9-10,12-15,22,28-29,36H,7-8,11,16-21,35H2,1-4H3,(H,37,41)(H,38,44)/t28-,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells |
Bioorg Med Chem Lett 11: 2731-4 (2001)
BindingDB Entry DOI: 10.7270/Q2JW8D5S |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50105469

((S)-6-Amino-2-((R)-3-(1H-indol-3-yl)-2-{[4-(2-oxo-...)Show SMILES CC(C)(C)OC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C34H45N7O5/c1-34(2,3)46-31(43)27(13-8-9-17-35)37-30(42)28(20-22-21-36-25-11-5-4-10-24(22)25)39-32(44)40-18-15-23(16-19-40)41-29-14-7-6-12-26(29)38-33(41)45/h4-7,10-12,14,21,23,27-28,36H,8-9,13,15-20,35H2,1-3H3,(H,37,42)(H,38,45)(H,39,44)/t27-,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Antagonistic activity of the compound towards sst2 receptor in GH4C1 cells a concentration of 1-2 x 10e6/mL incubated for 20 minutes |
Bioorg Med Chem Lett 11: 2731-4 (2001)
BindingDB Entry DOI: 10.7270/Q2JW8D5S |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50105469

((S)-6-Amino-2-((R)-3-(1H-indol-3-yl)-2-{[4-(2-oxo-...)Show SMILES CC(C)(C)OC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C34H45N7O5/c1-34(2,3)46-31(43)27(13-8-9-17-35)37-30(42)28(20-22-21-36-25-11-5-4-10-24(22)25)39-32(44)40-18-15-23(16-19-40)41-29-14-7-6-12-26(29)38-33(41)45/h4-7,10-12,14,21,23,27-28,36H,8-9,13,15-20,35H2,1-3H3,(H,37,42)(H,38,45)(H,39,44)/t27-,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Antagonistic activity of the compound towards sst2 receptor in GH4C1 cells a concentration of 1-2 x 10e6/mL incubated for 20 minutes |
Bioorg Med Chem Lett 11: 2731-4 (2001)
BindingDB Entry DOI: 10.7270/Q2JW8D5S |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50105468

((S)-6-Amino-2-[(R)-2-[(4-benzoyl-piperazine-1-carb...)Show SMILES CC(C)(C)OC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)N1CCN(CC1)C(=O)c1ccccc1 Show InChI InChI=1S/C33H44N6O5/c1-33(2,3)44-31(42)27(15-9-10-16-34)36-29(40)28(21-24-22-35-26-14-8-7-13-25(24)26)37-32(43)39-19-17-38(18-20-39)30(41)23-11-5-4-6-12-23/h4-8,11-14,22,27-28,35H,9-10,15-21,34H2,1-3H3,(H,36,40)(H,37,43)/t27-,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells |
Bioorg Med Chem Lett 11: 2731-4 (2001)
BindingDB Entry DOI: 10.7270/Q2JW8D5S |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50105468

((S)-6-Amino-2-[(R)-2-[(4-benzoyl-piperazine-1-carb...)Show SMILES CC(C)(C)OC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)N1CCN(CC1)C(=O)c1ccccc1 Show InChI InChI=1S/C33H44N6O5/c1-33(2,3)44-31(42)27(15-9-10-16-34)36-29(40)28(21-24-22-35-26-14-8-7-13-25(24)26)37-32(43)39-19-17-38(18-20-39)30(41)23-11-5-4-6-12-23/h4-8,11-14,22,27-28,35H,9-10,15-21,34H2,1-3H3,(H,36,40)(H,37,43)/t27-,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells |
Bioorg Med Chem Lett 11: 2731-4 (2001)
BindingDB Entry DOI: 10.7270/Q2JW8D5S |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity on human eosinophil phosphodiesterase 4. |
J Med Chem 41: 2268-77 (1998)
Article DOI: 10.1021/jm9800090
BindingDB Entry DOI: 10.7270/Q27S7RH6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50360292
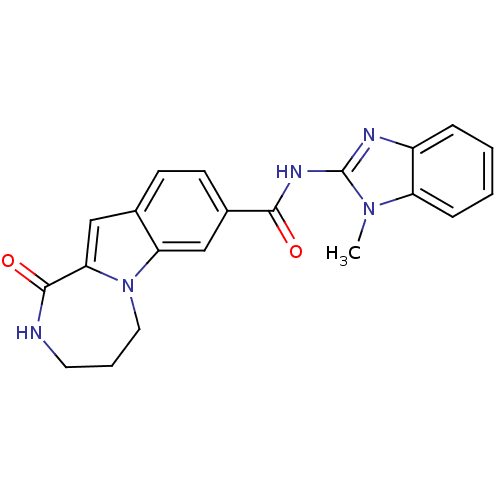
(CHEMBL1933279 | US9150577, 64)Show SMILES Cn1c(NC(=O)c2ccc3cc4C(=O)NCCCn4c3c2)nc2ccccc12 Show InChI InChI=1S/C21H19N5O2/c1-25-16-6-3-2-5-15(16)23-21(25)24-19(27)14-8-7-13-11-18-20(28)22-9-4-10-26(18)17(13)12-14/h2-3,5-8,11-12H,4,9-10H2,1H3,(H,22,28)(H,23,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 phosphorylation by luminescence assay |
Bioorg Med Chem Lett 22: 733-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.030
BindingDB Entry DOI: 10.7270/Q2XW4K7N |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50105471
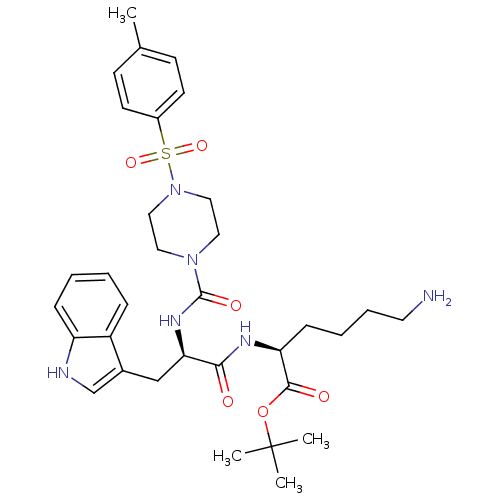
((S)-6-Amino-2-((R)-3-(1H-indol-3-yl)-2-{[4-(toluen...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCN(CC1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C Show InChI InChI=1S/C33H46N6O6S/c1-23-12-14-25(15-13-23)46(43,44)39-19-17-38(18-20-39)32(42)37-29(21-24-22-35-27-10-6-5-9-26(24)27)30(40)36-28(11-7-8-16-34)31(41)45-33(2,3)4/h5-6,9-10,12-15,22,28-29,35H,7-8,11,16-21,34H2,1-4H3,(H,36,40)(H,37,42)/t28-,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells |
Bioorg Med Chem Lett 11: 2731-4 (2001)
BindingDB Entry DOI: 10.7270/Q2JW8D5S |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50062381
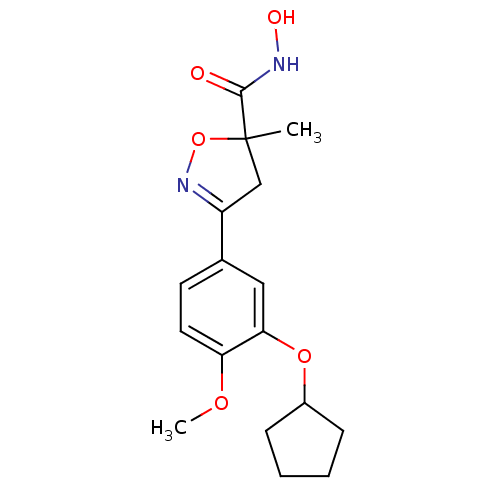
(3-(3-Cyclopentyloxy-4-methoxy-phenyl)-5-methyl-4,5...)Show SMILES COc1ccc(cc1OC1CCCC1)C1=NOC(C)(C1)C(=O)NO |t:16| Show InChI InChI=1S/C17H22N2O5/c1-17(16(20)18-21)10-13(19-24-17)11-7-8-14(22-2)15(9-11)23-12-5-3-4-6-12/h7-9,12,21H,3-6,10H2,1-2H3,(H,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase type 4A (PDE4A). |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50062381

(3-(3-Cyclopentyloxy-4-methoxy-phenyl)-5-methyl-4,5...)Show SMILES COc1ccc(cc1OC1CCCC1)C1=NOC(C)(C1)C(=O)NO |t:16| Show InChI InChI=1S/C17H22N2O5/c1-17(16(20)18-21)10-13(19-24-17)11-7-8-14(22-2)15(9-11)23-12-5-3-4-6-12/h7-9,12,21H,3-6,10H2,1-2H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase 4D |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase type 4A (PDE4A). |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50062381

(3-(3-Cyclopentyloxy-4-methoxy-phenyl)-5-methyl-4,5...)Show SMILES COc1ccc(cc1OC1CCCC1)C1=NOC(C)(C1)C(=O)NO |t:16| Show InChI InChI=1S/C17H22N2O5/c1-17(16(20)18-21)10-13(19-24-17)11-7-8-14(22-2)15(9-11)23-12-5-3-4-6-12/h7-9,12,21H,3-6,10H2,1-2H3,(H,18,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Phosphodiesterase 4B |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50360288

(CHEMBL1933148 | US9150577, 23)Show InChI InChI=1S/C17H17N5O2/c1-21-10-13(9-19-21)20-16(23)12-4-3-11-7-15-17(24)18-5-2-6-22(15)14(11)8-12/h3-4,7-10H,2,5-6H2,1H3,(H,18,24)(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 phosphorylation by luminescence assay |
Bioorg Med Chem Lett 22: 733-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.030
BindingDB Entry DOI: 10.7270/Q2XW4K7N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Homo sapiens (Human)) | BDBM50062383
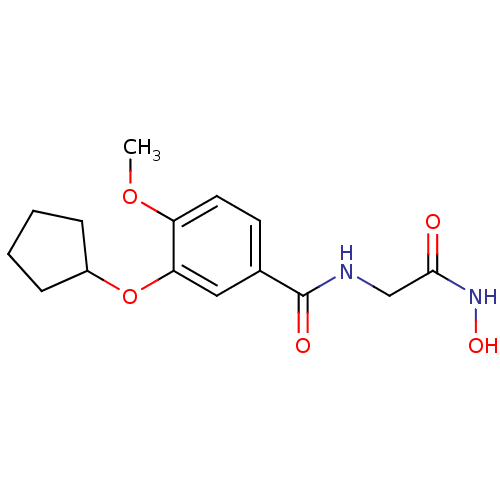
(3-Cyclopentyloxy-N-hydroxycarbamoylmethyl-4-methox...)Show InChI InChI=1S/C15H20N2O5/c1-21-12-7-6-10(15(19)16-9-14(18)17-20)8-13(12)22-11-4-2-3-5-11/h6-8,11,20H,2-5,9H2,1H3,(H,16,19)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase 4C |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50062383

(3-Cyclopentyloxy-N-hydroxycarbamoylmethyl-4-methox...)Show InChI InChI=1S/C15H20N2O5/c1-21-12-7-6-10(15(19)16-9-14(18)17-20)8-13(12)22-11-4-2-3-5-11/h6-8,11,20H,2-5,9H2,1H3,(H,16,19)(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase 4D |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50062383

(3-Cyclopentyloxy-N-hydroxycarbamoylmethyl-4-methox...)Show InChI InChI=1S/C15H20N2O5/c1-21-12-7-6-10(15(19)16-9-14(18)17-20)8-13(12)22-11-4-2-3-5-11/h6-8,11,20H,2-5,9H2,1H3,(H,16,19)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Phosphodiesterase 4B |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Homo sapiens (Human)) | BDBM50062387

(3-(3-Cyclopentyloxy-4-methoxy-phenyl)-4,5-dihydro-...)Show SMILES COc1ccc(cc1OC1CCCC1)C1=NOC(C1)C(=O)NO |t:16| Show InChI InChI=1S/C16H20N2O5/c1-21-13-7-6-10(8-14(13)22-11-4-2-3-5-11)12-9-15(23-18-12)16(19)17-20/h6-8,11,15,20H,2-5,9H2,1H3,(H,17,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase 4C |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50360282
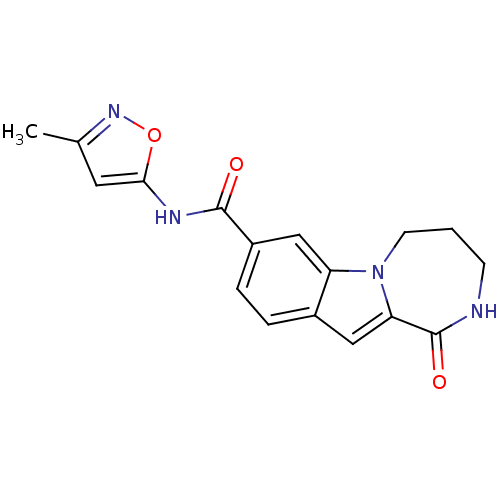
(CHEMBL1933143 | US9150577, 193)Show InChI InChI=1S/C17H16N4O3/c1-10-7-15(24-20-10)19-16(22)12-4-3-11-8-14-17(23)18-5-2-6-21(14)13(11)9-12/h3-4,7-9H,2,5-6H2,1H3,(H,18,23)(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 phosphorylation by luminescence assay |
Bioorg Med Chem Lett 22: 733-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.030
BindingDB Entry DOI: 10.7270/Q2XW4K7N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50062379

(3-Cyclopentyloxy-N-(1-hydroxycarbamoyl-ethyl)-4-me...)Show InChI InChI=1S/C16H22N2O5/c1-10(15(19)18-21)17-16(20)11-7-8-13(22-2)14(9-11)23-12-5-3-4-6-12/h7-10,12,21H,3-6H2,1-2H3,(H,17,20)(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase 4D |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50361841

(CHEMBL1938786 | US9150577, 215)Show SMILES NC(=O)c1ccccc1NC(=O)c1ccc2cc3C(=O)NCCCn3c2c1 Show InChI InChI=1S/C20H18N4O3/c21-18(25)14-4-1-2-5-15(14)23-19(26)13-7-6-12-10-17-20(27)22-8-3-9-24(17)16(12)11-13/h1-2,4-7,10-11H,3,8-9H2,(H2,21,25)(H,22,27)(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 phosphorylation by luminescence assay |
Bioorg Med Chem Lett 22: 733-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.030
BindingDB Entry DOI: 10.7270/Q2XW4K7N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50062379

(3-Cyclopentyloxy-N-(1-hydroxycarbamoyl-ethyl)-4-me...)Show InChI InChI=1S/C16H22N2O5/c1-10(15(19)18-21)17-16(20)11-7-8-13(22-2)14(9-11)23-12-5-3-4-6-12/h7-10,12,21H,3-6H2,1-2H3,(H,17,20)(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Phosphodiesterase 4B |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50361845
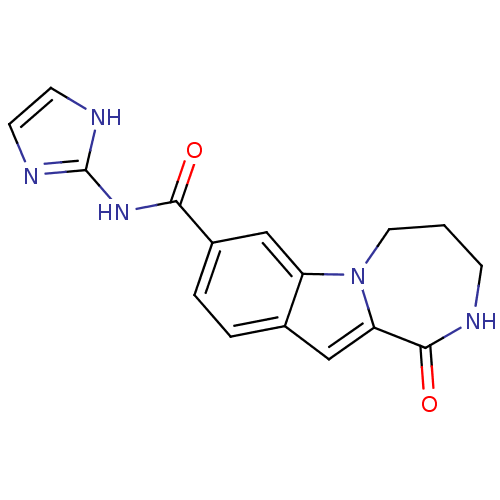
(CHEMBL1938792 | US9150577, 108)Show InChI InChI=1S/C16H15N5O2/c22-14(20-16-18-5-6-19-16)11-3-2-10-8-13-15(23)17-4-1-7-21(13)12(10)9-11/h2-3,5-6,8-9H,1,4,7H2,(H,17,23)(H2,18,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 phosphorylation by luminescence assay |
Bioorg Med Chem Lett 22: 733-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.030
BindingDB Entry DOI: 10.7270/Q2XW4K7N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50062383

(3-Cyclopentyloxy-N-hydroxycarbamoylmethyl-4-methox...)Show InChI InChI=1S/C15H20N2O5/c1-21-12-7-6-10(15(19)16-9-14(18)17-20)8-13(12)22-11-4-2-3-5-11/h6-8,11,20H,2-5,9H2,1H3,(H,16,19)(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase type 4A (PDE4A). |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50105466

((S)-6-Amino-2-[(R)-2-[(4-benzenesulfonyl-piperazin...)Show SMILES CC(C)(C)OC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)N1CCN(CC1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C32H44N6O6S/c1-32(2,3)44-30(40)27(15-9-10-16-33)35-29(39)28(21-23-22-34-26-14-8-7-13-25(23)26)36-31(41)37-17-19-38(20-18-37)45(42,43)24-11-5-4-6-12-24/h4-8,11-14,22,27-28,34H,9-10,15-21,33H2,1-3H3,(H,35,39)(H,36,41)/t27-,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound towards binding of sst2 receptor using [125I]-somatostatin as radioligand in Neuro2A cells |
Bioorg Med Chem Lett 11: 2731-4 (2001)
BindingDB Entry DOI: 10.7270/Q2JW8D5S |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase 4D |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50361842
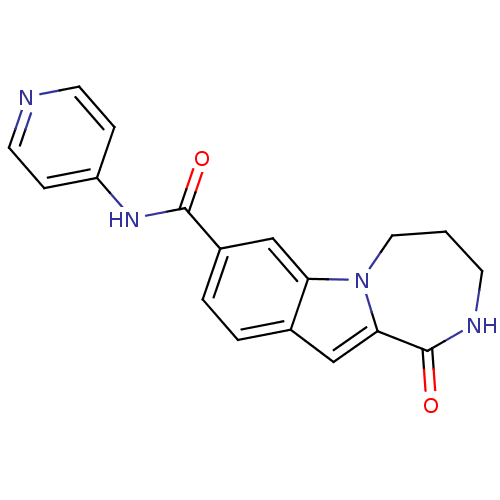
(CHEMBL1938787 | US9150577, 156)Show InChI InChI=1S/C18H16N4O2/c23-17(21-14-4-7-19-8-5-14)13-3-2-12-10-16-18(24)20-6-1-9-22(16)15(12)11-13/h2-5,7-8,10-11H,1,6,9H2,(H,20,24)(H,19,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 phosphorylation by luminescence assay |
Bioorg Med Chem Lett 22: 733-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.030
BindingDB Entry DOI: 10.7270/Q2XW4K7N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50471675
(CHEMBL307678)Show InChI InChI=1S/C20H25N3O2/c1-3-18-17-11-12-22(15-9-6-10-16(13-15)25-2)20(24)19(17)23(21-18)14-7-4-5-8-14/h6,9-10,13-14H,3-5,7-8,11-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity on human eosinophil phosphodiesterase 4. |
J Med Chem 41: 2268-77 (1998)
Article DOI: 10.1021/jm9800090
BindingDB Entry DOI: 10.7270/Q27S7RH6 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50361847
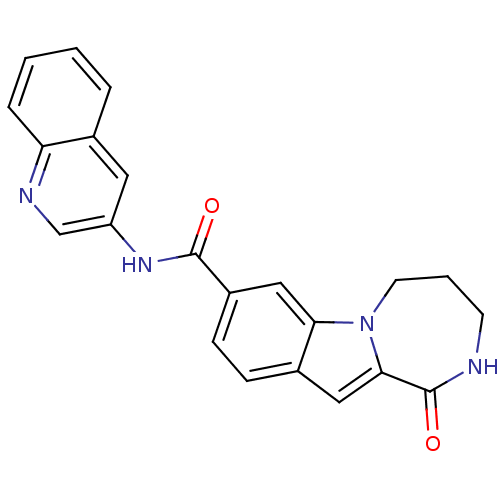
(CHEMBL1938798 | US9150577, 190)Show SMILES O=C(Nc1cnc2ccccc2c1)c1ccc2cc3C(=O)NCCCn3c2c1 Show InChI InChI=1S/C22H18N4O2/c27-21(25-17-10-14-4-1-2-5-18(14)24-13-17)16-7-6-15-11-20-22(28)23-8-3-9-26(20)19(15)12-16/h1-2,4-7,10-13H,3,8-9H2,(H,23,28)(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 phosphorylation by luminescence assay |
Bioorg Med Chem Lett 22: 733-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.030
BindingDB Entry DOI: 10.7270/Q2XW4K7N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 35.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Phosphodiesterase 4B |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50471668

(CHEMBL309803)Show InChI InChI=1S/C19H22ClN3O/c1-2-17-16-10-11-22(15-9-5-6-13(20)12-15)19(24)18(16)23(21-17)14-7-3-4-8-14/h5-6,9,12,14H,2-4,7-8,10-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity on human eosinophil phosphodiesterase 4. |
J Med Chem 41: 2268-77 (1998)
Article DOI: 10.1021/jm9800090
BindingDB Entry DOI: 10.7270/Q27S7RH6 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50361835
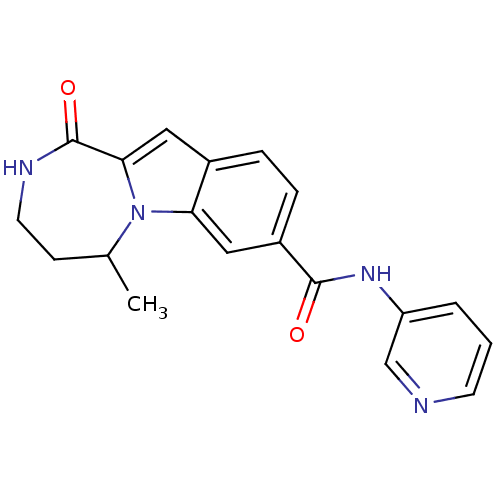
(CHEMBL1938765 | US9150577, 10)Show InChI InChI=1S/C19H18N4O2/c1-12-6-8-21-19(25)17-9-13-4-5-14(10-16(13)23(12)17)18(24)22-15-3-2-7-20-11-15/h2-5,7,9-12H,6,8H2,1H3,(H,21,25)(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 phosphorylation by luminescence assay |
Bioorg Med Chem Lett 22: 733-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.030
BindingDB Entry DOI: 10.7270/Q2XW4K7N |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50361846
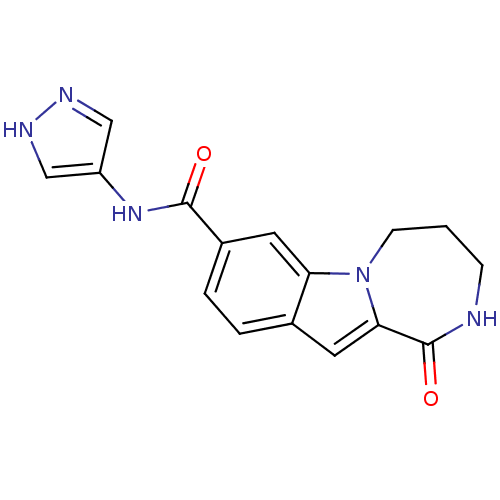
(CHEMBL1938794 | US9150577, 15)Show InChI InChI=1S/C16H15N5O2/c22-15(20-12-8-18-19-9-12)11-3-2-10-6-14-16(23)17-4-1-5-21(14)13(10)7-11/h2-3,6-9H,1,4-5H2,(H,17,23)(H,18,19)(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 phosphorylation by luminescence assay |
Bioorg Med Chem Lett 22: 733-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.030
BindingDB Entry DOI: 10.7270/Q2XW4K7N |
More data for this
Ligand-Target Pair | |
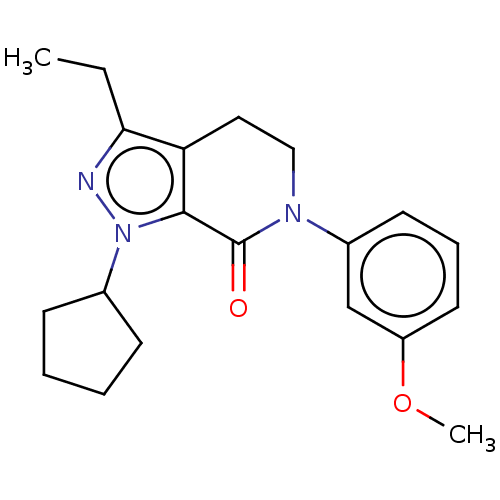
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50361839

(CHEMBL1938776)Show SMILES C[C@@H]1NC(=O)c2cc3ccc(cc3n2[C@@H]1C)C(=O)Nc1cccnc1 |r| Show InChI InChI=1S/C19H18N4O2/c1-11-12(2)23-16-9-14(18(24)22-15-4-3-7-20-10-15)6-5-13(16)8-17(23)19(25)21-11/h3-12H,1-2H3,(H,21,25)(H,22,24)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 phosphorylation by luminescence assay |
Bioorg Med Chem Lett 22: 733-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.030
BindingDB Entry DOI: 10.7270/Q2XW4K7N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50471677

(CHEMBL76066)Show InChI InChI=1S/C21H27N3O2/c1-3-19-18-12-13-23(16-10-7-11-17(14-16)26-2)21(25)20(18)24(22-19)15-8-5-4-6-9-15/h7,10-11,14-15H,3-6,8-9,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity on human eosinophil phosphodiesterase 4. |
J Med Chem 41: 2268-77 (1998)
Article DOI: 10.1021/jm9800090
BindingDB Entry DOI: 10.7270/Q27S7RH6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50062379

(3-Cyclopentyloxy-N-(1-hydroxycarbamoyl-ethyl)-4-me...)Show InChI InChI=1S/C16H22N2O5/c1-10(15(19)18-21)17-16(20)11-7-8-13(22-2)14(9-11)23-12-5-3-4-6-12/h7-10,12,21H,3-6H2,1-2H3,(H,17,20)(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase type 4A (PDE4A). |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50361867
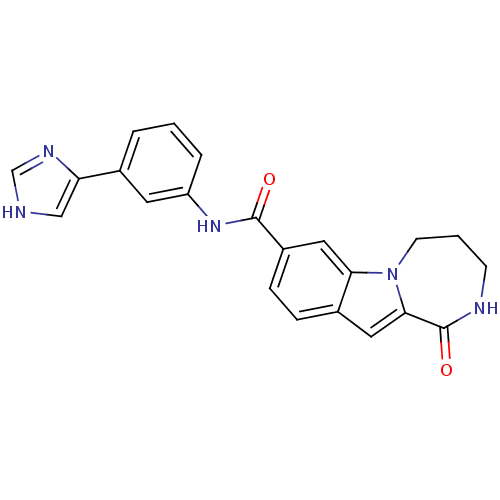
(CHEMBL1938791 | US9150577, 226)Show SMILES O=C(Nc1cccc(c1)-c1c[nH]cn1)c1ccc2cc3C(=O)NCCCn3c2c1 Show InChI InChI=1S/C22H19N5O2/c28-21(26-17-4-1-3-14(9-17)18-12-23-13-25-18)16-6-5-15-10-20-22(29)24-7-2-8-27(20)19(15)11-16/h1,3-6,9-13H,2,7-8H2,(H,23,25)(H,24,29)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 phosphorylation by luminescence assay |
Bioorg Med Chem Lett 22: 733-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.030
BindingDB Entry DOI: 10.7270/Q2XW4K7N |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50361848

(CHEMBL1938799 | US9150577, 19)Show SMILES CC(C)n1ncc2cc(NC(=O)c3ccc4cc5C(=O)NCCCn5c4c3)cnc12 Show InChI InChI=1S/C22H22N6O2/c1-13(2)28-20-16(11-25-28)8-17(12-24-20)26-21(29)15-5-4-14-9-19-22(30)23-6-3-7-27(19)18(14)10-15/h4-5,8-13H,3,6-7H2,1-2H3,(H,23,30)(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 phosphorylation by luminescence assay |
Bioorg Med Chem Lett 22: 733-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.030
BindingDB Entry DOI: 10.7270/Q2XW4K7N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50471683

(CHEMBL74597)Show InChI InChI=1S/C17H21N3OS/c1-2-14-13-9-10-19(15-8-5-11-22-15)17(21)16(13)20(18-14)12-6-3-4-7-12/h5,8,11-12H,2-4,6-7,9-10H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity on human eosinophil phosphodiesterase 4. |
J Med Chem 41: 2268-77 (1998)
Article DOI: 10.1021/jm9800090
BindingDB Entry DOI: 10.7270/Q27S7RH6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50105470

((S)-6-Amino-2-((R)-3-(1H-indol-3-yl)-2-{[4-(4-meth...)Show SMILES Cc1ccc(cc1)C(=O)N1CCN(CC1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCCN)C(=O)OC(C)(C)C Show InChI InChI=1S/C34H46N6O5/c1-23-12-14-24(15-13-23)31(42)39-17-19-40(20-18-39)33(44)38-29(21-25-22-36-27-10-6-5-9-26(25)27)30(41)37-28(11-7-8-16-35)32(43)45-34(2,3)4/h5-6,9-10,12-15,22,28-29,36H,7-8,11,16-21,35H2,1-4H3,(H,37,41)(H,38,44)/t28-,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Antagonistic activity of the compound towards sst2 receptor in GH4C1 cells a concentration of 1-2 x 10e6/mL incubated for 20 minutes |
Bioorg Med Chem Lett 11: 2731-4 (2001)
BindingDB Entry DOI: 10.7270/Q2JW8D5S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data