 Found 307 hits with Last Name = 'dillard' and Initial = 'rd'
Found 307 hits with Last Name = 'dillard' and Initial = 'rd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50055367
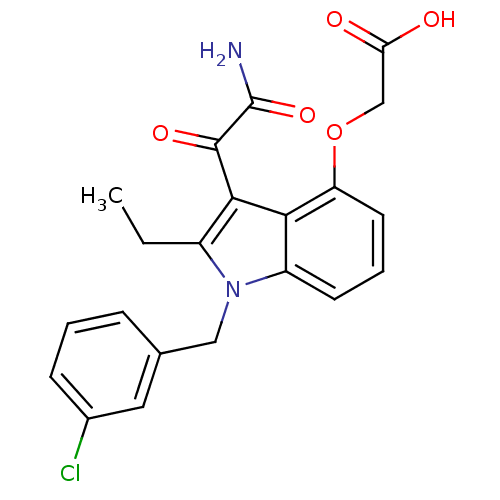
(CHEMBL345986 | [3-Aminooxalyl-1-(3-chloro-benzyl)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1cccc(Cl)c1 Show InChI InChI=1S/C21H19ClN2O5/c1-2-14-19(20(27)21(23)28)18-15(7-4-8-16(18)29-11-17(25)26)24(14)10-12-5-3-6-13(22)9-12/h3-9H,2,10-11H2,1H3,(H2,23,28)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Compound wastested for inhibition of porcine secreted pancreatic PLA2 |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055371
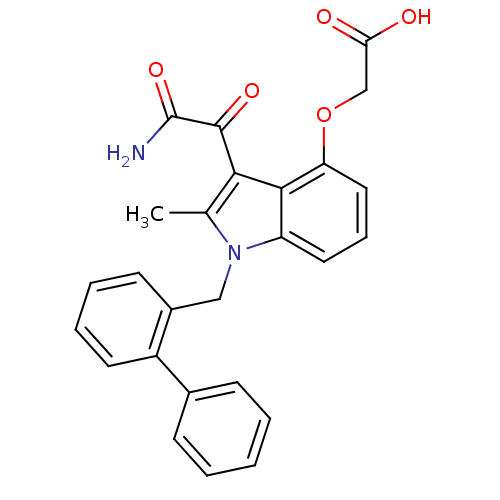
((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(12-7-13-21(24)33-15-22(29)30)28(16)14-18-10-5-6-11-19(18)17-8-3-2-4-9-17/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055374
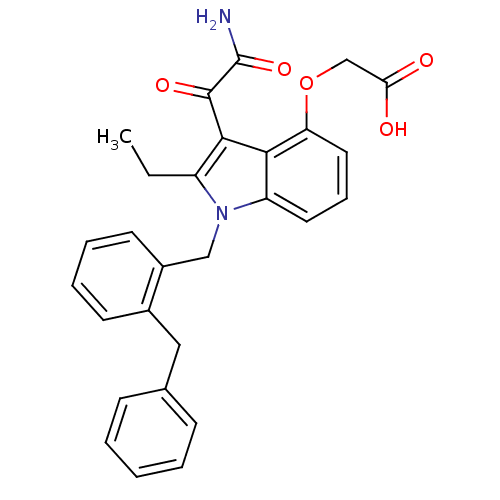
(CHEMBL436456 | [3-Aminooxalyl-1-(2-benzyl-benzyl)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1Cc1ccccc1 Show InChI InChI=1S/C28H26N2O5/c1-2-21-26(27(33)28(29)34)25-22(13-8-14-23(25)35-17-24(31)32)30(21)16-20-12-7-6-11-19(20)15-18-9-4-3-5-10-18/h3-14H,2,15-17H2,1H3,(H2,29,34)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055374

(CHEMBL436456 | [3-Aminooxalyl-1-(2-benzyl-benzyl)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1Cc1ccccc1 Show InChI InChI=1S/C28H26N2O5/c1-2-21-26(27(33)28(29)34)25-22(13-8-14-23(25)35-17-24(31)32)30(21)16-20-12-7-6-11-19(20)15-18-9-4-3-5-10-18/h3-14H,2,15-17H2,1H3,(H2,29,34)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055371

((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(12-7-13-21(24)33-15-22(29)30)28(16)14-18-10-5-6-11-19(18)17-8-3-2-4-9-17/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055379

((3-Aminooxalyl-1-biphenyl-2-ylmethyl-2-cyclopropyl...)Show SMILES NC(=O)C(=O)c1c(C2CC2)n(Cc2ccccc2-c2ccccc2)c2cccc(OCC(O)=O)c12 Show InChI InChI=1S/C28H24N2O5/c29-28(34)27(33)25-24-21(11-6-12-22(24)35-16-23(31)32)30(26(25)18-13-14-18)15-19-9-4-5-10-20(19)17-7-2-1-3-8-17/h1-12,18H,13-16H2,(H2,29,34)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055401

((3-Aminooxalyl-1-biphenyl-2-ylmethyl-2-ethyl-1H-in...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C27H24N2O5/c1-2-20-25(26(32)27(28)33)24-21(13-8-14-22(24)34-16-23(30)31)29(20)15-18-11-6-7-12-19(18)17-9-4-3-5-10-17/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055378

(CHEMBL345488 | [3-Aminooxalyl-1-(2,6-dichloro-benz...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1c(Cl)cccc1Cl Show InChI InChI=1S/C20H16Cl2N2O5/c1-10-17(19(27)20(23)28)18-14(6-3-7-15(18)29-9-16(25)26)24(10)8-11-12(21)4-2-5-13(11)22/h2-7H,8-9H2,1H3,(H2,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055401

((3-Aminooxalyl-1-biphenyl-2-ylmethyl-2-ethyl-1H-in...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C27H24N2O5/c1-2-20-25(26(32)27(28)33)24-21(13-8-14-22(24)34-16-23(30)31)29(20)15-18-11-6-7-12-19(18)17-9-4-3-5-10-17/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055387

((R)-2-(3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-...)Show SMILES C[C@@H](Oc1cccc2n(Cc3ccccc3)c(C)c(C(=O)C(N)=O)c12)C(O)=O Show InChI InChI=1S/C21H20N2O5/c1-12-17(19(24)20(22)25)18-15(23(12)11-14-7-4-3-5-8-14)9-6-10-16(18)28-13(2)21(26)27/h3-10,13H,11H2,1-2H3,(H2,22,25)(H,26,27)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055367

(CHEMBL345986 | [3-Aminooxalyl-1-(3-chloro-benzyl)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1cccc(Cl)c1 Show InChI InChI=1S/C21H19ClN2O5/c1-2-14-19(20(27)21(23)28)18-15(7-4-8-16(18)29-11-17(25)26)24(14)10-12-5-3-6-13(22)9-12/h3-9H,2,10-11H2,1H3,(H2,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50055384
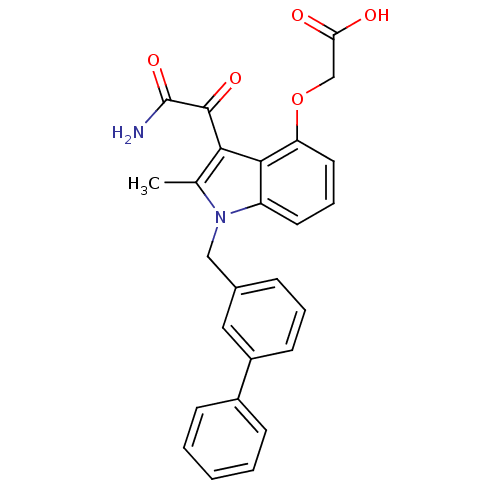
((3-Aminooxalyl-1-biphenyl-3-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(11-6-12-21(24)33-15-22(29)30)28(16)14-17-7-5-10-19(13-17)18-8-3-2-4-9-18/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of porcine secretory pancreatic Phospholipase A2 |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50226788
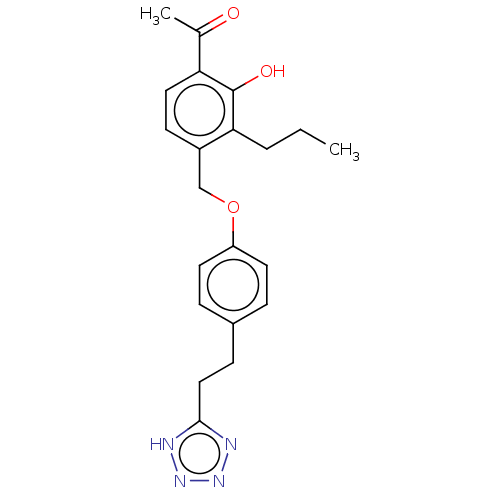
(CHEMBL170413)Show SMILES CCCc1c(COc2ccc(CCc3nnn[nH]3)cc2)ccc(C(C)=O)c1O Show InChI InChI=1S/C21H24N4O3/c1-3-4-19-16(8-11-18(14(2)26)21(19)27)13-28-17-9-5-15(6-10-17)7-12-20-22-24-25-23-20/h5-6,8-11,27H,3-4,7,12-13H2,1-2H3,(H,22,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LTD4-induced contraction in guinea pig ileum |
J Med Chem 30: 911-8 (1987)
BindingDB Entry DOI: 10.7270/Q25X2B45 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055380
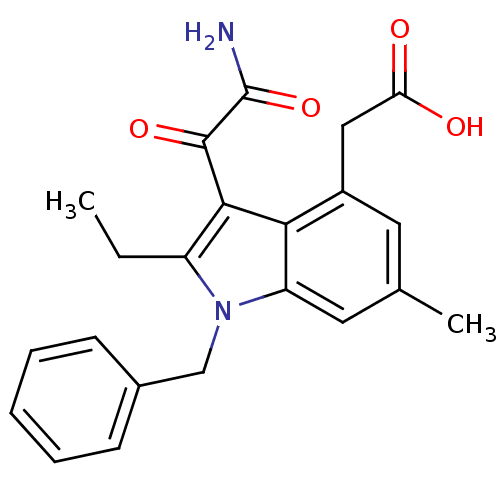
((3-Aminooxalyl-1-benzyl-2-ethyl-6-methyl-1H-indol-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(CC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O4/c1-3-16-20(21(27)22(23)28)19-15(11-18(25)26)9-13(2)10-17(19)24(16)12-14-7-5-4-6-8-14/h4-10H,3,11-12H2,1-2H3,(H2,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055370
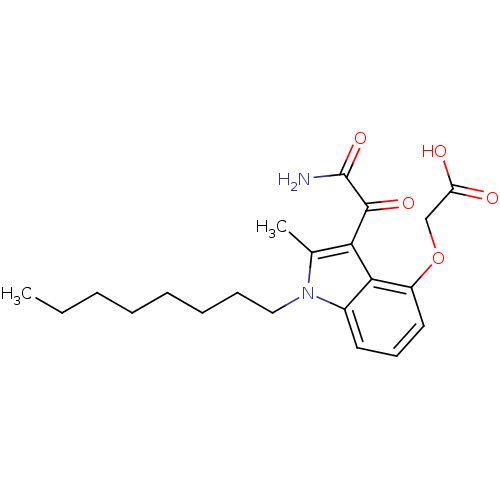
((3-Aminooxalyl-2-methyl-1-octyl-1H-indol-4-yloxy)-...)Show SMILES CCCCCCCCn1c(C)c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc12 Show InChI InChI=1S/C21H28N2O5/c1-3-4-5-6-7-8-12-23-14(2)18(20(26)21(22)27)19-15(23)10-9-11-16(19)28-13-17(24)25/h9-11H,3-8,12-13H2,1-2H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055380

((3-Aminooxalyl-1-benzyl-2-ethyl-6-methyl-1H-indol-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(CC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O4/c1-3-16-20(21(27)22(23)28)19-15(11-18(25)26)9-13(2)10-17(19)24(16)12-14-7-5-4-6-8-14/h4-10H,3,11-12H2,1-2H3,(H2,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055384

((3-Aminooxalyl-1-biphenyl-3-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(11-6-12-21(24)33-15-22(29)30)28(16)14-17-7-5-10-19(13-17)18-8-3-2-4-9-18/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055384

((3-Aminooxalyl-1-biphenyl-3-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(11-6-12-21(24)33-15-22(29)30)28(16)14-17-7-5-10-19(13-17)18-8-3-2-4-9-18/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055368

((3-Aminooxalyl-2-methyl-1-naphthalen-1-ylmethyl-1H...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1cccc2ccccc12 Show InChI InChI=1S/C24H20N2O5/c1-14-21(23(29)24(25)30)22-18(10-5-11-19(22)31-13-20(27)28)26(14)12-16-8-4-7-15-6-2-3-9-17(15)16/h2-11H,12-13H2,1H3,(H2,25,30)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055394
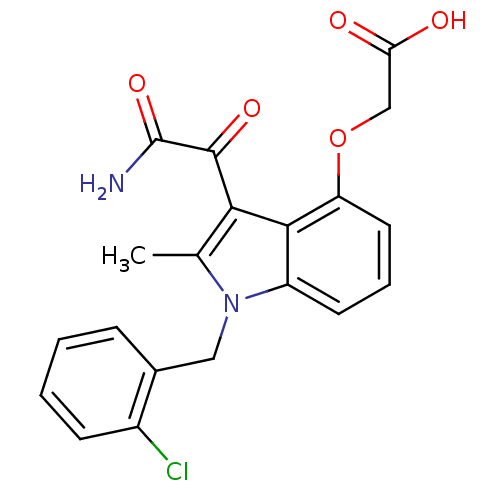
(CHEMBL148795 | [3-Aminooxalyl-1-(2-chloro-benzyl)-...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1Cl Show InChI InChI=1S/C20H17ClN2O5/c1-11-17(19(26)20(22)27)18-14(7-4-8-15(18)28-10-16(24)25)23(11)9-12-5-2-3-6-13(12)21/h2-8H,9-10H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055385
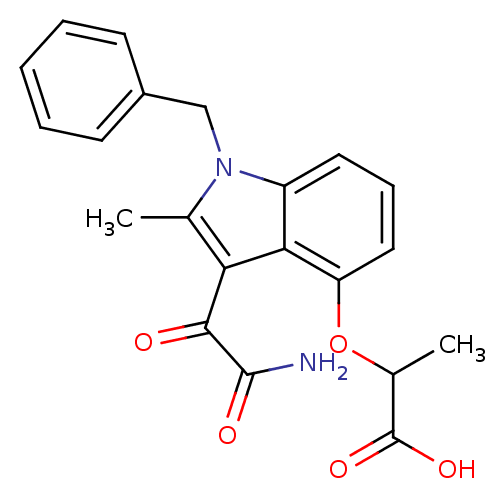
(2-(3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-ylox...)Show SMILES CC(Oc1cccc2n(Cc3ccccc3)c(C)c(C(=O)C(N)=O)c12)C(O)=O Show InChI InChI=1S/C21H20N2O5/c1-12-17(19(24)20(22)25)18-15(23(12)11-14-7-4-3-5-8-14)9-6-10-16(18)28-13(2)21(26)27/h3-10,13H,11H2,1-2H3,(H2,22,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055305
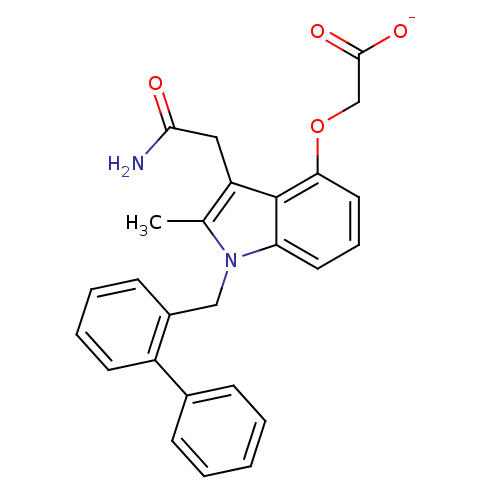
(CHEMBL423762 | Sodium; (1-biphenyl-2-ylmethyl-3-ca...)Show SMILES Cc1c(CC(N)=O)c2c(OCC([O-])=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H24N2O4/c1-17-21(14-24(27)29)26-22(12-7-13-23(26)32-16-25(30)31)28(17)15-19-10-5-6-11-20(19)18-8-3-2-4-9-18/h2-13H,14-16H2,1H3,(H2,27,29)(H,30,31)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human non-pancreatic secretory phospholipase A2 (PLA2) in a chromogenic assay |
J Med Chem 39: 5137-58 (1997)
Article DOI: 10.1021/jm960486n
BindingDB Entry DOI: 10.7270/Q2639NV0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055385

(2-(3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-ylox...)Show SMILES CC(Oc1cccc2n(Cc3ccccc3)c(C)c(C(=O)C(N)=O)c12)C(O)=O Show InChI InChI=1S/C21H20N2O5/c1-12-17(19(24)20(22)25)18-15(23(12)11-14-7-4-3-5-8-14)9-6-10-16(18)28-13(2)21(26)27/h3-10,13H,11H2,1-2H3,(H2,22,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50226795
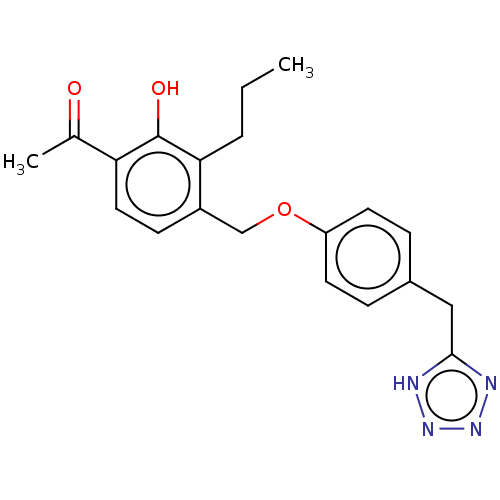
(LY-163443)Show SMILES CCCc1c(COc2ccc(Cc3nnn[nH]3)cc2)ccc(C(C)=O)c1O Show InChI InChI=1S/C20H22N4O3/c1-3-4-18-15(7-10-17(13(2)25)20(18)26)12-27-16-8-5-14(6-9-16)11-19-21-23-24-22-19/h5-10,26H,3-4,11-12H2,1-2H3,(H,21,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LTD4-induced contraction in guinea pig ileum |
J Med Chem 30: 911-8 (1987)
BindingDB Entry DOI: 10.7270/Q25X2B45 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50226779

(CHEMBL172086)Show SMILES CCCc1c(COc2cccc(OCc3nn[nH]n3)c2)ccc(C(C)=O)c1O Show InChI InChI=1S/C20H22N4O4/c1-3-5-18-14(8-9-17(13(2)25)20(18)26)11-27-15-6-4-7-16(10-15)28-12-19-21-23-24-22-19/h4,6-10,26H,3,5,11-12H2,1-2H3,(H,21,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LTD4-induced contraction in guinea pig ileum |
J Med Chem 30: 911-8 (1987)
BindingDB Entry DOI: 10.7270/Q25X2B45 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50226781
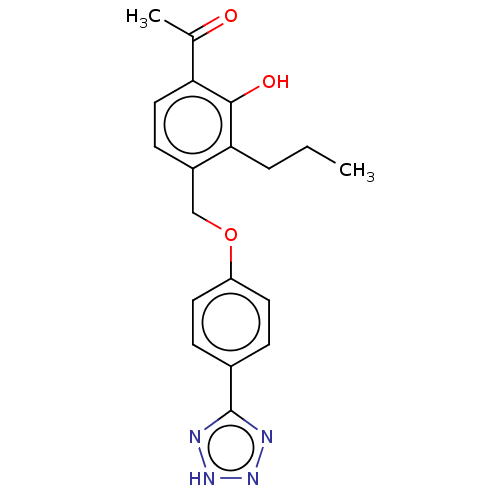
(CHEMBL171093)Show SMILES CCCc1c(COc2ccc(cc2)-c2nn[nH]n2)ccc(C(C)=O)c1O Show InChI InChI=1S/C19H20N4O3/c1-3-4-17-14(7-10-16(12(2)24)18(17)25)11-26-15-8-5-13(6-9-15)19-20-22-23-21-19/h5-10,25H,3-4,11H2,1-2H3,(H,20,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LTD4-induced contraction in guinea pig ileum |
J Med Chem 30: 911-8 (1987)
BindingDB Entry DOI: 10.7270/Q25X2B45 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055391

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-ind...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OC(C)C(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-19(20(25)21(23)26)18-16(24(15)12-14-8-5-4-6-9-14)10-7-11-17(18)29-13(2)22(27)28/h4-11,13H,3,12H2,1-2H3,(H2,23,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055387

((R)-2-(3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-...)Show SMILES C[C@@H](Oc1cccc2n(Cc3ccccc3)c(C)c(C(=O)C(N)=O)c12)C(O)=O Show InChI InChI=1S/C21H20N2O5/c1-12-17(19(24)20(22)25)18-15(23(12)11-14-7-4-3-5-8-14)9-6-10-16(18)28-13(2)21(26)27/h3-10,13H,11H2,1-2H3,(H2,22,25)(H,26,27)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055383

((3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-yloxy)...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C20H18N2O5/c1-12-17(19(25)20(21)26)18-14(8-5-9-15(18)27-11-16(23)24)22(12)10-13-6-3-2-4-7-13/h2-9H,10-11H2,1H3,(H2,21,26)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055391

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-ind...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OC(C)C(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-19(20(25)21(23)26)18-16(24(15)12-14-8-5-4-6-9-14)10-7-11-17(18)29-13(2)22(27)28/h4-11,13H,3,12H2,1-2H3,(H2,23,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50226772
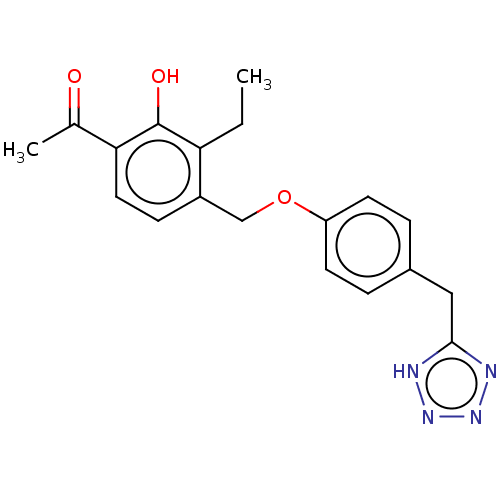
(CHEMBL170213)Show SMILES CCc1c(COc2ccc(Cc3nnn[nH]3)cc2)ccc(C(C)=O)c1O Show InChI InChI=1S/C19H20N4O3/c1-3-16-14(6-9-17(12(2)24)19(16)25)11-26-15-7-4-13(5-8-15)10-18-20-22-23-21-18/h4-9,25H,3,10-11H2,1-2H3,(H,20,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LTD4-induced contraction in guinea pig ileum |
J Med Chem 30: 911-8 (1987)
BindingDB Entry DOI: 10.7270/Q25X2B45 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50226776
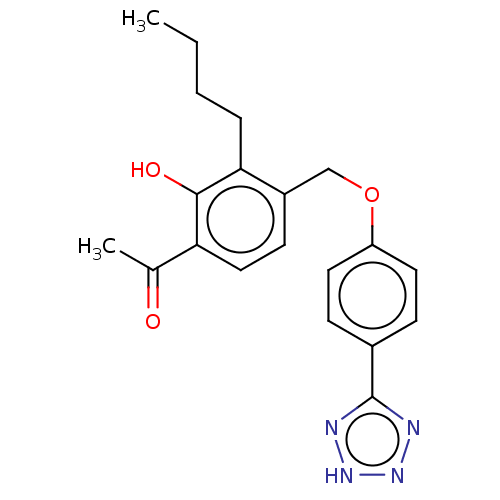
(CHEMBL172225)Show SMILES CCCCc1c(COc2ccc(cc2)-c2nn[nH]n2)ccc(C(C)=O)c1O Show InChI InChI=1S/C20H22N4O3/c1-3-4-5-18-15(8-11-17(13(2)25)19(18)26)12-27-16-9-6-14(7-10-16)20-21-23-24-22-20/h6-11,26H,3-5,12H2,1-2H3,(H,21,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LTD4-induced contraction in guinea pig ileum |
J Med Chem 30: 911-8 (1987)
BindingDB Entry DOI: 10.7270/Q25X2B45 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50226784
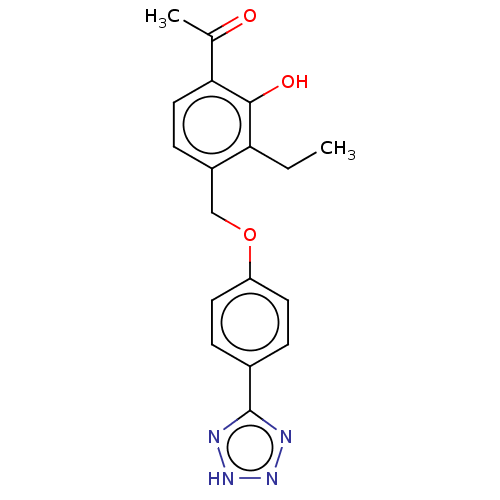
(CHEMBL353667)Show SMILES CCc1c(COc2ccc(cc2)-c2nn[nH]n2)ccc(C(C)=O)c1O Show InChI InChI=1S/C18H18N4O3/c1-3-15-13(6-9-16(11(2)23)17(15)24)10-25-14-7-4-12(5-8-14)18-19-21-22-20-18/h4-9,24H,3,10H2,1-2H3,(H,19,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LTD4-induced contraction in guinea pig ileum |
J Med Chem 30: 911-8 (1987)
BindingDB Entry DOI: 10.7270/Q25X2B45 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50226790
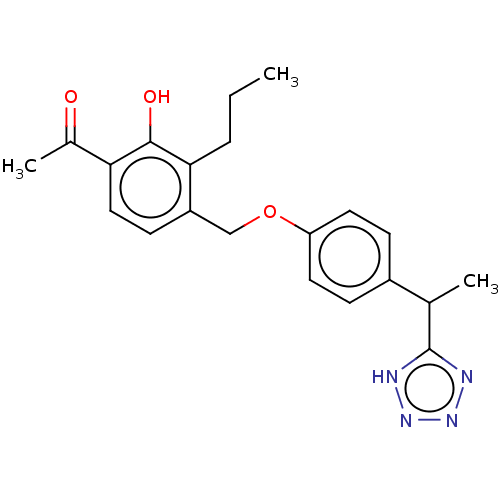
(CHEMBL353279)Show SMILES CCCc1c(COc2ccc(cc2)C(C)c2nnn[nH]2)ccc(C(C)=O)c1O Show InChI InChI=1S/C21H24N4O3/c1-4-5-19-16(8-11-18(14(3)26)20(19)27)12-28-17-9-6-15(7-10-17)13(2)21-22-24-25-23-21/h6-11,13,27H,4-5,12H2,1-3H3,(H,22,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LTD4-induced contraction in guinea pig ileum |
J Med Chem 30: 911-8 (1987)
BindingDB Entry DOI: 10.7270/Q25X2B45 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055305

(CHEMBL423762 | Sodium; (1-biphenyl-2-ylmethyl-3-ca...)Show SMILES Cc1c(CC(N)=O)c2c(OCC([O-])=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H24N2O4/c1-17-21(14-24(27)29)26-22(12-7-13-23(26)32-16-25(30)31)28(17)15-19-10-5-6-11-20(19)18-8-3-2-4-9-18/h2-13H,14-16H2,1H3,(H2,27,29)(H,30,31)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against porcine pancreatic Phospholipase A2 |
J Med Chem 39: 5137-58 (1997)
Article DOI: 10.1021/jm960486n
BindingDB Entry DOI: 10.7270/Q2639NV0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055315

(CHEMBL359304 | [3-(1-Benzyl-3-carbamoylmethyl-2-et...)Show SMILES CCc1c(CC(N)=O)c2cc(OCCCP(O)(O)=O)c(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C23H29N2O5P/c1-3-20-19(14-23(24)26)18-13-22(30-10-7-11-31(27,28)29)16(2)12-21(18)25(20)15-17-8-5-4-6-9-17/h4-6,8-9,12-13H,3,7,10-11,14-15H2,1-2H3,(H2,24,26)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human non-pancreatic secretory phospholipase A2 (PLA2) in a chromogenic assay |
J Med Chem 39: 5137-58 (1997)
Article DOI: 10.1021/jm960486n
BindingDB Entry DOI: 10.7270/Q2639NV0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50055383

((3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-yloxy)...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C20H18N2O5/c1-12-17(19(25)20(21)26)18-14(8-5-9-15(18)27-11-16(23)24)22(12)10-13-6-3-2-4-7-13/h2-9H,10-11H2,1H3,(H2,21,26)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of porcine secretory pancreatic Phospholipase A2 |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055396
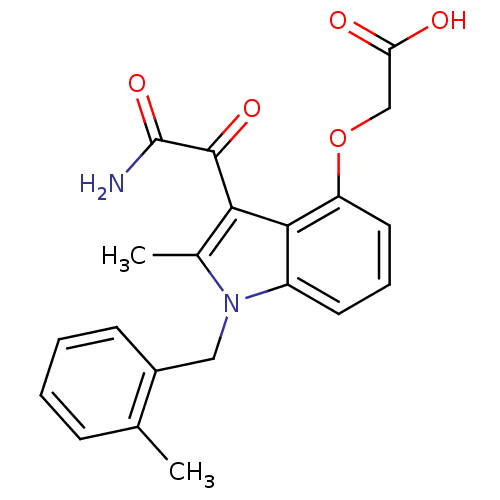
(CHEMBL423219 | [3-Aminooxalyl-2-methyl-1-(2-methyl...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1C Show InChI InChI=1S/C21H20N2O5/c1-12-6-3-4-7-14(12)10-23-13(2)18(20(26)21(22)27)19-15(23)8-5-9-16(19)28-11-17(24)25/h3-9H,10-11H2,1-2H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055355

(CHEMBL148601 | {3-[3-Carbamoylmethyl-1-(3-chloro-b...)Show SMILES CCc1c(CC(N)=O)c2cc(OCCCP(O)(O)=O)ccc2n1Cc1cccc(Cl)c1 Show InChI InChI=1S/C22H26ClN2O5P/c1-2-20-19(13-22(24)26)18-12-17(30-9-4-10-31(27,28)29)7-8-21(18)25(20)14-15-5-3-6-16(23)11-15/h3,5-8,11-12H,2,4,9-10,13-14H2,1H3,(H2,24,26)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human non-pancreatic secretory phospholipase A2 (PLA2) in a chromogenic assay |
J Med Chem 39: 5137-58 (1997)
Article DOI: 10.1021/jm960486n
BindingDB Entry DOI: 10.7270/Q2639NV0 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50226789

(CHEMBL170719)Show SMILES CCCCc1c(COc2ccc(Cc3nnn[nH]3)cc2)ccc(C(C)=O)c1O Show InChI InChI=1S/C21H24N4O3/c1-3-4-5-19-16(8-11-18(14(2)26)21(19)27)13-28-17-9-6-15(7-10-17)12-20-22-24-25-23-20/h6-11,27H,3-5,12-13H2,1-2H3,(H,22,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LTD4-induced contraction in guinea pig ileum |
J Med Chem 30: 911-8 (1987)
BindingDB Entry DOI: 10.7270/Q25X2B45 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50226791
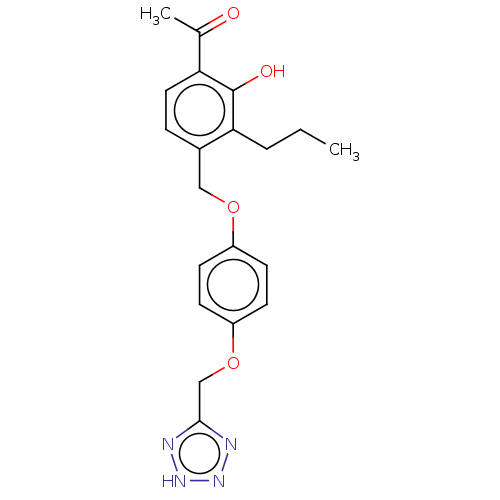
(CHEMBL173751)Show SMILES CCCc1c(COc2ccc(OCc3nn[nH]n3)cc2)ccc(C(C)=O)c1O Show InChI InChI=1S/C20H22N4O4/c1-3-4-18-14(5-10-17(13(2)25)20(18)26)11-27-15-6-8-16(9-7-15)28-12-19-21-23-24-22-19/h5-10,26H,3-4,11-12H2,1-2H3,(H,21,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LTD4-induced contraction in guinea pig ileum |
J Med Chem 30: 911-8 (1987)
BindingDB Entry DOI: 10.7270/Q25X2B45 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055368

((3-Aminooxalyl-2-methyl-1-naphthalen-1-ylmethyl-1H...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1cccc2ccccc12 Show InChI InChI=1S/C24H20N2O5/c1-14-21(23(29)24(25)30)22-18(10-5-11-19(22)31-13-20(27)28)26(14)12-16-8-4-7-15-6-2-3-9-17(15)16/h2-11H,12-13H2,1H3,(H2,25,30)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055370

((3-Aminooxalyl-2-methyl-1-octyl-1H-indol-4-yloxy)-...)Show SMILES CCCCCCCCn1c(C)c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc12 Show InChI InChI=1S/C21H28N2O5/c1-3-4-5-6-7-8-12-23-14(2)18(20(26)21(22)27)19-15(23)10-9-11-16(19)28-13-17(24)25/h9-11H,3-8,12-13H2,1-2H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055383

((3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-yloxy)...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C20H18N2O5/c1-12-17(19(25)20(21)26)18-14(8-5-9-15(18)27-11-16(23)24)22(12)10-13-6-3-2-4-7-13/h2-9H,10-11H2,1H3,(H2,21,26)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055334
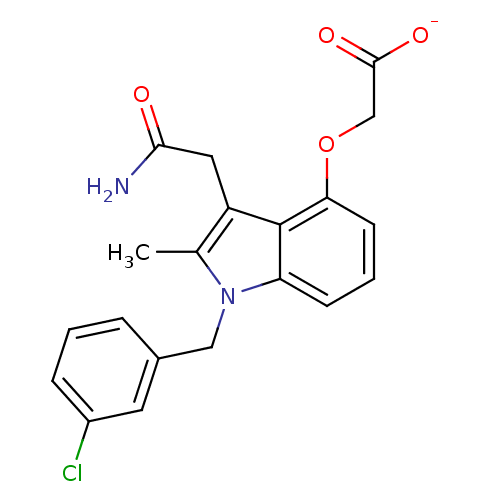
(CHEMBL148479 | Sodium; [3-carbamoylmethyl-1-(3-chl...)Show SMILES Cc1c(CC(N)=O)c2c(OCC([O-])=O)cccc2n1Cc1cccc(Cl)c1 Show InChI InChI=1S/C20H19ClN2O4/c1-12-15(9-18(22)24)20-16(6-3-7-17(20)27-11-19(25)26)23(12)10-13-4-2-5-14(21)8-13/h2-8H,9-11H2,1H3,(H2,22,24)(H,25,26)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against porcine pancreatic Phospholipase A2 |
J Med Chem 39: 5137-58 (1997)
Article DOI: 10.1021/jm960486n
BindingDB Entry DOI: 10.7270/Q2639NV0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055351

(4-(1-Benzyl-2-bromo-3-carbamoylmethyl-6-chloro-1H-...)Show SMILES NC(=O)Cc1c(Br)n(Cc2ccccc2)c2cc(Cl)c(OCCCC(O)=O)cc12 Show InChI InChI=1S/C21H20BrClN2O4/c22-21-15(10-19(24)26)14-9-18(29-8-4-7-20(27)28)16(23)11-17(14)25(21)12-13-5-2-1-3-6-13/h1-3,5-6,9,11H,4,7-8,10,12H2,(H2,24,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human non-pancreatic secretory phospholipase A2 (PLA2) in a chromogenic assay |
J Med Chem 39: 5137-58 (1997)
Article DOI: 10.1021/jm960486n
BindingDB Entry DOI: 10.7270/Q2639NV0 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50226771

(CHEMBL171575)Show SMILES CCCc1c(CSc2ccc(cc2)-c2nn[nH]n2)ccc(C(C)=O)c1O Show InChI InChI=1S/C19H20N4O2S/c1-3-4-17-14(7-10-16(12(2)24)18(17)25)11-26-15-8-5-13(6-9-15)19-20-22-23-21-19/h5-10,25H,3-4,11H2,1-2H3,(H,20,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity was evaluated against Cysteinyl leukotriene D4 receptor induced contraction in guinea pig ileum |
J Med Chem 30: 911-8 (1987)
BindingDB Entry DOI: 10.7270/Q25X2B45 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50226786
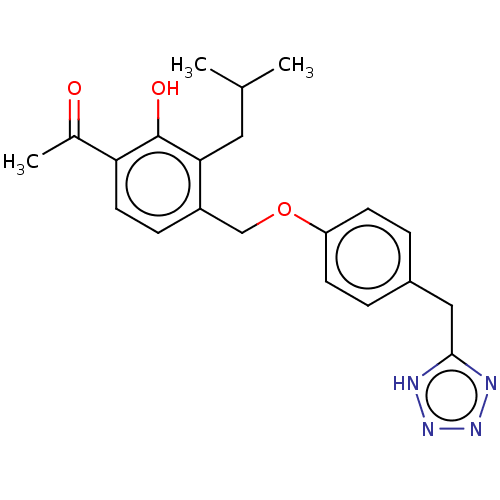
(CHEMBL174432)Show SMILES CC(C)Cc1c(COc2ccc(Cc3nnn[nH]3)cc2)ccc(C(C)=O)c1O Show InChI InChI=1S/C21H24N4O3/c1-13(2)10-19-16(6-9-18(14(3)26)21(19)27)12-28-17-7-4-15(5-8-17)11-20-22-24-25-23-20/h4-9,13,27H,10-12H2,1-3H3,(H,22,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LTD4-induced contraction in guinea pig ileum |
J Med Chem 30: 911-8 (1987)
BindingDB Entry DOI: 10.7270/Q25X2B45 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data