

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469565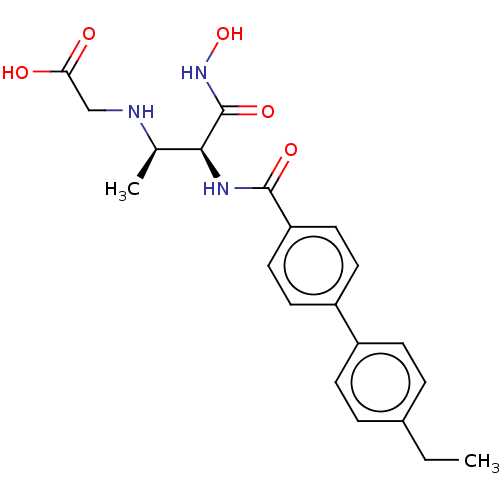 (CHEMBL4082918) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469558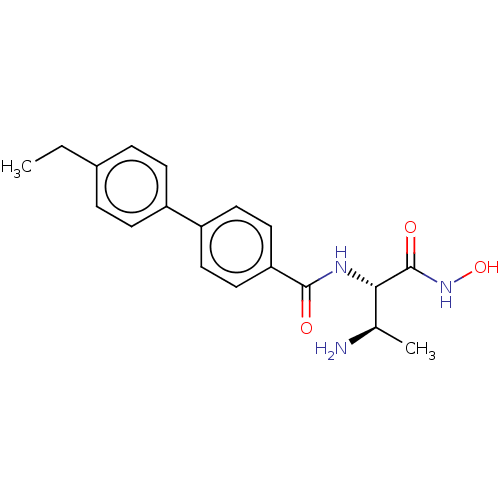 (CHEMBL4061041) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.46 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50249583 (CHEMBL4097399) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469562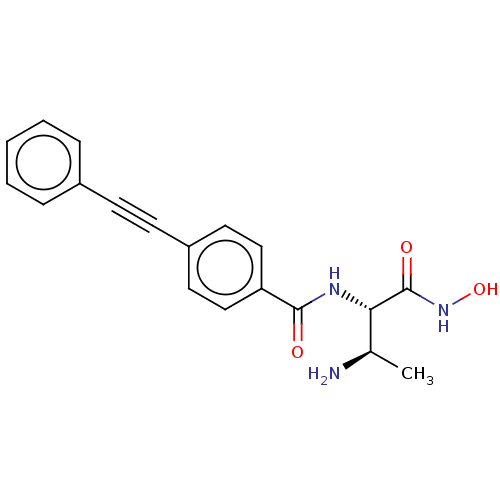 (CHEMBL4069725) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469559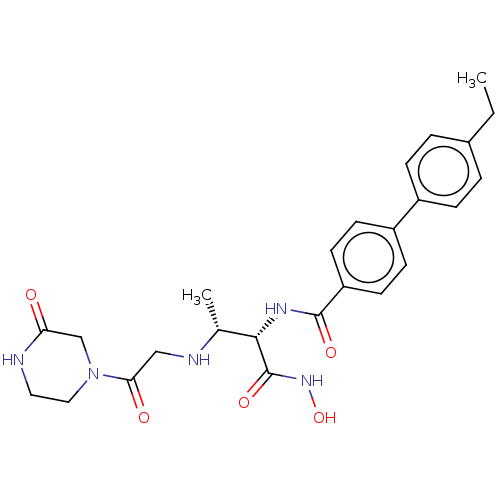 (CHEMBL4063087) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469555 (CHEMBL4090716) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469560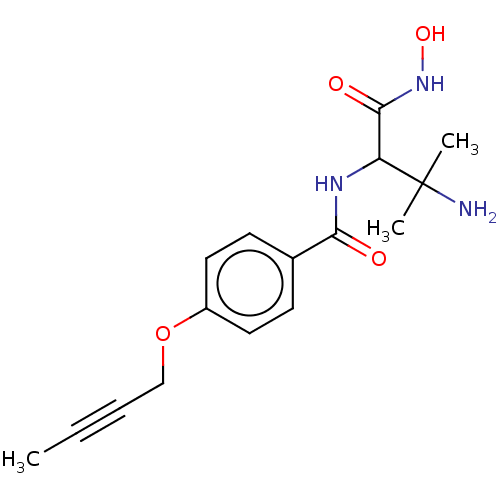 (CHEMBL4083624) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469563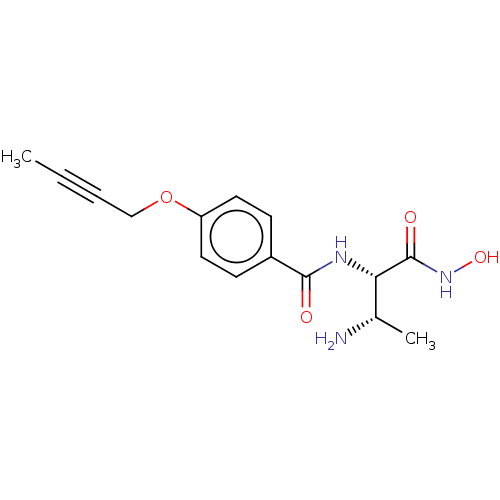 (CHEMBL4079368) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469550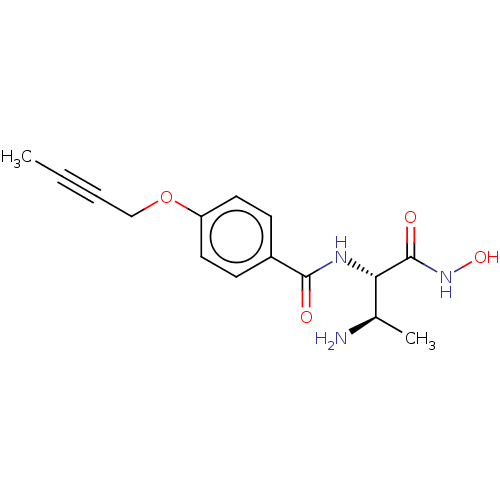 (CHEMBL4070478) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469557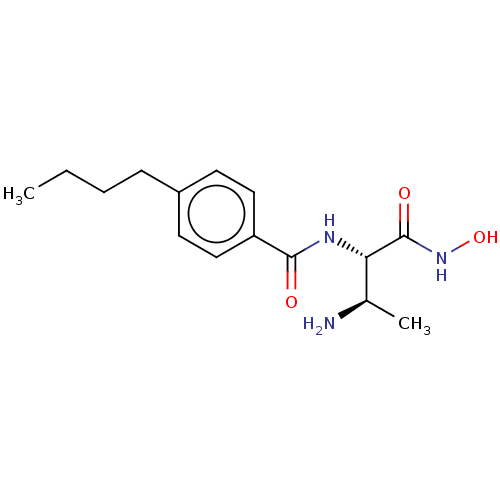 (CHEMBL4091408) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469554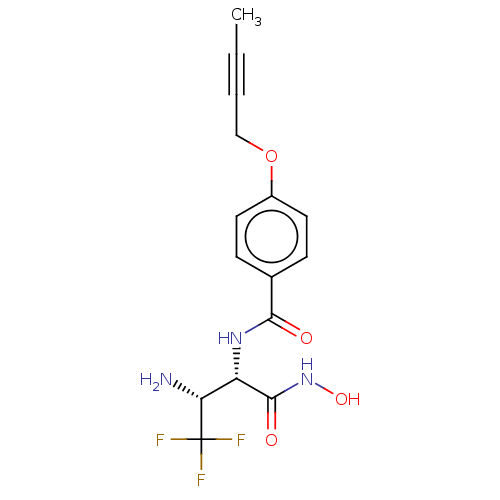 (CHEMBL4061854) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372752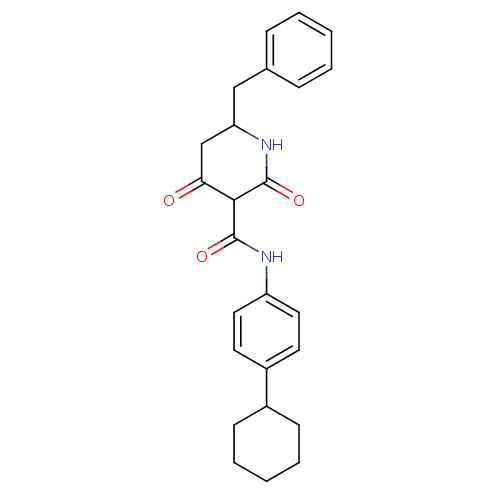 (CHEMBL272547) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372771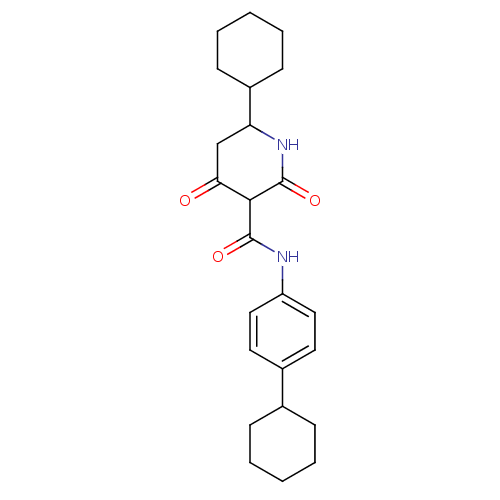 (CHEMBL404127) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372766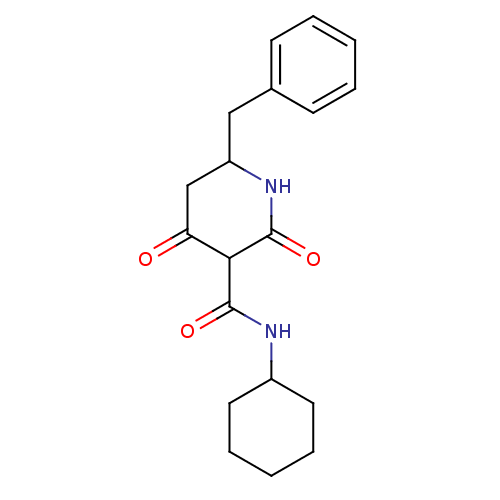 (CHEMBL271482) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372754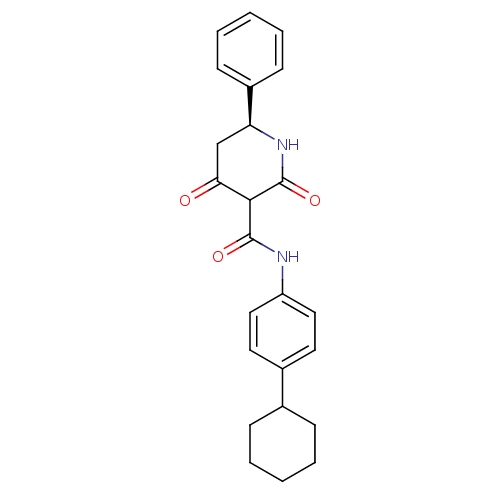 (CHEMBL272054) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469553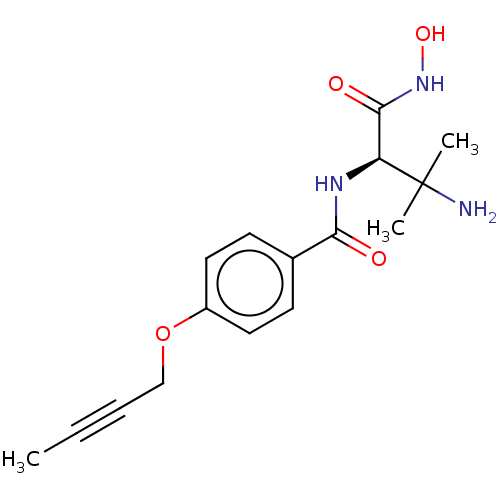 (CHEMBL4102769) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 81.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469552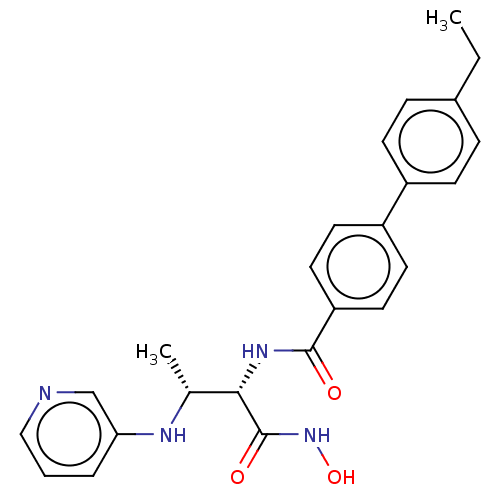 (CHEMBL4072428) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372751 (CHEMBL256222) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372750 (CHEMBL272914) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469548 (CHEMBL4073216) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372760 (CHEMBL257948) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372767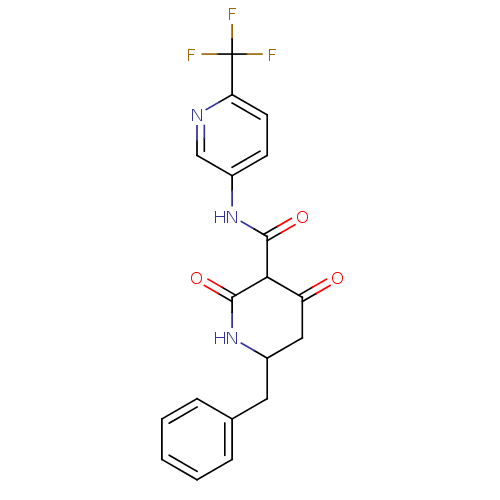 (CHEMBL272913) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372762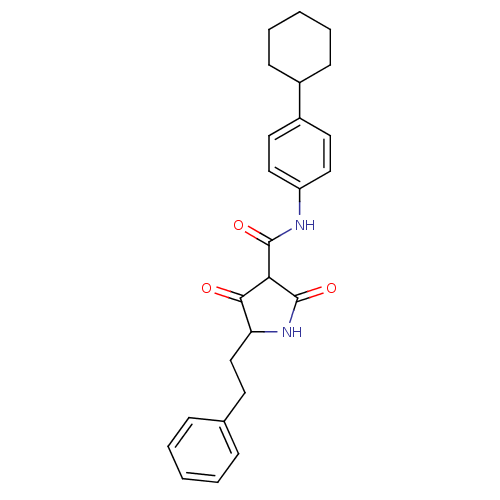 (CHEMBL272162) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372753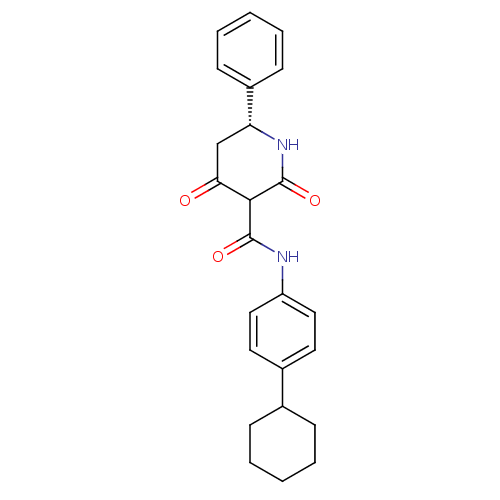 (CHEMBL271845) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372780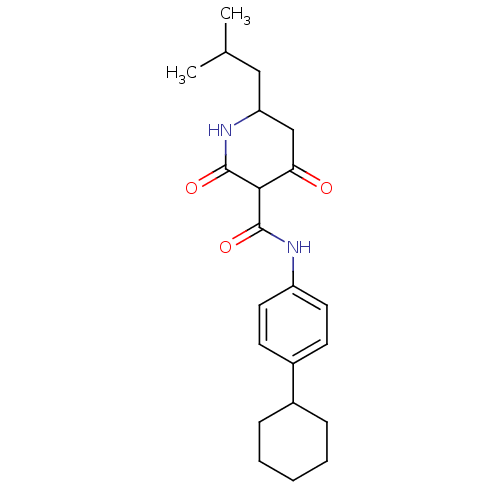 (CHEMBL404126) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372753 (CHEMBL271845) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469549 (CHEMBL4092311) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 268 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372758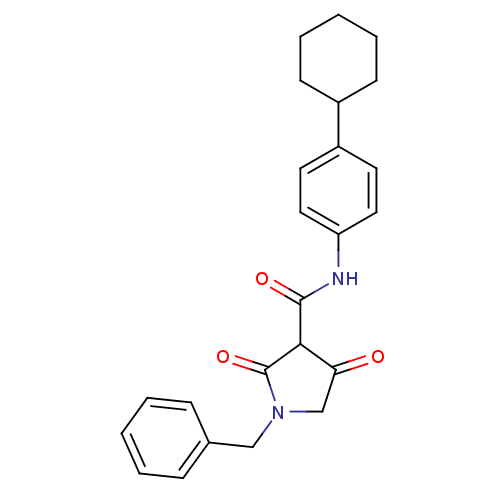 (CHEMBL269960) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469551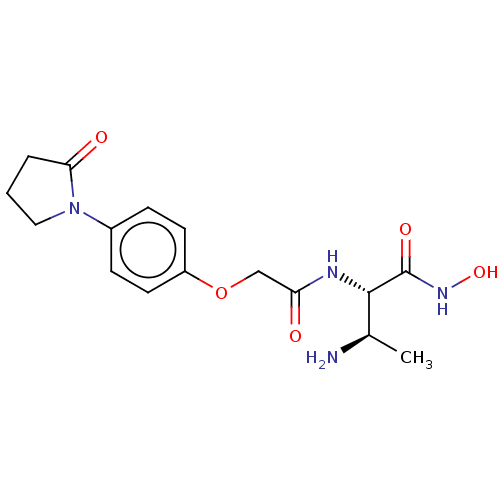 (CHEMBL4064630) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 342 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469561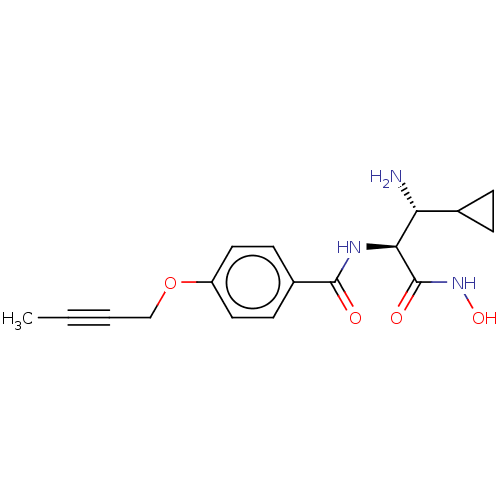 (CHEMBL4065657) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 362 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372755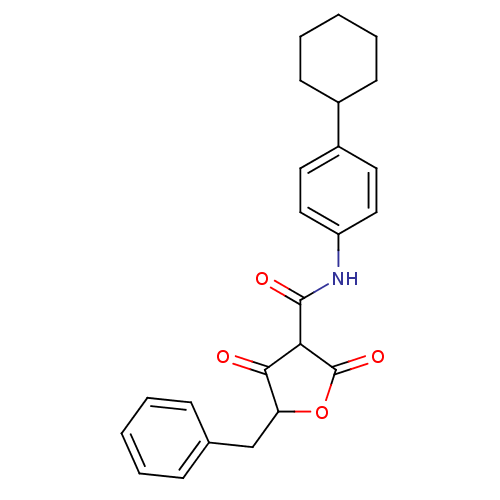 (CHEMBL255828) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372768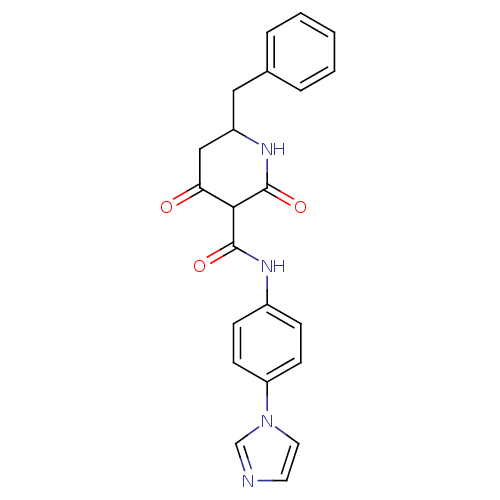 (CHEMBL272696) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372759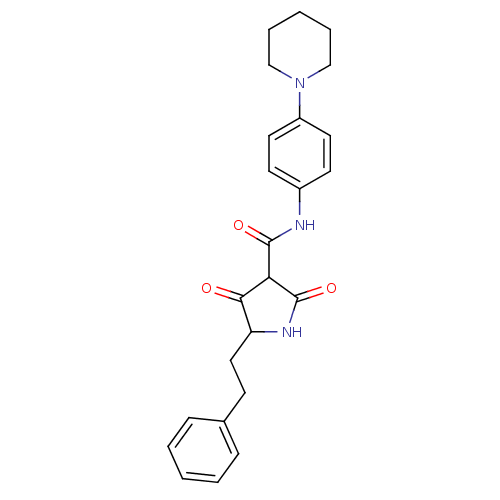 (CHEMBL256476) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469556 (CHEMBL4087085) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 899 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372772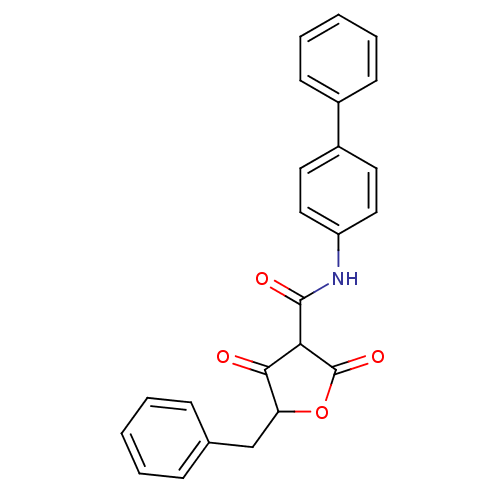 (CHEMBL271293) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372770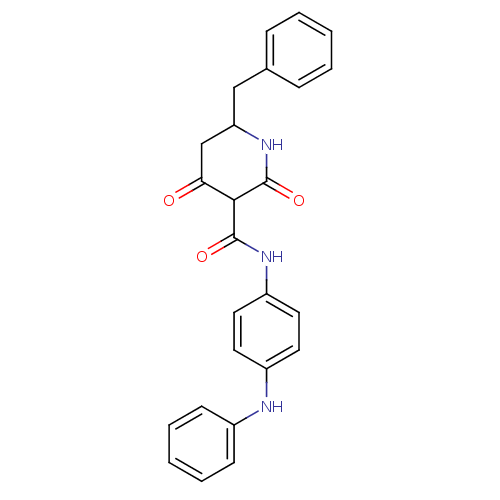 (CHEMBL256223) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372761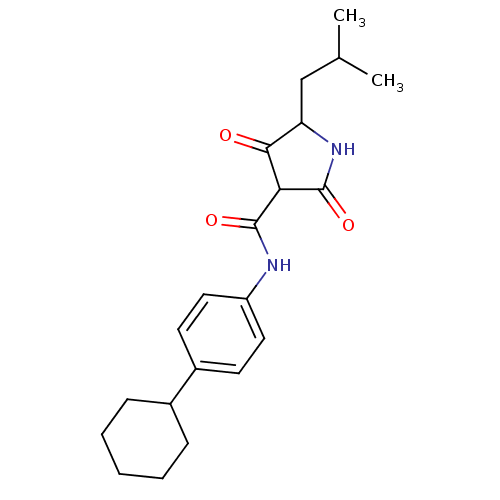 (CHEMBL272163) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469564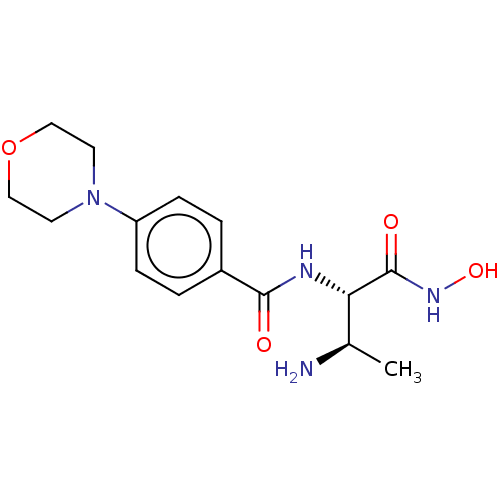 (CHEMBL4100062) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372778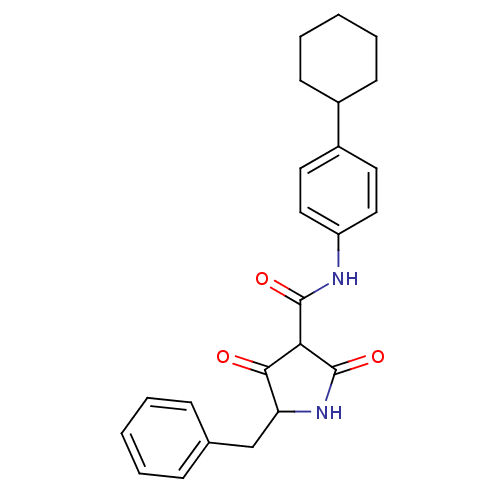 (CHEMBL403226) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372756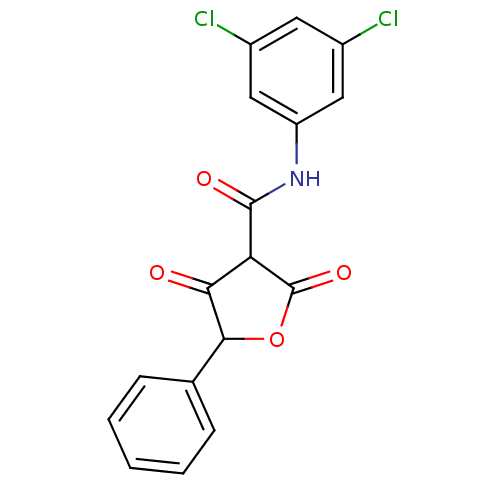 (CHEMBL404337) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372765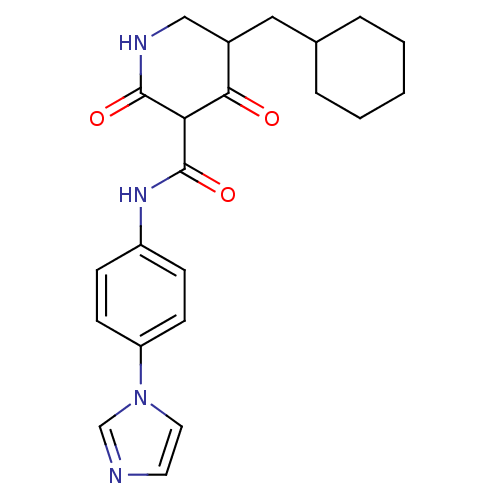 (CHEMBL256483) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372774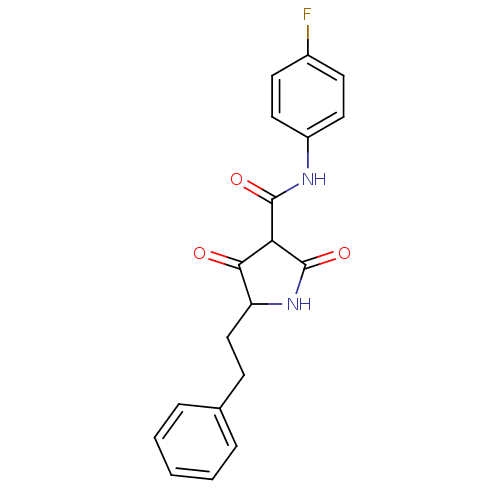 (CHEMBL270170) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372763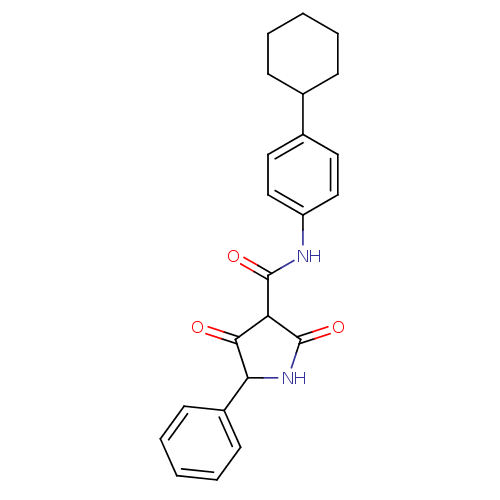 (CHEMBL272377) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372776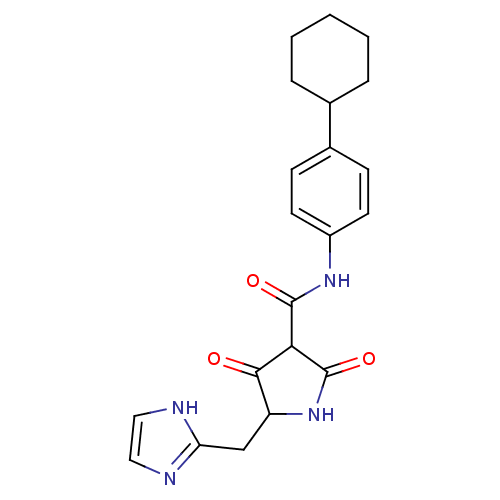 (CHEMBL428250) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50372758 (CHEMBL269960) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human FPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372769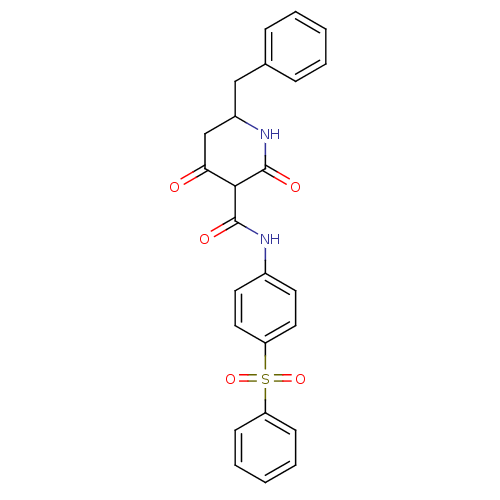 (CHEMBL256017) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372779 (CHEMBL271282) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372764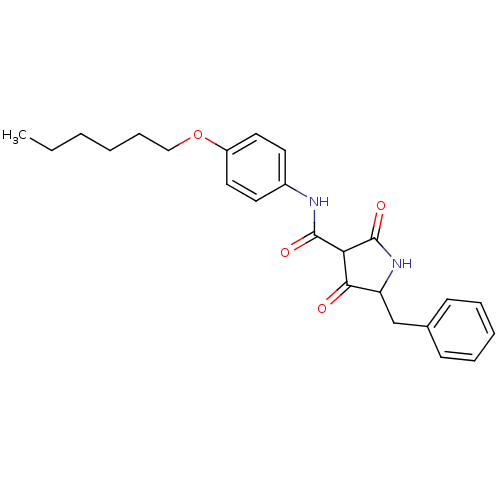 (CHEMBL272703) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoprenyl transferase (Streptococcus pneumoniae) | BDBM50372757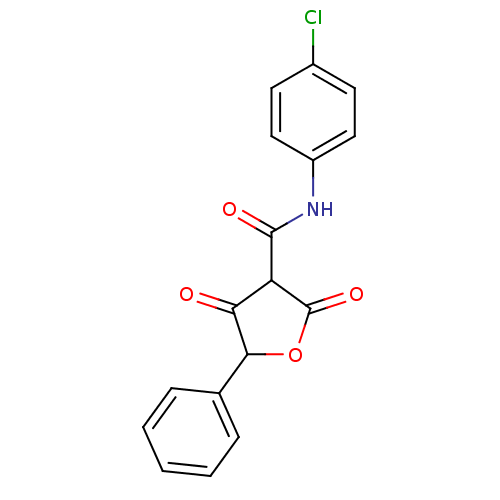 (CHEMBL256033) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae UPPS | Bioorg Med Chem Lett 18: 1840-4 (2008) Article DOI: 10.1016/j.bmcl.2008.02.009 BindingDB Entry DOI: 10.7270/Q2CF9QZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50249583 (CHEMBL4097399) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human HDAC6 | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 80 total ) | Next | Last >> |