 Found 132 hits with Last Name = 'ebeltoft' and Initial = 's'
Found 132 hits with Last Name = 'ebeltoft' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50054539
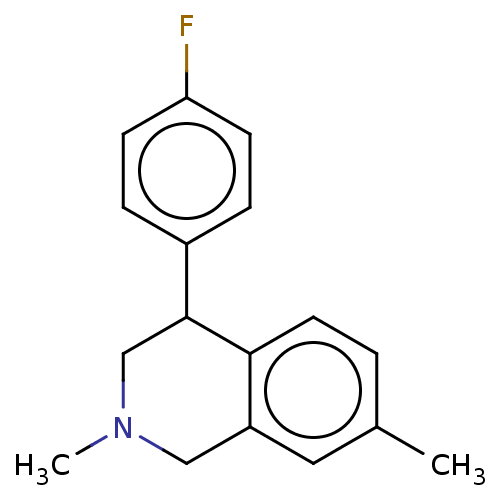
(CHEMBL3323088)Show InChI InChI=1S/C17H18FN/c1-12-3-8-16-14(9-12)10-19(2)11-17(16)13-4-6-15(18)7-5-13/h3-9,17H,10-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50062912
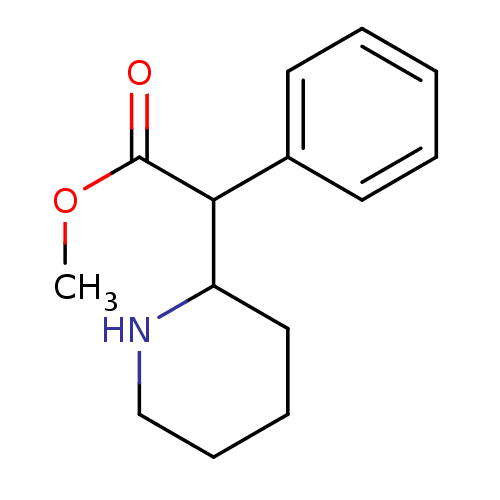
(CHEMBL796 | Methylphenidate | alpha-phenyl-2-piper...)Show InChI InChI=1S/C14H19NO2/c1-17-14(16)13(11-7-3-2-4-8-11)12-9-5-6-10-15-12/h2-4,7-8,12-13,15H,5-6,9-10H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN 35,428 from human DAT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50054539

(CHEMBL3323088)Show InChI InChI=1S/C17H18FN/c1-12-3-8-16-14(9-12)10-19(2)11-17(16)13-4-6-15(18)7-5-13/h3-9,17H,10-11H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN 35,428 from human DAT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50054539

(CHEMBL3323088)Show InChI InChI=1S/C17H18FN/c1-12-3-8-16-14(9-12)10-19(2)11-17(16)13-4-6-15(18)7-5-13/h3-9,17H,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Citolapram from human SERT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50062912

(CHEMBL796 | Methylphenidate | alpha-phenyl-2-piper...)Show InChI InChI=1S/C14H19NO2/c1-17-14(16)13(11-7-3-2-4-8-11)12-9-5-6-10-15-12/h2-4,7-8,12-13,15H,5-6,9-10H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50062912

(CHEMBL796 | Methylphenidate | alpha-phenyl-2-piper...)Show InChI InChI=1S/C14H19NO2/c1-17-14(16)13(11-7-3-2-4-8-11)12-9-5-6-10-15-12/h2-4,7-8,12-13,15H,5-6,9-10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Citolapram from human SERT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM20977
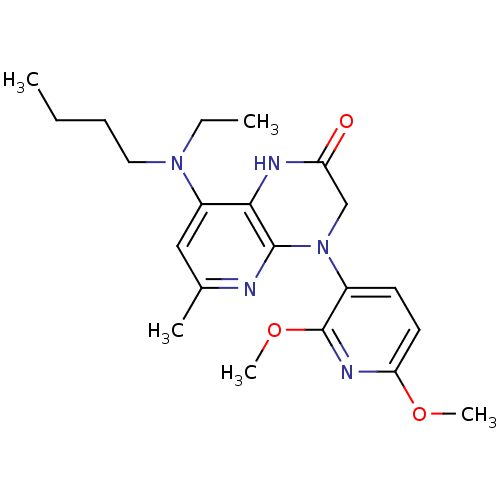
(8-[butyl(ethyl)amino]-4-(2,6-dimethoxypyridin-3-yl...)Show SMILES CCCCN(CC)c1cc(C)nc2N(CC(=O)Nc12)c1ccc(OC)nc1OC Show InChI InChI=1S/C21H29N5O3/c1-6-8-11-25(7-2)16-12-14(3)22-20-19(16)23-17(27)13-26(20)15-9-10-18(28-4)24-21(15)29-5/h9-10,12H,6-8,11,13H2,1-5H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Bristol-Myers Squibb Company
| Assay Description
The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. |
J Med Chem 50: 2269-72 (2007)
Article DOI: 10.1021/jm0611410
BindingDB Entry DOI: 10.7270/Q25Q4TCS |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM20973

(8-[butyl(ethyl)amino]-4-(4-methoxy-2-methylphenyl)...)Show SMILES CCCCN(CC)c1cc(C)nc2N(CC(=O)Nc12)c1ccc(OC)cc1C Show InChI InChI=1S/C22H30N4O2/c1-6-8-11-25(7-2)19-13-16(4)23-22-21(19)24-20(27)14-26(22)18-10-9-17(28-5)12-15(18)3/h9-10,12-13H,6-8,11,14H2,1-5H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Bristol-Myers Squibb Company
| Assay Description
The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. |
J Med Chem 50: 2269-72 (2007)
Article DOI: 10.1021/jm0611410
BindingDB Entry DOI: 10.7270/Q25Q4TCS |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM170441
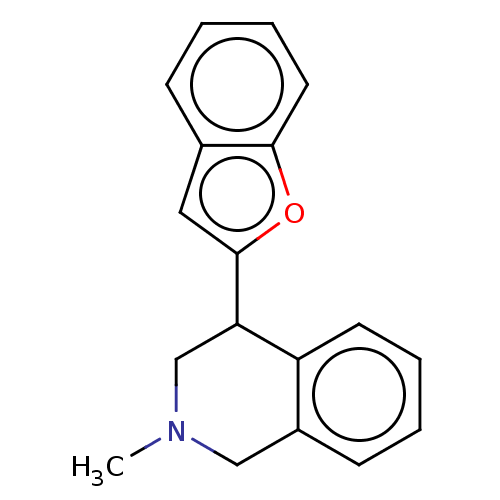
(US9085531, 2)Show InChI InChI=1S/C18H17NO/c1-19-11-14-7-2-4-8-15(14)16(12-19)18-10-13-6-3-5-9-17(13)20-18/h2-10,16H,11-12H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50054533
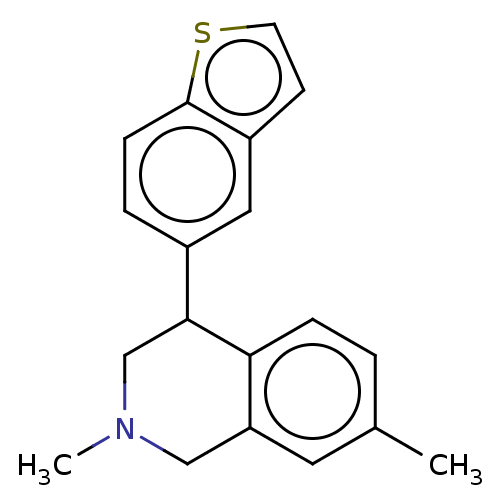
(CHEMBL3323173)Show InChI InChI=1S/C19H19NS/c1-13-3-5-17-16(9-13)11-20(2)12-18(17)14-4-6-19-15(10-14)7-8-21-19/h3-10,18H,11-12H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM170440
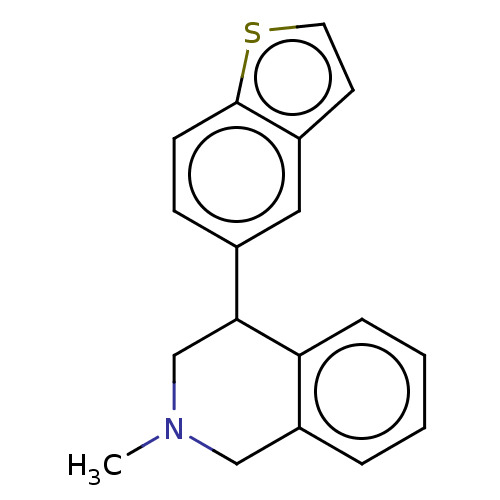
(US9085531, 40)Show InChI InChI=1S/C18H17NS/c1-19-11-15-4-2-3-5-16(15)17(12-19)13-6-7-18-14(10-13)8-9-20-18/h2-10,17H,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant SERT (unknown origin) expressed in HEK cells by saturation binding analysis |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM20978
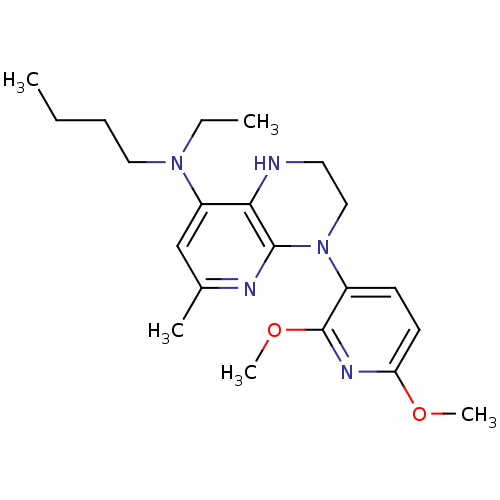
(N-butyl-4-(2,6-dimethoxypyridin-3-yl)-N-ethyl-6-me...)Show InChI InChI=1S/C21H31N5O2/c1-6-8-12-25(7-2)17-14-15(3)23-20-19(17)22-11-13-26(20)16-9-10-18(27-4)24-21(16)28-5/h9-10,14,22H,6-8,11-13H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Bristol-Myers Squibb Company
| Assay Description
The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. |
J Med Chem 50: 2269-72 (2007)
Article DOI: 10.1021/jm0611410
BindingDB Entry DOI: 10.7270/Q25Q4TCS |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM170444
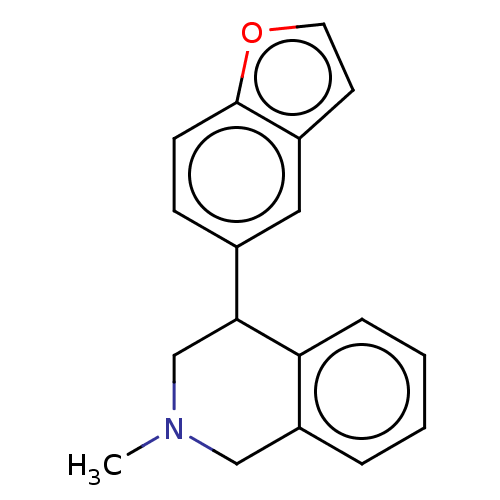
(US9085531, 14)Show InChI InChI=1S/C18H17NO/c1-19-11-15-4-2-3-5-16(15)17(12-19)13-6-7-18-14(10-13)8-9-20-18/h2-10,17H,11-12H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50054529
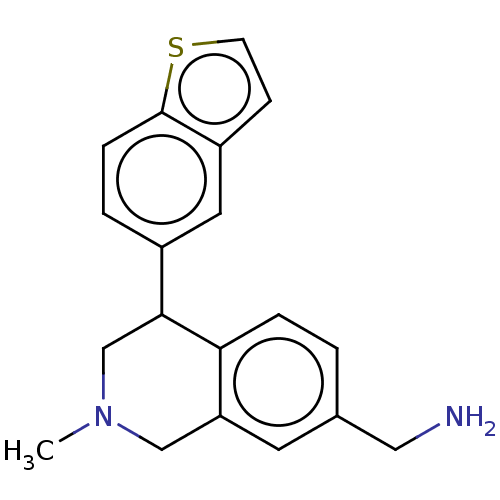
(CHEMBL3323178)Show InChI InChI=1S/C19H20N2S/c1-21-11-16-8-13(10-20)2-4-17(16)18(12-21)14-3-5-19-15(9-14)6-7-22-19/h2-9,18H,10-12,20H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50054522

(CHEMBL3323181)Show SMILES CN1C[C@H](c2ccc3sccc3c2)c2ccc(cc2C1)N1CCOCC1 |r| Show InChI InChI=1S/C22H24N2OS/c1-23-14-18-13-19(24-7-9-25-10-8-24)3-4-20(18)21(15-23)16-2-5-22-17(12-16)6-11-26-22/h2-6,11-13,21H,7-10,14-15H2,1H3/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant SERT (unknown origin) expressed in HEK cells by saturation binding analysis |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM170454
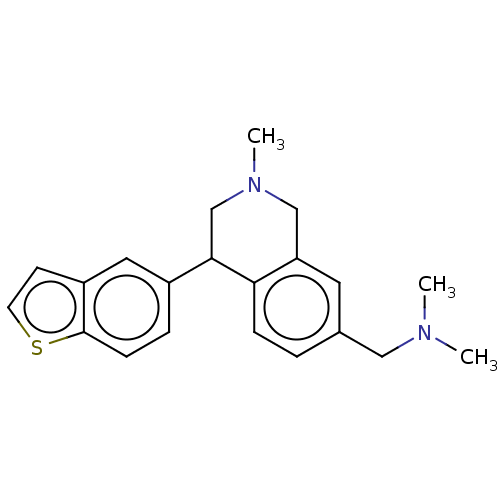
(US9085531, 102)Show InChI InChI=1S/C21H24N2S/c1-22(2)12-15-4-6-19-18(10-15)13-23(3)14-20(19)16-5-7-21-17(11-16)8-9-24-21/h4-11,20H,12-14H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50054522

(CHEMBL3323181)Show SMILES CN1C[C@H](c2ccc3sccc3c2)c2ccc(cc2C1)N1CCOCC1 |r| Show InChI InChI=1S/C22H24N2OS/c1-23-14-18-13-19(24-7-9-25-10-8-24)3-4-20(18)21(15-23)16-2-5-22-17(12-16)6-11-26-22/h2-6,11-13,21H,7-10,14-15H2,1H3/t21-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant DAT (unknown origin) expressed in HEK cells by saturation binding analysis |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM20985

(4-{8-[butyl(ethyl)amino]-6-methyl-2-oxo-1H,2H,3H,4...)Show SMILES CCCCN(CC)c1cc(C)nc2N(CC(=O)Nc12)c1ccc(cc1C)C#N Show InChI InChI=1S/C22H27N5O/c1-5-7-10-26(6-2)19-12-16(4)24-22-21(19)25-20(28)14-27(22)18-9-8-17(13-23)11-15(18)3/h8-9,11-12H,5-7,10,14H2,1-4H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Bristol-Myers Squibb Company
| Assay Description
The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. |
J Med Chem 50: 2269-72 (2007)
Article DOI: 10.1021/jm0611410
BindingDB Entry DOI: 10.7270/Q25Q4TCS |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM170440

(US9085531, 40)Show InChI InChI=1S/C18H17NS/c1-19-11-15-4-2-3-5-16(15)17(12-19)13-6-7-18-14(10-13)8-9-20-18/h2-10,17H,11-12H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant NET (unknown origin) expressed in HEK cells by saturation binding analysis |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM20986

(4-{8-[butyl(ethyl)amino]-6-methyl-1H,2H,3H,4H-pyri...)Show InChI InChI=1S/C22H29N5/c1-5-7-11-26(6-2)20-14-17(4)25-22-21(20)24-10-12-27(22)19-9-8-18(15-23)13-16(19)3/h8-9,13-14,24H,5-7,10-12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Bristol-Myers Squibb Company
| Assay Description
The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. |
J Med Chem 50: 2269-72 (2007)
Article DOI: 10.1021/jm0611410
BindingDB Entry DOI: 10.7270/Q25Q4TCS |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM20974
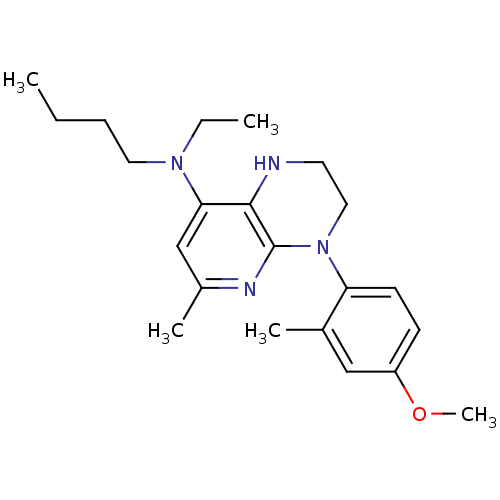
(N-butyl-N-ethyl-4-(4-methoxy-2-methylphenyl)-6-met...)Show InChI InChI=1S/C22H32N4O/c1-6-8-12-25(7-2)20-15-17(4)24-22-21(20)23-11-13-26(22)19-10-9-18(27-5)14-16(19)3/h9-10,14-15,23H,6-8,11-13H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Bristol-Myers Squibb Company
| Assay Description
The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. |
J Med Chem 50: 2269-72 (2007)
Article DOI: 10.1021/jm0611410
BindingDB Entry DOI: 10.7270/Q25Q4TCS |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM170454

(US9085531, 102)Show InChI InChI=1S/C21H24N2S/c1-22(2)12-15-4-6-19-18(10-15)13-23(3)14-20(19)16-5-7-21-17(11-16)8-9-24-21/h4-11,20H,12-14H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Citolapram from human SERT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM60507
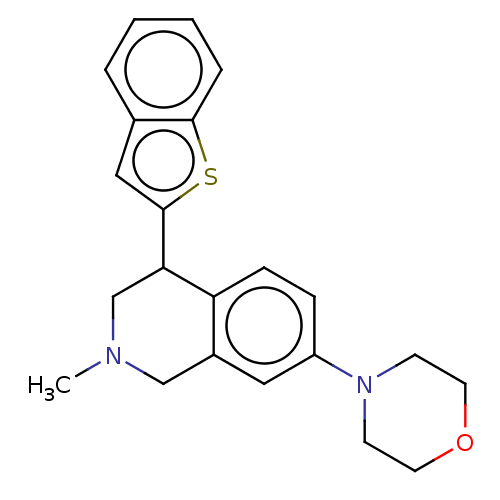
(BDBM50054521 | US9085531, 75)Show InChI InChI=1S/C22H24N2OS/c1-23-14-17-12-18(24-8-10-25-11-9-24)6-7-19(17)20(15-23)22-13-16-4-2-3-5-21(16)26-22/h2-7,12-13,20H,8-11,14-15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant SERT (unknown origin) expressed in HEK cells by saturation binding analysis |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM170446
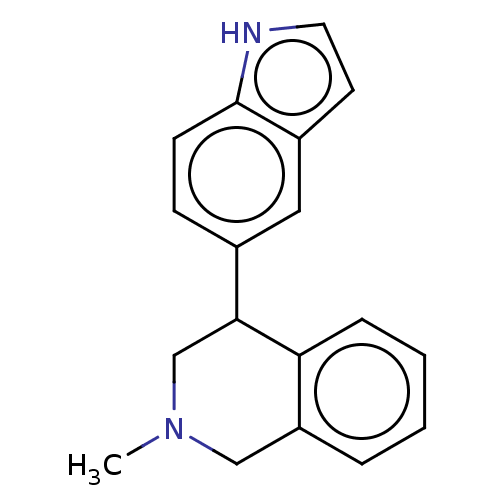
(US9085531, 49) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN 35,428 from human DAT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50054535
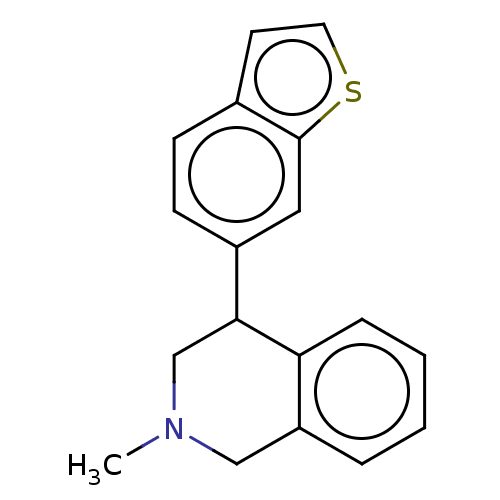
(CHEMBL3323096)Show InChI InChI=1S/C18H17NS/c1-19-11-15-4-2-3-5-16(15)17(12-19)14-7-6-13-8-9-20-18(13)10-14/h2-10,17H,11-12H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN 35,428 from human DAT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50054528
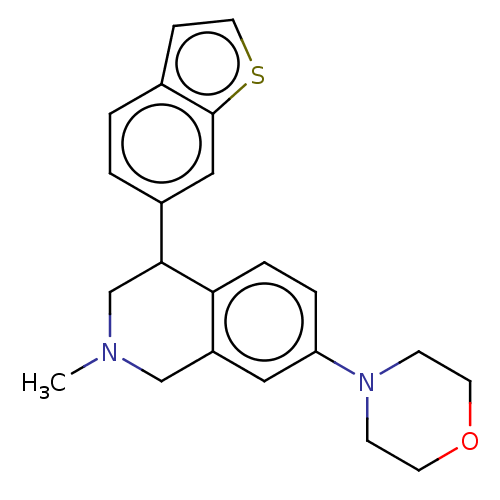
(CHEMBL3323183)Show InChI InChI=1S/C22H24N2OS/c1-23-14-18-12-19(24-7-9-25-10-8-24)4-5-20(18)21(15-23)17-3-2-16-6-11-26-22(16)13-17/h2-6,11-13,21H,7-10,14-15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Citolapram from human SERT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50054533

(CHEMBL3323173)Show InChI InChI=1S/C19H19NS/c1-13-3-5-17-16(9-13)11-20(2)12-18(17)14-4-6-19-15(10-14)7-8-21-19/h3-10,18H,11-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Citolapram from human SERT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM170453
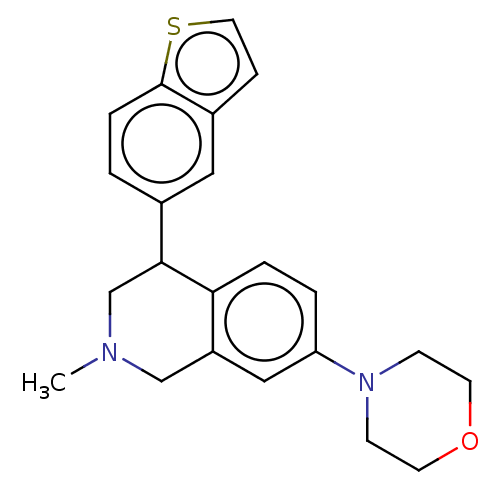
(US9085531, 100)Show InChI InChI=1S/C22H24N2OS/c1-23-14-18-13-19(24-7-9-25-10-8-24)3-4-20(18)21(15-23)16-2-5-22-17(12-16)6-11-26-22/h2-6,11-13,21H,7-10,14-15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant SERT (unknown origin) expressed in HEK cells by saturation binding analysis |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50054532

(CHEMBL3323174)Show InChI InChI=1S/C19H19NOS/c1-20-11-15-10-16(21-2)4-5-17(15)18(12-20)13-3-6-19-14(9-13)7-8-22-19/h3-10,18H,11-12H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM170440

(US9085531, 40)Show InChI InChI=1S/C18H17NS/c1-19-11-15-4-2-3-5-16(15)17(12-19)13-6-7-18-14(10-13)8-9-20-18/h2-10,17H,11-12H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant DAT (unknown origin) expressed in HEK cells by saturation binding analysis |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM170445
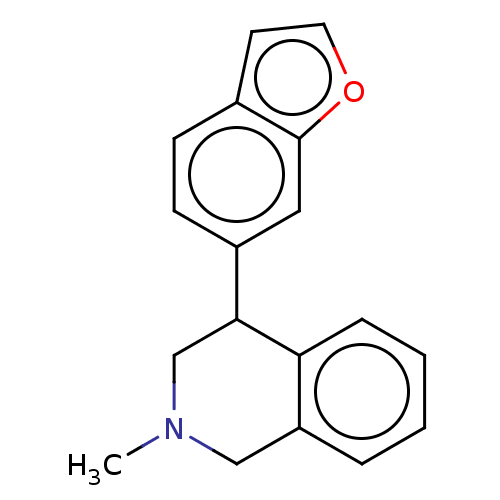
(US9085531, 17)Show InChI InChI=1S/C18H17NO/c1-19-11-15-4-2-3-5-16(15)17(12-19)14-7-6-13-8-9-20-18(13)10-14/h2-10,17H,11-12H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50054532

(CHEMBL3323174)Show InChI InChI=1S/C19H19NOS/c1-20-11-15-10-16(21-2)4-5-17(15)18(12-20)13-3-6-19-14(9-13)7-8-22-19/h3-10,18H,11-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Citolapram from human SERT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM170454

(US9085531, 102)Show InChI InChI=1S/C21H24N2S/c1-22(2)12-15-4-6-19-18(10-15)13-23(3)14-20(19)16-5-7-21-17(11-16)8-9-24-21/h4-11,20H,12-14H2,1-3H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN 35,428 from human DAT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50054530
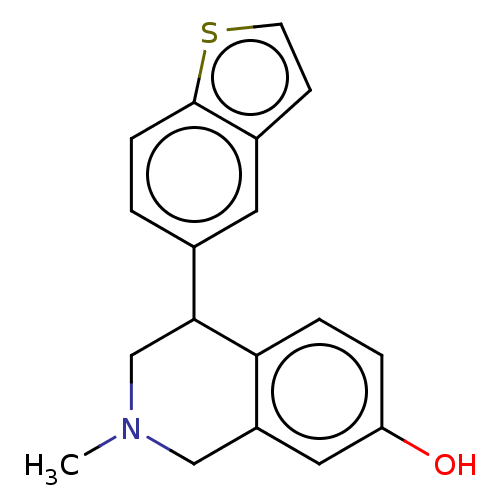
(CHEMBL3323175)Show InChI InChI=1S/C18H17NOS/c1-19-10-14-9-15(20)3-4-16(14)17(11-19)12-2-5-18-13(8-12)6-7-21-18/h2-9,17,20H,10-11H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM170444

(US9085531, 14)Show InChI InChI=1S/C18H17NO/c1-19-11-15-4-2-3-5-16(15)17(12-19)13-6-7-18-14(10-13)8-9-20-18/h2-10,17H,11-12H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN 35,428 from human DAT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM60507

(BDBM50054521 | US9085531, 75)Show InChI InChI=1S/C22H24N2OS/c1-23-14-17-12-18(24-8-10-25-11-9-24)6-7-19(17)20(15-23)22-13-16-4-2-3-5-21(16)26-22/h2-7,12-13,20H,8-11,14-15H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant DAT (unknown origin) expressed in HEK cells by saturation binding analysis |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM20989
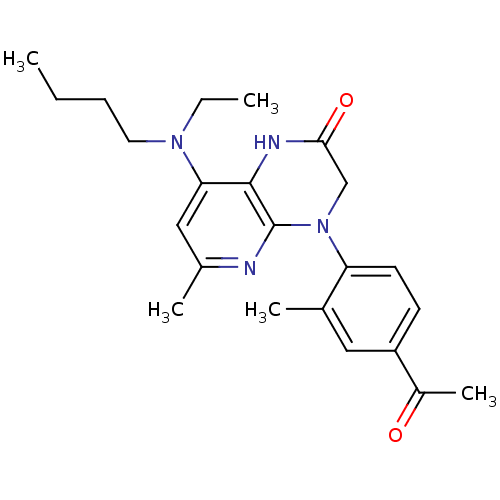
(8-[butyl(ethyl)amino]-4-(4-acetyl-2-methylphenyl)-...)Show SMILES CCCCN(CC)c1cc(C)nc2N(CC(=O)Nc12)c1ccc(cc1C)C(C)=O Show InChI InChI=1S/C23H30N4O2/c1-6-8-11-26(7-2)20-13-16(4)24-23-22(20)25-21(29)14-27(23)19-10-9-18(17(5)28)12-15(19)3/h9-10,12-13H,6-8,11,14H2,1-5H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Bristol-Myers Squibb Company
| Assay Description
The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. |
J Med Chem 50: 2269-72 (2007)
Article DOI: 10.1021/jm0611410
BindingDB Entry DOI: 10.7270/Q25Q4TCS |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM170446

(US9085531, 49) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM170456
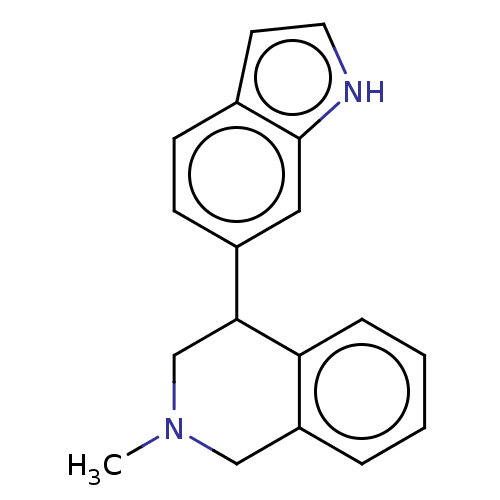
(US9085531, 119) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant SERT (unknown origin) expressed in HEK cells by saturation binding analysis |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50054515
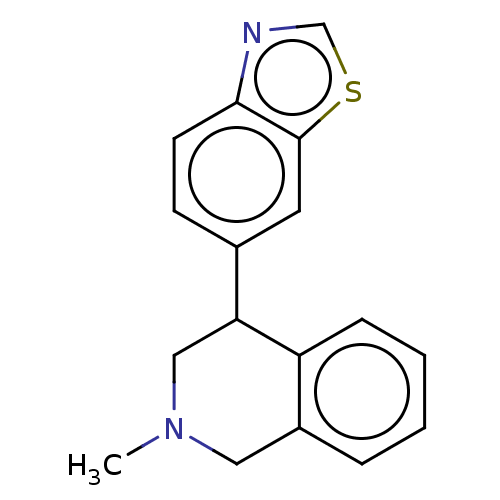
(CHEMBL3323106)Show InChI InChI=1S/C17H16N2S/c1-19-9-13-4-2-3-5-14(13)15(10-19)12-6-7-16-17(8-12)20-11-18-16/h2-8,11,15H,9-10H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant NET (unknown origin) expressed in HEK cells by saturation binding analysis |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50054526
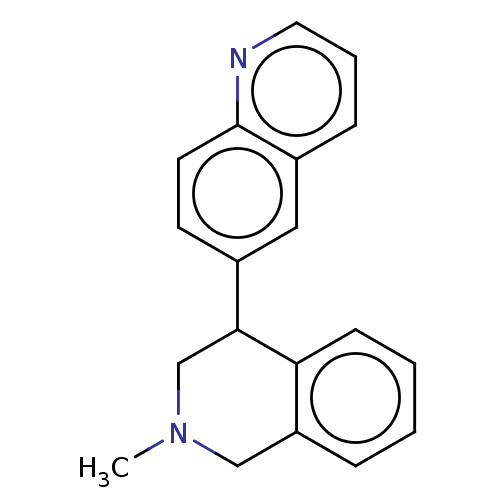
(CHEMBL3323109)Show InChI InChI=1S/C19H18N2/c1-21-12-16-5-2-3-7-17(16)18(13-21)14-8-9-19-15(11-14)6-4-10-20-19/h2-11,18H,12-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant SERT (unknown origin) expressed in HEK cells by saturation binding analysis |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50054530

(CHEMBL3323175)Show InChI InChI=1S/C18H17NOS/c1-19-10-14-9-15(20)3-4-16(14)17(11-19)12-2-5-18-13(8-12)6-7-21-18/h2-9,17,20H,10-11H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN 35,428 from human DAT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM170445

(US9085531, 17)Show InChI InChI=1S/C18H17NO/c1-19-11-15-4-2-3-5-16(15)17(12-19)14-7-6-13-8-9-20-18(13)10-14/h2-10,17H,11-12H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN 35,428 from human DAT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50054520

(CHEMBL3323100)Show InChI InChI=1S/C18H17NS/c1-19-11-14-5-2-3-7-15(14)17(12-19)16-8-4-6-13-9-10-20-18(13)16/h2-10,17H,11-12H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant NET (unknown origin) expressed in HEK cells by saturation binding analysis |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM20990
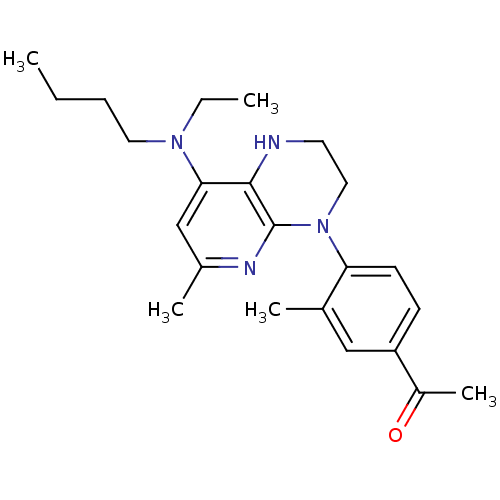
(1-(4-{8-[butyl(ethyl)amino]-6-methyl-1H,2H,3H,4H-p...)Show SMILES CCCCN(CC)c1cc(C)nc2N(CCNc12)c1ccc(cc1C)C(C)=O Show InChI InChI=1S/C23H32N4O/c1-6-8-12-26(7-2)21-15-17(4)25-23-22(21)24-11-13-27(23)20-10-9-19(18(5)28)14-16(20)3/h9-10,14-15,24H,6-8,11-13H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Bristol-Myers Squibb Company
| Assay Description
The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. |
J Med Chem 50: 2269-72 (2007)
Article DOI: 10.1021/jm0611410
BindingDB Entry DOI: 10.7270/Q25Q4TCS |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50054522

(CHEMBL3323181)Show SMILES CN1C[C@H](c2ccc3sccc3c2)c2ccc(cc2C1)N1CCOCC1 |r| Show InChI InChI=1S/C22H24N2OS/c1-23-14-18-13-19(24-7-9-25-10-8-24)3-4-20(18)21(15-23)16-2-5-22-17(12-16)6-11-26-22/h2-6,11-13,21H,7-10,14-15H2,1H3/t21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant NET (unknown origin) expressed in HEK cells by saturation binding analysis |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50054524

(CHEMBL3323176)Show InChI InChI=1S/C18H16FNS/c1-20-10-14-9-15(19)3-4-16(14)17(11-20)12-2-5-18-13(8-12)6-7-21-18/h2-9,17H,10-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant SERT (unknown origin) expressed in HEK cells by saturation binding analysis |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM170456

(US9085531, 119) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [125I]-RTI-55 from recombinant NET (unknown origin) expressed in HEK cells by saturation binding analysis |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50054532

(CHEMBL3323174)Show InChI InChI=1S/C19H19NOS/c1-20-11-15-10-16(21-2)4-5-17(15)18(12-20)13-3-6-19-14(9-13)7-8-22-19/h3-10,18H,11-12H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN 35,428 from human DAT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM20980
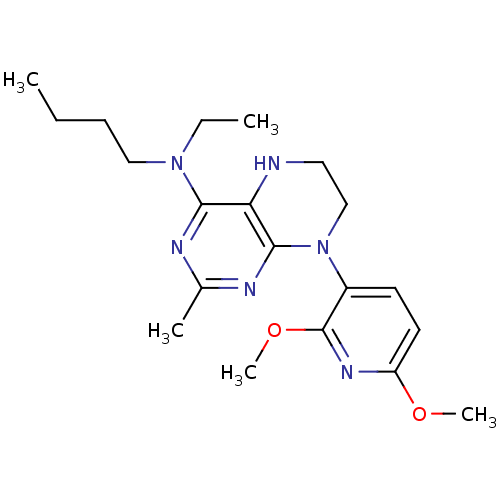
(N-butyl-8-(2,6-dimethoxypyridin-3-yl)-N-ethyl-2-me...)Show InChI InChI=1S/C20H30N6O2/c1-6-8-12-25(7-2)18-17-19(23-14(3)22-18)26(13-11-21-17)15-9-10-16(27-4)24-20(15)28-5/h9-10,21H,6-8,11-13H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | 7.0 | 23 |
Bristol-Myers Squibb Company
| Assay Description
The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. |
J Med Chem 50: 2269-72 (2007)
Article DOI: 10.1021/jm0611410
BindingDB Entry DOI: 10.7270/Q25Q4TCS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data