 Found 672 hits with Last Name = 'engel' and Initial = 'j'
Found 672 hits with Last Name = 'engel' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238182
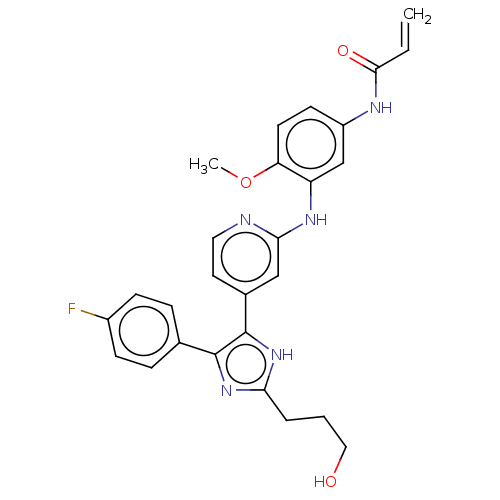
(CHEMBL4100860)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(CCCO)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C27H26FN5O3/c1-3-25(35)30-20-10-11-22(36-2)21(16-20)31-24-15-18(12-13-29-24)27-26(17-6-8-19(28)9-7-17)32-23(33-27)5-4-14-34/h3,6-13,15-16,34H,1,4-5,14H2,2H3,(H,29,31)(H,30,35)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H][D-Ala2,D-Leu5]enkephalin to Opioid receptor delta 1 in the rat brain homogenate |
J Med Chem 60: 5613-5637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00316
BindingDB Entry DOI: 10.7270/Q2V98BCK |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238177

(CHEMBL4098072)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H22FN5O2S/c1-4-22(32)28-18-9-10-20(33-2)19(14-18)29-21-13-16(11-12-27-21)24-23(30-25(31-24)34-3)15-5-7-17(26)8-6-15/h4-14H,1H2,2-3H3,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal GST-fused EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus using TK-substrate-biotin incubated ... |
J Med Chem 60: 5613-5637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00316
BindingDB Entry DOI: 10.7270/Q2V98BCK |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238183
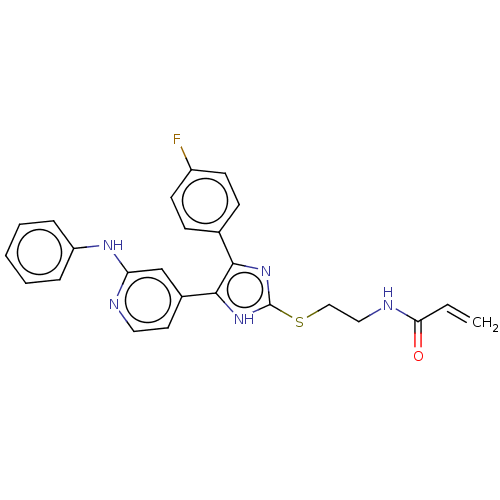
(CHEMBL4071012)Show SMILES Fc1ccc(cc1)-c1nc(SCCNC(=O)C=C)[nH]c1-c1ccnc(Nc2ccccc2)c1 Show InChI InChI=1S/C25H22FN5OS/c1-2-22(32)28-14-15-33-25-30-23(17-8-10-19(26)11-9-17)24(31-25)18-12-13-27-21(16-18)29-20-6-4-3-5-7-20/h2-13,16H,1,14-15H2,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal GST-fused EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus using TK-substrate-biotin incubated ... |
J Med Chem 60: 5613-5637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00316
BindingDB Entry DOI: 10.7270/Q2V98BCK |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237140

(CHEMBL4068763)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1n[nH]c2cc(Nc3cccc(F)c3)ccc12 Show InChI InChI=1S/C28H32FN7O2/c1-6-27(37)31-23-16-24(26(38-5)17-25(23)36(4)13-12-35(2)3)32-28-21-11-10-20(15-22(21)33-34-28)30-19-9-7-8-18(29)14-19/h6-11,14-17,30H,1,12-13H2,2-5H3,(H,31,37)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Reversible inhibition of wild-type human N-terminal GST-tagged EGFR cytoplasmic domain (669 to 1210 end amino acid residues) expressed in baculovirus... |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal GST-fused EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus using TK-substrate-biotin incubated ... |
J Med Chem 60: 5613-5637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00316
BindingDB Entry DOI: 10.7270/Q2V98BCK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Reversible inhibition of wild-type human N-terminal GST-tagged EGFR cytoplasmic domain (669 to 1210 end amino acid residues) expressed in baculovirus... |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM149404
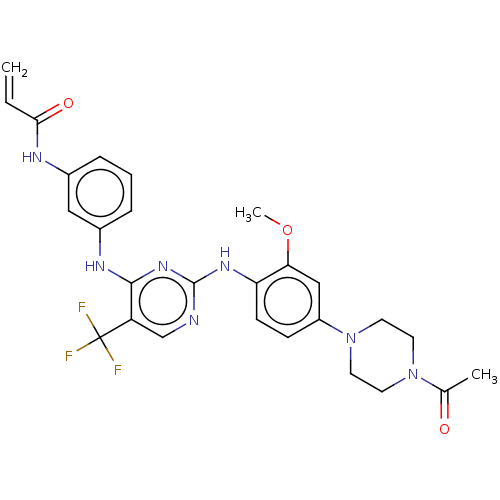
(AVL-301 | CHEMBL3545308 | CNX-419 | CO-1686 | Roci...)Show SMILES COc1cc(ccc1Nc1ncc(c(Nc2cccc(NC(=O)C=C)c2)n1)C(F)(F)F)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C27H28F3N7O3/c1-4-24(39)32-18-6-5-7-19(14-18)33-25-21(27(28,29)30)16-31-26(35-25)34-22-9-8-20(15-23(22)40-3)37-12-10-36(11-13-37)17(2)38/h4-9,14-16H,1,10-13H2,2-3H3,(H,32,39)(H2,31,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Reversible inhibition of wild-type human N-terminal GST-tagged EGFR cytoplasmic domain (669 to 1210 end amino acid residues) expressed in baculovirus... |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cruzipain
(Trypanosoma cruzi) | BDBM50306596
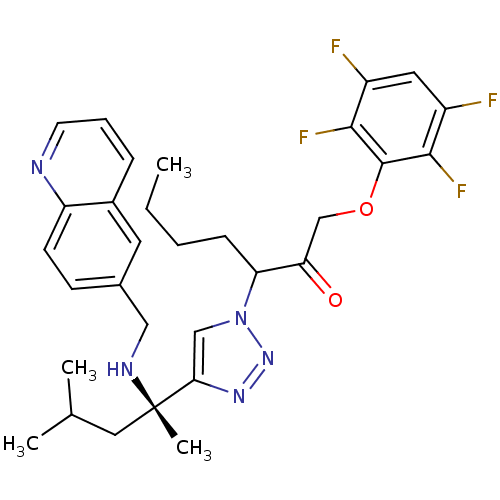
(3-(4-((S)-4-methyl-2-(quinolin-6-ylmethylamino)pen...)Show SMILES CCCCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@](C)(CC(C)C)NCc1ccc2ncccc2c1 |r| Show InChI InChI=1S/C31H35F4N5O2/c1-5-6-9-25(26(41)18-42-30-28(34)22(32)14-23(33)29(30)35)40-17-27(38-39-40)31(4,15-19(2)3)37-16-20-10-11-24-21(13-20)8-7-12-36-24/h7-8,10-14,17,19,25,37H,5-6,9,15-16,18H2,1-4H3/t25?,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306600
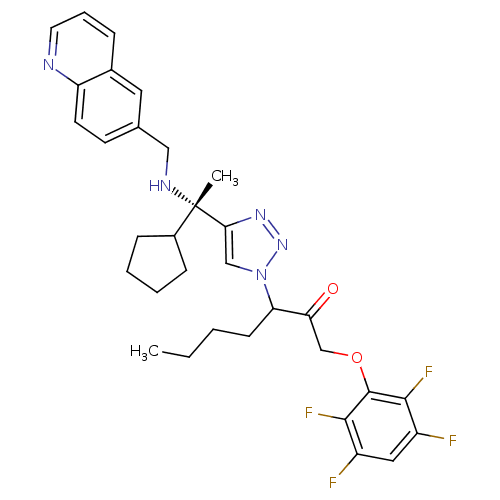
(3-(4-((S)-1-cyclopentyl-1-(quinolin-6-ylmethylamin...)Show SMILES CCCCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@@](C)(NCc1ccc2ncccc2c1)C1CCCC1 |r| Show InChI InChI=1S/C32H35F4N5O2/c1-3-4-11-26(27(42)19-43-31-29(35)23(33)16-24(34)30(31)36)41-18-28(39-40-41)32(2,22-9-5-6-10-22)38-17-20-12-13-25-21(15-20)8-7-14-37-25/h7-8,12-16,18,22,26,38H,3-6,9-11,17,19H2,1-2H3/t26?,32-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306598
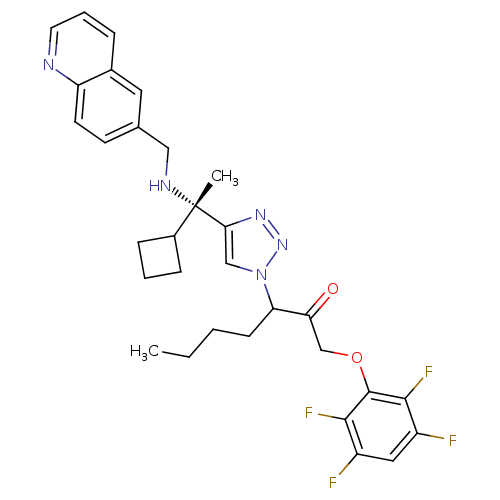
(3-(4-((S)-1-cyclobutyl-1-(quinolin-6-ylmethylamino...)Show SMILES CCCCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@@](C)(NCc1ccc2ncccc2c1)C1CCC1 |r| Show InChI InChI=1S/C31H33F4N5O2/c1-3-4-10-25(26(41)18-42-30-28(34)22(32)15-23(33)29(30)35)40-17-27(38-39-40)31(2,21-8-5-9-21)37-16-19-11-12-24-20(14-19)7-6-13-36-24/h6-7,11-15,17,21,25,37H,3-5,8-10,16,18H2,1-2H3/t25?,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237139

(CHEMBL4089863)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1n[nH]c2ccc(cc12)-c1ccc2ccccc2c1 Show InChI InChI=1S/C32H34N6O2/c1-6-31(39)33-27-19-28(30(40-5)20-29(27)38(4)16-15-37(2)3)34-32-25-18-24(13-14-26(25)35-36-32)23-12-11-21-9-7-8-10-22(21)17-23/h6-14,17-20H,1,15-16H2,2-5H3,(H,33,39)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards 5-hydroxytryptamine 3 receptor was determined |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306597
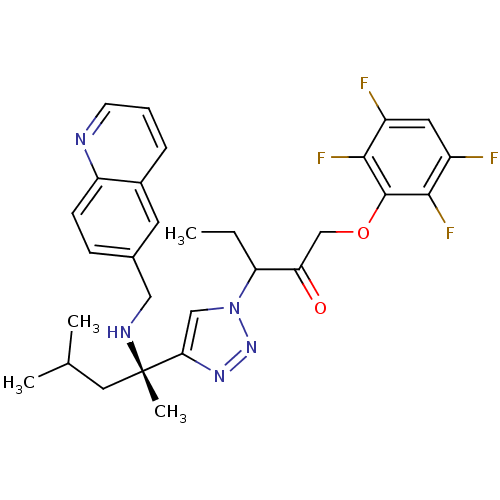
(3-(4-((S)-4-methyl-2-(quinolin-6-ylmethylamino)pen...)Show SMILES CCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@](C)(CC(C)C)NCc1ccc2ncccc2c1 |r| Show InChI InChI=1S/C29H31F4N5O2/c1-5-23(24(39)16-40-28-26(32)20(30)12-21(31)27(28)33)38-15-25(36-37-38)29(4,13-17(2)3)35-14-18-8-9-22-19(11-18)7-6-10-34-22/h6-12,15,17,23,35H,5,13-14,16H2,1-4H3/t23?,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237153
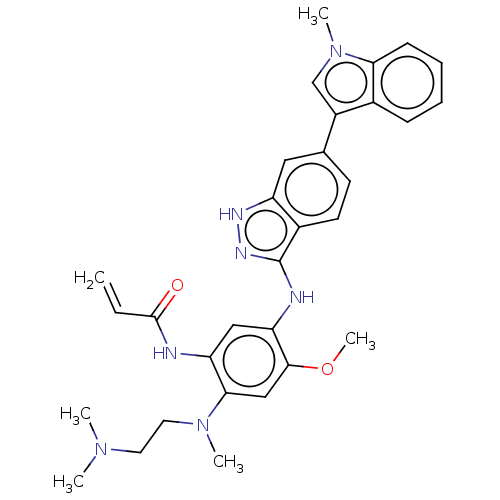
(CHEMBL4098444)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1n[nH]c2cc(ccc12)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C31H35N7O2/c1-7-30(39)32-25-17-26(29(40-6)18-28(25)37(4)15-14-36(2)3)33-31-22-13-12-20(16-24(22)34-35-31)23-19-38(5)27-11-9-8-10-21(23)27/h7-13,16-19H,1,14-15H2,2-6H3,(H,32,39)(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Inhibition of cloned isozyme, human carbonic anhydrase II |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306599
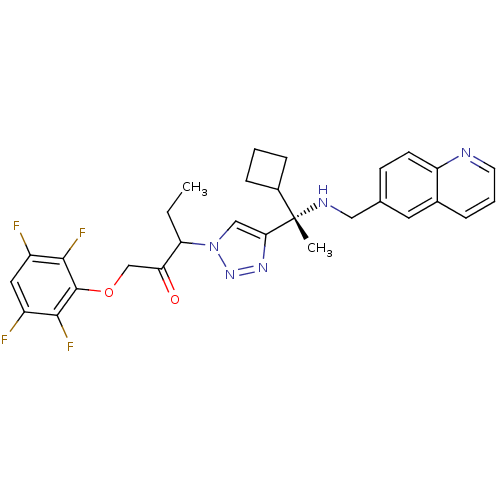
(3-(4-((S)-1-cyclobutyl-1-(quinolin-6-ylmethylamino...)Show SMILES CCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@@](C)(NCc1ccc2ncccc2c1)C1CCC1 |r| Show InChI InChI=1S/C29H29F4N5O2/c1-3-23(24(39)16-40-28-26(32)20(30)13-21(31)27(28)33)38-15-25(36-37-38)29(2,19-7-4-8-19)35-14-17-9-10-22-18(12-17)6-5-11-34-22/h5-6,9-13,15,19,23,35H,3-4,7-8,14,16H2,1-2H3/t23?,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306592
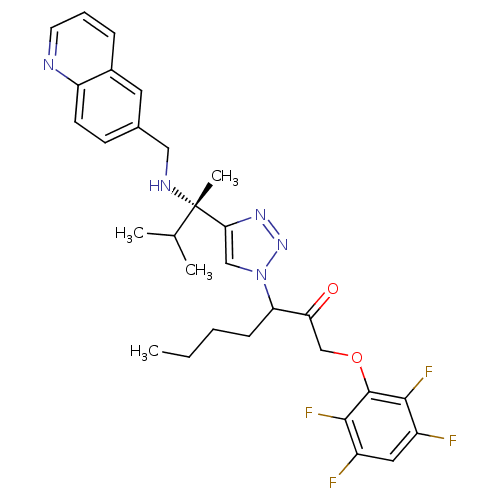
(3-(4-((S)-3-methyl-2-(quinolin-6-ylmethylamino)but...)Show SMILES CCCCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@@](C)(NCc1ccc2ncccc2c1)C(C)C |r| Show InChI InChI=1S/C30H33F4N5O2/c1-5-6-9-24(25(40)17-41-29-27(33)21(31)14-22(32)28(29)34)39-16-26(37-38-39)30(4,18(2)3)36-15-19-10-11-23-20(13-19)8-7-12-35-23/h7-8,10-14,16,18,24,36H,5-6,9,15,17H2,1-4H3/t24?,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128869
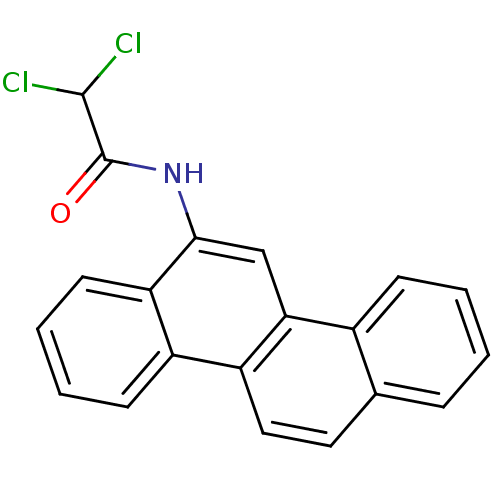
(2,2-Dichloro-N-chrysen-6-yl-acetamide | CHEMBL8805...)Show InChI InChI=1S/C20H13Cl2NO/c21-19(22)20(24)23-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11,19H,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128871
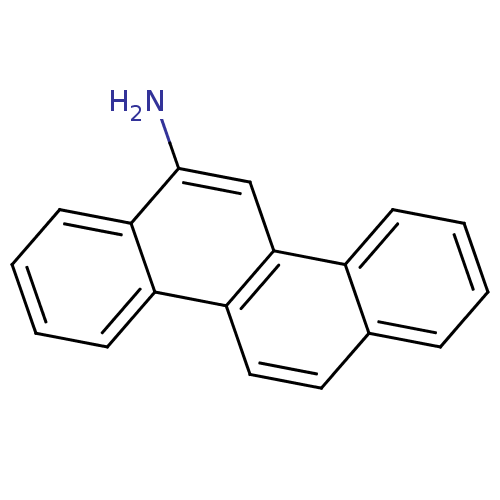
(CHEMBL313154 | Chrysen-6-ylamine)Show InChI InChI=1S/C18H13N/c19-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11H,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306595
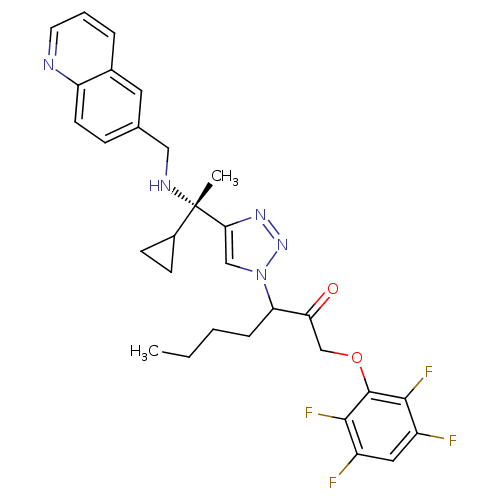
(3-(4-((S)-1-cyclopropyl-1-(quinolin-6-ylmethylamin...)Show SMILES CCCCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@@](C)(NCc1ccc2ncccc2c1)C1CC1 |r| Show InChI InChI=1S/C30H31F4N5O2/c1-3-4-7-24(25(40)17-41-29-27(33)21(31)14-22(32)28(29)34)39-16-26(37-38-39)30(2,20-9-10-20)36-15-18-8-11-23-19(13-18)6-5-12-35-23/h5-6,8,11-14,16,20,24,36H,3-4,7,9-10,15,17H2,1-2H3/t24?,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306593
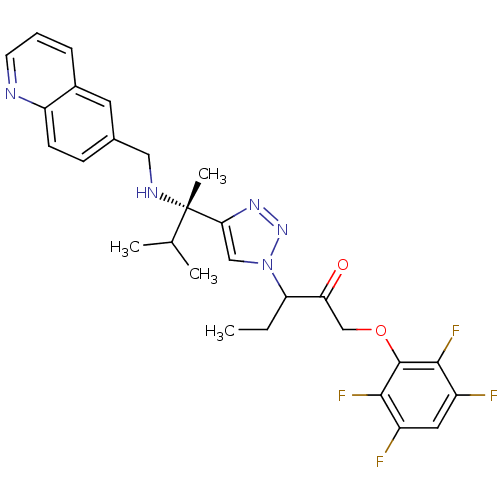
(3-(4-((S)-3-methyl-2-(quinolin-6-ylmethylamino)but...)Show SMILES CCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@@](C)(NCc1ccc2ncccc2c1)C(C)C |r| Show InChI InChI=1S/C28H29F4N5O2/c1-5-22(23(38)15-39-27-25(31)19(29)12-20(30)26(27)32)37-14-24(35-36-37)28(4,16(2)3)34-13-17-8-9-21-18(11-17)7-6-10-33-21/h6-12,14,16,22,34H,5,13,15H2,1-4H3/t22?,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306601
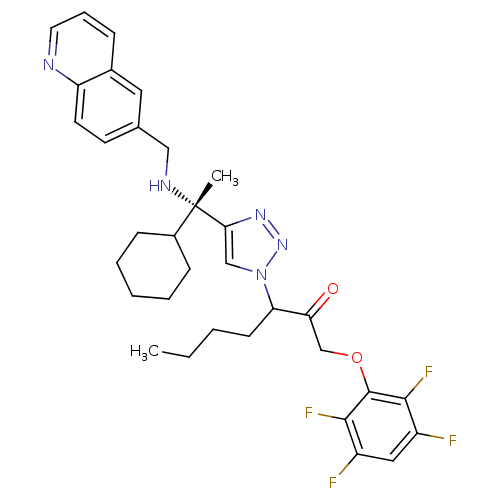
(3-(4-((S)-1-cyclohexyl-1-(quinolin-6-ylmethylamino...)Show SMILES CCCCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@@](C)(NCc1ccc2ncccc2c1)C1CCCCC1 |r| Show InChI InChI=1S/C33H37F4N5O2/c1-3-4-12-27(28(43)20-44-32-30(36)24(34)17-25(35)31(32)37)42-19-29(40-41-42)33(2,23-10-6-5-7-11-23)39-18-21-13-14-26-22(16-21)9-8-15-38-26/h8-9,13-17,19,23,27,39H,3-7,10-12,18,20H2,1-2H3/t27?,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128867
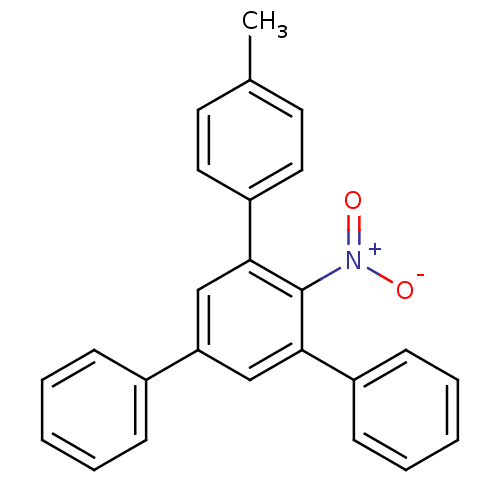
(3,5-diphenyl-4'-methyl-2-nitrobiphenyl | CHEMBL875...)Show SMILES Cc1ccc(cc1)-c1cc(cc(-c2ccccc2)c1[N+]([O-])=O)-c1ccccc1 Show InChI InChI=1S/C25H19NO2/c1-18-12-14-21(15-13-18)24-17-22(19-8-4-2-5-9-19)16-23(25(24)26(27)28)20-10-6-3-7-11-20/h2-17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128866

(2-(2,4,7-Trinitro-fluoren-9-ylidene)-malononitrile...)Show SMILES [#8-]-[#7+](=O)-c1ccc-2c(c1)\[#6](=[#6](/C#N)C#N)-c1cc(cc(c-21)-[#7+](-[#8-])=O)-[#7+](-[#8-])=O Show InChI InChI=1S/C16H5N5O6/c17-6-8(7-18)15-12-3-9(19(22)23)1-2-11(12)16-13(15)4-10(20(24)25)5-14(16)21(26)27/h1-5H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128872
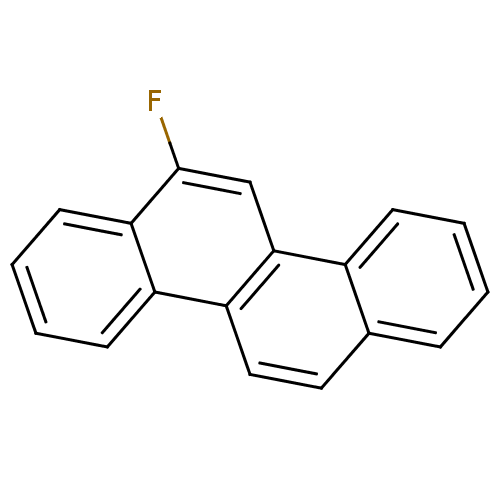
(6-Fluoro-chrysene | CHEMBL83242)Show InChI InChI=1S/C18H11F/c19-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128866

(2-(2,4,7-Trinitro-fluoren-9-ylidene)-malononitrile...)Show SMILES [#8-]-[#7+](=O)-c1ccc-2c(c1)\[#6](=[#6](/C#N)C#N)-c1cc(cc(c-21)-[#7+](-[#8-])=O)-[#7+](-[#8-])=O Show InChI InChI=1S/C16H5N5O6/c17-6-8(7-18)15-12-3-9(19(22)23)1-2-11(12)16-13(15)4-10(20(24)25)5-14(16)21(26)27/h1-5H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM236497
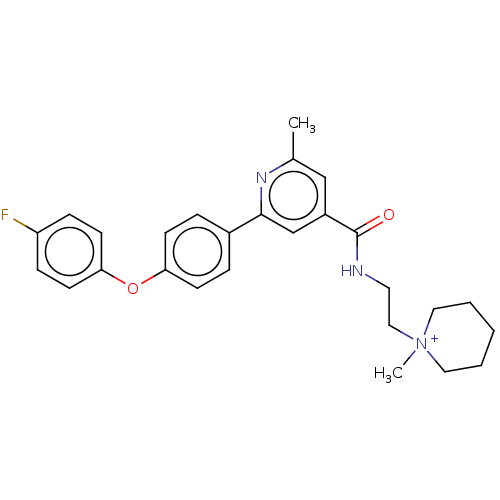
(US9388137, 1)Show SMILES Cc1cc(cc(n1)-c1ccc(Oc2ccc(F)cc2)cc1)C(=O)NCC[N+]1(C)CCCCC1 Show InChI InChI=1S/C27H30FN3O2/c1-20-18-22(27(32)29-14-17-31(2)15-4-3-5-16-31)19-26(30-20)21-6-10-24(11-7-21)33-25-12-8-23(28)9-13-25/h6-13,18-19H,3-5,14-17H2,1-2H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 6.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
US Patent
| Assay Description
On the day of experimentation, the 35 mm dish was placed on the stage of an inverted microscope equipped with a perfusion system that continuously pe... |
US Patent US9388137 (2016)
BindingDB Entry DOI: 10.7270/Q2542MG1 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128865

(6-Nitro-chrysene | CHEMBL82858)Show InChI InChI=1S/C18H11NO2/c20-19(21)18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306594
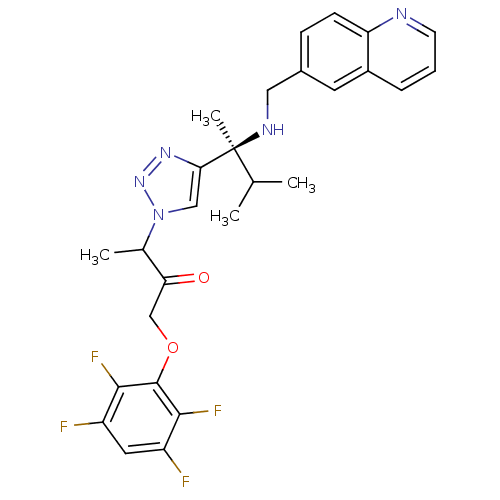
(3-(4-((S)-3-methyl-2-(quinolin-6-ylmethylamino)but...)Show SMILES CC(C)[C@](C)(NCc1ccc2ncccc2c1)c1cn(nn1)C(C)C(=O)COc1c(F)c(F)cc(F)c1F |r| Show InChI InChI=1S/C27H27F4N5O2/c1-15(2)27(4,33-12-17-7-8-21-18(10-17)6-5-9-32-21)23-13-36(35-34-23)16(3)22(37)14-38-26-24(30)19(28)11-20(29)25(26)31/h5-11,13,15-16,33H,12,14H2,1-4H3/t16?,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128870

(CHEMBL85685 | chrysene)Show InChI InChI=1S/C18H12/c1-3-7-15-13(5-1)9-11-18-16-8-4-2-6-14(16)10-12-17(15)18/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128867

(3,5-diphenyl-4'-methyl-2-nitrobiphenyl | CHEMBL875...)Show SMILES Cc1ccc(cc1)-c1cc(cc(-c2ccccc2)c1[N+]([O-])=O)-c1ccccc1 Show InChI InChI=1S/C25H19NO2/c1-18-12-14-21(15-13-18)24-17-22(19-8-4-2-5-9-19)16-23(25(24)26(27)28)20-10-6-3-7-11-20/h2-17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128868

(5-(2,4-Dichloro-phenoxy)-3-(2-fluoro-phenyl)-[1,2,...)Show SMILES Fc1ccccc1-c1nnc2c3ccccc3nc(Oc3ccc(Cl)cc3Cl)n12 Show InChI InChI=1S/C21H11Cl2FN4O/c22-12-9-10-18(15(23)11-12)29-21-25-17-8-4-2-6-14(17)20-27-26-19(28(20)21)13-5-1-3-7-16(13)24/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128867

(3,5-diphenyl-4'-methyl-2-nitrobiphenyl | CHEMBL875...)Show SMILES Cc1ccc(cc1)-c1cc(cc(-c2ccccc2)c1[N+]([O-])=O)-c1ccccc1 Show InChI InChI=1S/C25H19NO2/c1-18-12-14-21(15-13-18)24-17-22(19-8-4-2-5-9-19)16-23(25(24)26(27)28)20-10-6-3-7-11-20/h2-17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with bacterial Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128869

(2,2-Dichloro-N-chrysen-6-yl-acetamide | CHEMBL8805...)Show InChI InChI=1S/C20H13Cl2NO/c21-19(22)20(24)23-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11,19H,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128869

(2,2-Dichloro-N-chrysen-6-yl-acetamide | CHEMBL8805...)Show InChI InChI=1S/C20H13Cl2NO/c21-19(22)20(24)23-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11,19H,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with bacterial Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128868

(5-(2,4-Dichloro-phenoxy)-3-(2-fluoro-phenyl)-[1,2,...)Show SMILES Fc1ccccc1-c1nnc2c3ccccc3nc(Oc3ccc(Cl)cc3Cl)n12 Show InChI InChI=1S/C21H11Cl2FN4O/c22-12-9-10-18(15(23)11-12)29-21-25-17-8-4-2-6-14(17)20-27-26-19(28(20)21)13-5-1-3-7-16(13)24/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128868

(5-(2,4-Dichloro-phenoxy)-3-(2-fluoro-phenyl)-[1,2,...)Show SMILES Fc1ccccc1-c1nnc2c3ccccc3nc(Oc3ccc(Cl)cc3Cl)n12 Show InChI InChI=1S/C21H11Cl2FN4O/c22-12-9-10-18(15(23)11-12)29-21-25-17-8-4-2-6-14(17)20-27-26-19(28(20)21)13-5-1-3-7-16(13)24/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with bacterial Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Growth hormone-releasing hormone receptor
(Homo sapiens (Human)) | BDBM36351
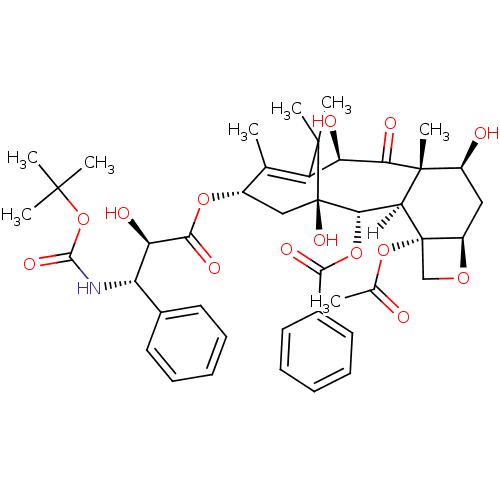
(CID148124 | Docetaxel)Show SMILES [H][C@]12[C@H](OC(=O)c3ccccc3)[C@]3(O)C[C@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)c4ccccc4)C(C)=C([C@@H](O)C(=O)[C@]1(C)[C@@H](O)C[C@H]1OC[C@@]21OC(C)=O)C3(C)C |t:39| Show InChI InChI=1S/C43H53NO14/c1-22-26(55-37(51)32(48)30(24-15-11-9-12-16-24)44-38(52)58-39(3,4)5)20-43(53)35(56-36(50)25-17-13-10-14-18-25)33-41(8,34(49)31(47)29(22)40(43,6)7)27(46)19-28-42(33,21-54-28)57-23(2)45/h9-18,26-28,30-33,35,46-48,53H,19-21H2,1-8H3,(H,44,52)/t26-,27-,28+,30-,31+,32+,33-,35-,41+,42-,43+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]JV1-42 from GHRH receptor expressed in human MX1 cells |
Proc Natl Acad Sci USA 104: 1943-6 (2007)
Article DOI: 10.1073/pnas.0610860104
BindingDB Entry DOI: 10.7270/Q28916RX |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund
Curated by ChEMBL
| Assay Description
Inhibition of wild type DDR2 (unknown origin) preincubated for 30 mins before substrate addition by FRET assay |
J Med Chem 57: 4252-62 (2014)
Article DOI: 10.1021/jm500167q
BindingDB Entry DOI: 10.7270/Q2Z039P4 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) expressed in Sf9 cells pre-incubated for 30 mins before substrate and ATP addition by homogeneous time-... |
J Med Chem 58: 6844-63 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01082
BindingDB Entry DOI: 10.7270/Q2WM1G59 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Inhibition of wild type N-terminal GST-fused human EGFR cytoplasmic domain expressed in baculovirus expression system preincubated for 30 mins follow... |
J Med Chem 60: 7725-7744 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00515
BindingDB Entry DOI: 10.7270/Q28W3GS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM153732

(K252a)Show SMILES [H]C12C[C@@](O)(C(=O)OC)[C@](C)(O1)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |TLB:13:12:11:3.2,19:35:11:3.2,THB:4:3:11:12.33.35.34,32:33:11:3.2,26:34:11:3.2| Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18?,26-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund
| Assay Description
IC50 determinations for TBK1 were performed with the KinEASE-STK assay from Cisbio according to the manufacturer's instructions. A biotinylated s... |
ACS Chem Biol 10: 289-98 (2015)
Article DOI: 10.1021/cb500908d
BindingDB Entry DOI: 10.7270/Q2WH2NRB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237140

(CHEMBL4068763)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1n[nH]c2cc(Nc3cccc(F)c3)ccc12 Show InChI InChI=1S/C28H32FN7O2/c1-6-27(37)31-23-16-24(26(38-5)17-25(23)36(4)13-12-35(2)3)32-28-21-11-10-20(15-22(21)33-34-28)30-19-9-7-8-18(29)14-19/h6-11,14-17,30H,1,12-13H2,2-5H3,(H,31,37)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards 5-hydroxytryptamine 3 receptor was determined |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards 5-hydroxytryptamine 3 receptor was determined |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Genome polyprotein
(Hepatitis C virus (HCV)) | BDBM50300504

(CHEMBL574455 | N-({3-[(5S)-5-tert-butyl-1-[(4-fluo...)Show SMILES CN(Cc1cccc2C(NS(=O)(=O)c12)=C1C(=O)[C@@H](N(Cc2ccc(F)cc2)C1=O)C(C)(C)C)S(N)(=O)=O |r,w:14.16| Show InChI InChI=1S/C24H27FN4O6S2/c1-24(2,3)22-20(30)18(23(31)29(22)12-14-8-10-16(25)11-9-14)19-17-7-5-6-15(13-28(4)37(26,34)35)21(17)36(32,33)27-19/h5-11,22,27H,12-13H2,1-4H3,(H2,26,34,35)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC
Curated by ChEMBL
| Assay Description
Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product |
Bioorg Med Chem Lett 19: 5652-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.022
BindingDB Entry DOI: 10.7270/Q2N016K0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50383274
(CHEMBL1229592 | US10167264, WZ4002 | US9670213, WZ...)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Oc2cccc(NC(=O)C=C)c2)n1)N1CCN(C)CC1 Show InChI InChI=1S/C25H27ClN6O3/c1-4-23(33)28-17-6-5-7-19(14-17)35-24-20(26)16-27-25(30-24)29-21-9-8-18(15-22(21)34-3)32-12-10-31(2)11-13-32/h4-9,14-16H,1,10-13H2,2-3H3,(H,28,33)(H,27,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Inhibition of wild type N-terminal GST-fused human EGFR cytoplasmic domain expressed in baculovirus expression system preincubated for 30 mins follow... |
J Med Chem 60: 7725-7744 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00515
BindingDB Entry DOI: 10.7270/Q28W3GS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50258974
(CHEMBL4090601)Show SMILES Nc1ncnc2n(nc(-c3ccc4ccccc4c3)c12)[C@@H]1CCCN(C1)C(=O)C=C |r| Show InChI InChI=1S/C23H22N6O/c1-2-19(30)28-11-5-8-18(13-28)29-23-20(22(24)25-14-26-23)21(27-29)17-10-9-15-6-3-4-7-16(15)12-17/h2-4,6-7,9-10,12,14,18H,1,5,8,11,13H2,(H2,24,25,26)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Inhibition of wild type N-terminal GST-fused human EGFR cytoplasmic domain expressed in baculovirus expression system preincubated for 30 mins follow... |
J Med Chem 60: 7725-7744 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00515
BindingDB Entry DOI: 10.7270/Q28W3GS9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238173

(CHEMBL4078963)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1cccs1 Show InChI InChI=1S/C23H21N5O2S2/c1-4-20(29)25-15-7-8-17(30-2)16(13-15)26-19-12-14(9-10-24-19)21-22(18-6-5-11-32-18)28-23(27-21)31-3/h4-13H,1H2,2-3H3,(H,24,26)(H,25,29)(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal GST-fused EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus using TK-substrate-biotin preincubat... |
J Med Chem 60: 5613-5637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00316
BindingDB Entry DOI: 10.7270/Q2V98BCK |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal GST-fused EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus using TK-substrate-biotin preincubat... |
J Med Chem 60: 5613-5637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00316
BindingDB Entry DOI: 10.7270/Q2V98BCK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238160
(CHEMBL4087268)Show SMILES CSc1nc(c([nH]1)-c1ccnc(Nc2cccc(NC(=O)C=C)c2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C24H20FN5OS/c1-3-21(31)28-19-6-4-5-18(14-19)27-20-13-16(11-12-26-20)23-22(29-24(30-23)32-2)15-7-9-17(25)10-8-15/h3-14H,1H2,2H3,(H,26,27)(H,28,31)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal GST-fused EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus using TK-substrate-biotin preincubat... |
J Med Chem 60: 5613-5637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00316
BindingDB Entry DOI: 10.7270/Q2V98BCK |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238165

(CHEMBL4060003)Show SMILES CSc1nc(c([nH]1)-c1ccnc(Nc2cc(NC(=O)C=C)cc(c2)N2CCOCC2)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C28H27FN6O2S/c1-3-25(36)32-22-15-21(16-23(17-22)35-10-12-37-13-11-35)31-24-14-19(8-9-30-24)27-26(33-28(34-27)38-2)18-4-6-20(29)7-5-18/h3-9,14-17H,1,10-13H2,2H3,(H,30,31)(H,32,36)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal GST-fused EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus using TK-substrate-biotin preincubat... |
J Med Chem 60: 5613-5637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00316
BindingDB Entry DOI: 10.7270/Q2V98BCK |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal GST-fused EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus using TK-substrate-biotin preincubat... |
J Med Chem 60: 5613-5637 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00316
BindingDB Entry DOI: 10.7270/Q2V98BCK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data