 Found 3513 hits with Last Name = 'fan' and Initial = 'a'
Found 3513 hits with Last Name = 'fan' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50146585
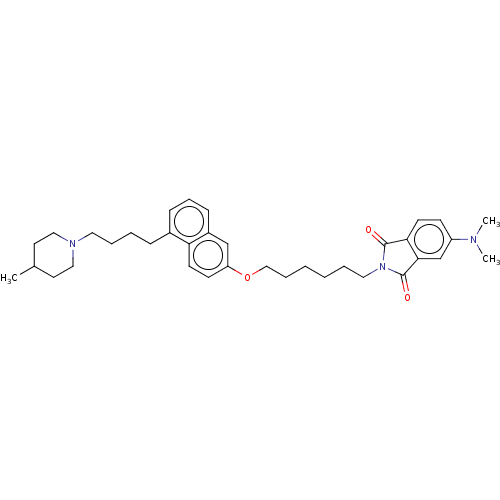
(CHEMBL3763396)Show SMILES CC1CCN(CCCCc2cccc3cc(OCCCCCCN4C(=O)c5ccc(cc5C4=O)N(C)C)ccc23)CC1 Show InChI InChI=1S/C36H47N3O3/c1-27-18-22-38(23-19-27)20-8-6-11-28-12-10-13-29-25-31(15-17-32(28)29)42-24-9-5-4-7-21-39-35(40)33-16-14-30(37(2)3)26-34(33)36(39)41/h10,12-17,25-27H,4-9,11,18-24H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50161342
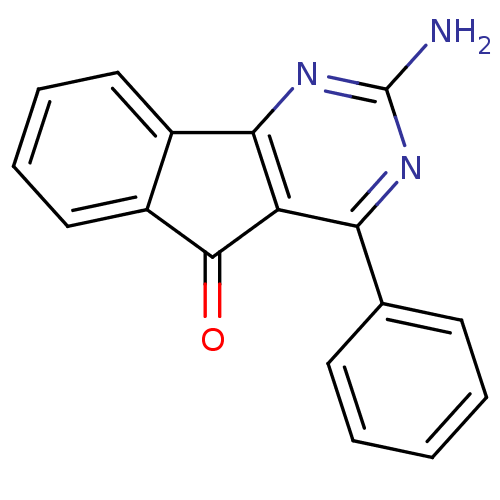
(2-Amino-4-phenyl-indeno[1,2-d]pyrimidin-5-one | 2-...)Show InChI InChI=1S/C17H11N3O/c18-17-19-14(10-6-2-1-3-7-10)13-15(20-17)11-8-4-5-9-12(11)16(13)21/h1-9H,(H2,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from A1 adenosine receptor (unknown origin) expressed in CHO cell membrane incubated for 60 mins at room temperature by NXT... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)-RAT) | BDBM50164588

(1-[1-(2-Chloro-phenyl)-cyclopropyl]-6-fluoro-2-met...)Show InChI InChI=1S/C19H19ClFNO/c1-22-9-6-12-10-16(21)17(23)11-13(12)18(22)19(7-8-19)14-4-2-3-5-15(14)20/h2-5,10-11,18,23H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ G. D'Annunzio
Curated by ChEMBL
| Assay Description
Inhibitory constant for Dopamine receptor D1-like |
J Med Chem 48: 2646-54 (2005)
Article DOI: 10.1021/jm040889k
BindingDB Entry DOI: 10.7270/Q2QR4XW2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50054088

((2R,3R)-2-(Methyl-propyl-amino)-3-phenyl-indan-5-o...)Show InChI InChI=1S/C19H23NO/c1-3-11-20(2)18-12-15-9-10-16(21)13-17(15)19(18)14-7-5-4-6-8-14/h4-10,13,18-19,21H,3,11-12H2,1-2H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity at Dopamine receptor D2 in rat striatal homogenate by [3H]-spiperone displacement. |
J Med Chem 39: 4238-46 (1996)
Article DOI: 10.1021/jm960318v
BindingDB Entry DOI: 10.7270/Q2WQ04FD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50054088

((2R,3R)-2-(Methyl-propyl-amino)-3-phenyl-indan-5-o...)Show InChI InChI=1S/C19H23NO/c1-3-11-20(2)18-12-15-9-10-16(21)13-17(15)19(18)14-7-5-4-6-8-14/h4-10,13,18-19,21H,3,11-12H2,1-2H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity at Dopamine receptor D2 in rat striatal homogenate by [3H]-spiperone displacement. |
J Med Chem 39: 4238-46 (1996)
Article DOI: 10.1021/jm960318v
BindingDB Entry DOI: 10.7270/Q2WQ04FD |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50251369
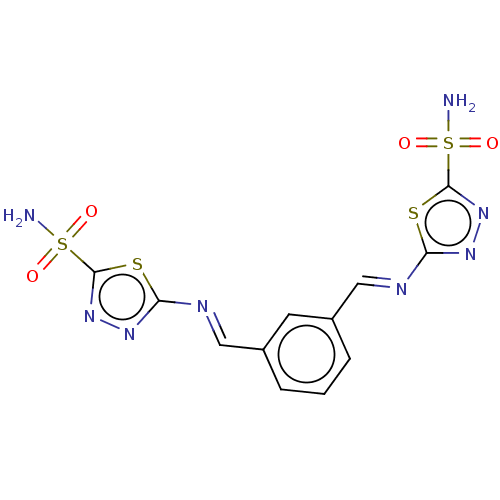
(CHEMBL4084006)Show SMILES NS(=O)(=O)c1nnc(\N=C\c2cccc(\C=N\c3nnc(s3)S(N)(=O)=O)c2)s1 Show InChI InChI=1S/C12H10N8O4S4/c13-27(21,22)11-19-17-9(25-11)15-5-7-2-1-3-8(4-7)6-16-10-18-20-12(26-10)28(14,23)24/h1-6H,(H2,13,21,22)(H2,14,23,24)/b15-5+,16-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Adiyaman University, 02040 Adiyaman, Turkey. Electronic address: akocaksuleyman@gmail.com.
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 incubated for 10 mins by stopped flow CO2 hydrase assay |
Bioorg Med Chem 25: 3093-3097 (2017)
Article DOI: 10.1016/j.bmc.2017.03.063
BindingDB Entry DOI: 10.7270/Q25T3NXJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50258734
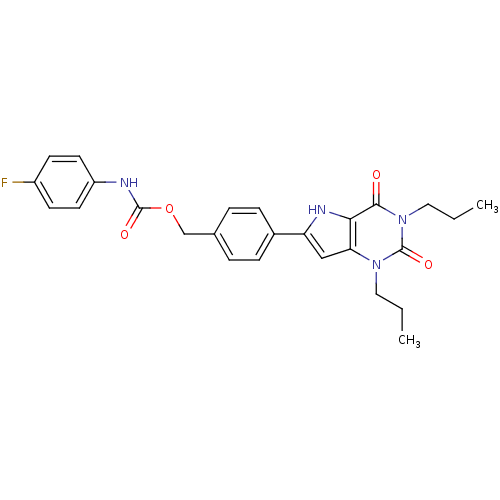
(CHEMBL512276 | [4-(2,3,4,5-Tetrahydro-2,4-dioxo-1,...)Show SMILES CCCn1c2cc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(COC(=O)Nc2ccc(F)cc2)cc1 Show InChI InChI=1S/C26H27FN4O4/c1-3-13-30-22-15-21(29-23(22)24(32)31(14-4-2)26(30)34)18-7-5-17(6-8-18)16-35-25(33)28-20-11-9-19(27)10-12-20/h5-12,15,29H,3-4,13-14,16H2,1-2H3,(H,28,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human recombinant adenosine A1 receptor expressed in CHO cells by beta scintillation counter |
Bioorg Med Chem 17: 3618-29 (2009)
Article DOI: 10.1016/j.bmc.2009.03.062
BindingDB Entry DOI: 10.7270/Q2Q81D07 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50054084

((2R,3R)-2-(Allyl-methyl-amino)-3-phenyl-indan-5-ol...)Show InChI InChI=1S/C19H21NO/c1-3-11-20(2)18-12-15-9-10-16(21)13-17(15)19(18)14-7-5-4-6-8-14/h3-10,13,18-19,21H,1,11-12H2,2H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity at Dopamine receptor D2 in rat striatal homogenate by [3H]-spiperone displacement. |
J Med Chem 39: 4238-46 (1996)
Article DOI: 10.1021/jm960318v
BindingDB Entry DOI: 10.7270/Q2WQ04FD |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM50613242
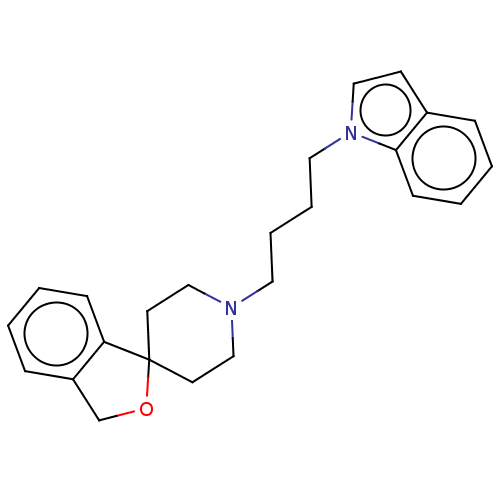
(CHEMBL5271751) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50054084

((2R,3R)-2-(Allyl-methyl-amino)-3-phenyl-indan-5-ol...)Show InChI InChI=1S/C19H21NO/c1-3-11-20(2)18-12-15-9-10-16(21)13-17(15)19(18)14-7-5-4-6-8-14/h3-10,13,18-19,21H,1,11-12H2,2H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity at Dopamine receptor D2 in rat striatal homogenate by [3H]-spiperone displacement. |
J Med Chem 39: 4238-46 (1996)
Article DOI: 10.1021/jm960318v
BindingDB Entry DOI: 10.7270/Q2WQ04FD |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50268107
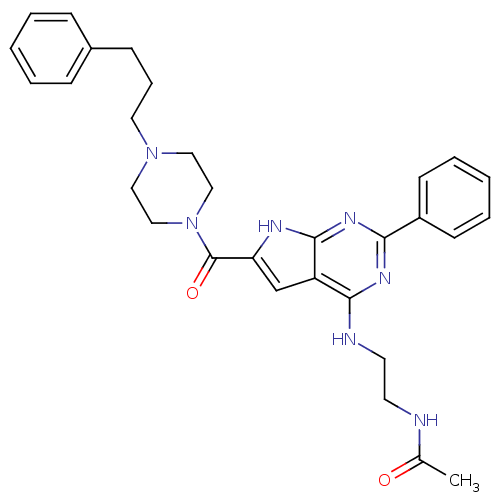
(CHEMBL485862 | CHEMBL500634 | N-(2-(2-phenyl-6-(4-...)Show SMILES CC(=O)NCCNc1nc(nc2[nH]c(cc12)C(=O)N1CCN(CCCc2ccccc2)CC1)-c1ccccc1 Show InChI InChI=1S/C30H35N7O2/c1-22(38)31-14-15-32-28-25-21-26(33-29(25)35-27(34-28)24-12-6-3-7-13-24)30(39)37-19-17-36(18-20-37)16-8-11-23-9-4-2-5-10-23/h2-7,9-10,12-13,21H,8,11,14-20H2,1H3,(H,31,38)(H2,32,33,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human A2B receptor |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01431
BindingDB Entry DOI: 10.7270/Q2JQ14S5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50251369

(CHEMBL4084006)Show SMILES NS(=O)(=O)c1nnc(\N=C\c2cccc(\C=N\c3nnc(s3)S(N)(=O)=O)c2)s1 Show InChI InChI=1S/C12H10N8O4S4/c13-27(21,22)11-19-17-9(25-11)15-5-7-2-1-3-8(4-7)6-16-10-18-20-12(26-10)28(14,23)24/h1-6H,(H2,13,21,22)(H2,14,23,24)/b15-5+,16-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Adiyaman University, 02040 Adiyaman, Turkey. Electronic address: akocaksuleyman@gmail.com.
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 incubated for 10 mins by stopped flow CO2 hydrase assay |
Bioorg Med Chem 25: 3093-3097 (2017)
Article DOI: 10.1016/j.bmc.2017.03.063
BindingDB Entry DOI: 10.7270/Q25T3NXJ |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50613240
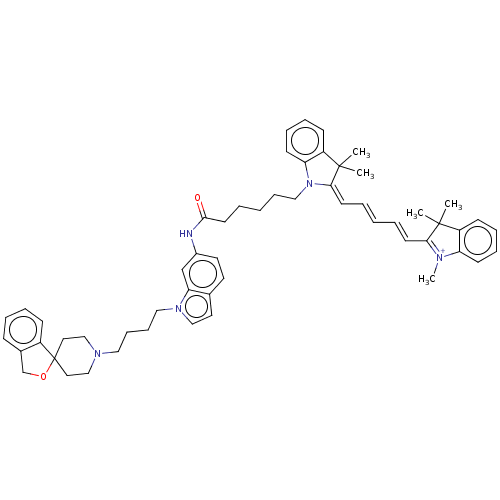
(CHEMBL5278003)Show SMILES [I-].C[N+]1=C(\C=C\C=C\C=C2\N(CCCCCC(=O)Nc3ccc4ccn(CCCCN5CCC6(CC5)OCc5ccccc65)c4c3)c3ccccc3C2(C)C)C(C)(C)c2ccccc12 |c:1| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50054089
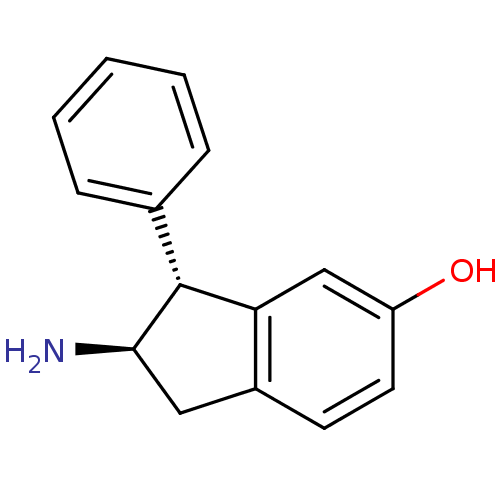
((2R,3R)-2-Amino-3-phenyl-indan-5-ol | CHEMBL135224)Show InChI InChI=1S/C15H15NO/c16-14-8-11-6-7-12(17)9-13(11)15(14)10-4-2-1-3-5-10/h1-7,9,14-15,17H,8,16H2/t14-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity at Dopamine receptor D2 in rat striatal homogenate by [3H]-spiperone displacement. |
J Med Chem 39: 4238-46 (1996)
Article DOI: 10.1021/jm960318v
BindingDB Entry DOI: 10.7270/Q2WQ04FD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50054089

((2R,3R)-2-Amino-3-phenyl-indan-5-ol | CHEMBL135224)Show InChI InChI=1S/C15H15NO/c16-14-8-11-6-7-12(17)9-13(11)15(14)10-4-2-1-3-5-10/h1-7,9,14-15,17H,8,16H2/t14-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity at Dopamine receptor D2 in rat striatal homogenate by [3H]-spiperone displacement. |
J Med Chem 39: 4238-46 (1996)
Article DOI: 10.1021/jm960318v
BindingDB Entry DOI: 10.7270/Q2WQ04FD |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50428089
(CHEMBL2323577)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@H](CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H](C)C(=O)NCC(=O)N[C@H](CC(N)=O)Cc1ccccc1)Cc1ccccc1 |r| Show InChI InChI=1S/C48H59N9O10/c1-29(53-47(66)39(49)23-33-13-17-37(58)18-14-33)45(64)51-28-44(63)56-36(22-32-11-7-4-8-12-32)26-42(61)57-40(24-34-15-19-38(59)20-16-34)48(67)54-30(2)46(65)52-27-43(62)55-35(25-41(50)60)21-31-9-5-3-6-10-31/h3-20,29-30,35-36,39-40,58-59H,21-28,49H2,1-2H3,(H2,50,60)(H,51,64)(H,52,65)(H,53,66)(H,54,67)(H,55,62)(H,56,63)(H,57,61)/t29-,30-,35+,36+,39+,40+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]Deltorphin from DOR in Sprague-Dawley rat brain membrane after 180 mins by scintillation counting analysis |
J Med Chem 56: 3419-23 (2013)
Article DOI: 10.1021/jm301456c
BindingDB Entry DOI: 10.7270/Q2X63P9H |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity at Dopamine receptor D1 in rat neostriatum by [3H]-SCH- 23390 displacement. |
J Med Chem 39: 4238-46 (1996)
Article DOI: 10.1021/jm960318v
BindingDB Entry DOI: 10.7270/Q2WQ04FD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding Affinity was determined against Dopamine receptor D1 in rat striatal membranes using [3H]- SCH 23390 radioligand. |
J Med Chem 43: 599-608 (2000)
Article DOI: 10.1021/jm991034o
BindingDB Entry DOI: 10.7270/Q2X35161 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50054084

((2R,3R)-2-(Allyl-methyl-amino)-3-phenyl-indan-5-ol...)Show InChI InChI=1S/C19H21NO/c1-3-11-20(2)18-12-15-9-10-16(21)13-17(15)19(18)14-7-5-4-6-8-14/h3-10,13,18-19,21H,1,11-12H2,2H3/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity at Dopamine receptor D1 in rat neostriatum by [3H]-SCH- 23390 displacement. |
J Med Chem 39: 4238-46 (1996)
Article DOI: 10.1021/jm960318v
BindingDB Entry DOI: 10.7270/Q2WQ04FD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50054084

((2R,3R)-2-(Allyl-methyl-amino)-3-phenyl-indan-5-ol...)Show InChI InChI=1S/C19H21NO/c1-3-11-20(2)18-12-15-9-10-16(21)13-17(15)19(18)14-7-5-4-6-8-14/h3-10,13,18-19,21H,1,11-12H2,2H3/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity at Dopamine receptor D1 in rat neostriatum by [3H]-SCH- 23390 displacement. |
J Med Chem 39: 4238-46 (1996)
Article DOI: 10.1021/jm960318v
BindingDB Entry DOI: 10.7270/Q2WQ04FD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21014
((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C46H56N10O10/c1-27(51-43(63)35(47)21-31-13-17-33(57)18-14-31)41(61)49-25-39(59)53-37(23-29-9-5-3-6-10-29)45(65)55-56-46(66)38(24-30-11-7-4-8-12-30)54-40(60)26-50-42(62)28(2)52-44(64)36(48)22-32-15-19-34(58)20-16-32/h3-20,27-28,35-38,57-58H,21-26,47-48H2,1-2H3,(H,49,61)(H,50,62)(H,51,63)(H,52,64)(H,53,59)(H,54,60)(H,55,65)(H,56,66)/t27-,28-,35+,36+,37+,38+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat whole brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 858-863 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00210
BindingDB Entry DOI: 10.7270/Q2S1853J |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21123
((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C23H29N5O5/c1-14(27-23(33)18(24)11-16-7-9-17(29)10-8-16)22(32)26-13-20(30)28-19(21(25)31)12-15-5-3-2-4-6-15/h2-10,14,18-19,29H,11-13,24H2,1H3,(H2,25,31)(H,26,32)(H,27,33)(H,28,30)/t14-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biological Research Centre of the Hungarian Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes after 60 mins by liquid scintillation analysis |
Eur J Med Chem 178: 571-588 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.037
BindingDB Entry DOI: 10.7270/Q2Z60SD8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50258672
(CHEMBL513681 | [4-(2,3,4,5-Tetrahydro-1,3-dimethyl...)Show SMILES Cn1c2cc([nH]c2c(=O)n(C)c1=O)-c1ccc(COC(=O)Nc2ccsc2)cc1 Show InChI InChI=1S/C20H18N4O4S/c1-23-16-9-15(22-17(16)18(25)24(2)20(23)27)13-5-3-12(4-6-13)10-28-19(26)21-14-7-8-29-11-14/h3-9,11,22H,10H2,1-2H3,(H,21,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human recombinant adenosine A2B receptor expressed in HEK293 cells by beta scintillation counter |
Bioorg Med Chem 17: 3618-29 (2009)
Article DOI: 10.1016/j.bmc.2009.03.062
BindingDB Entry DOI: 10.7270/Q2Q81D07 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50054091

((2R,3R)-2-Dipropylamino-3-phenyl-indan-5-ol | CHEM...)Show InChI InChI=1S/C21H27NO/c1-3-12-22(13-4-2)20-14-17-10-11-18(23)15-19(17)21(20)16-8-6-5-7-9-16/h5-11,15,20-21,23H,3-4,12-14H2,1-2H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity at Dopamine receptor D2 in rat striatal homogenate by [3H]-spiperone displacement. |
J Med Chem 39: 4238-46 (1996)
Article DOI: 10.1021/jm960318v
BindingDB Entry DOI: 10.7270/Q2WQ04FD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50054091

((2R,3R)-2-Dipropylamino-3-phenyl-indan-5-ol | CHEM...)Show InChI InChI=1S/C21H27NO/c1-3-12-22(13-4-2)20-14-17-10-11-18(23)15-19(17)21(20)16-8-6-5-7-9-16/h5-11,15,20-21,23H,3-4,12-14H2,1-2H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity at Dopamine receptor D2 in rat striatal homogenate by [3H]-spiperone displacement. |
J Med Chem 39: 4238-46 (1996)
Article DOI: 10.1021/jm960318v
BindingDB Entry DOI: 10.7270/Q2WQ04FD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50054088

((2R,3R)-2-(Methyl-propyl-amino)-3-phenyl-indan-5-o...)Show InChI InChI=1S/C19H23NO/c1-3-11-20(2)18-12-15-9-10-16(21)13-17(15)19(18)14-7-5-4-6-8-14/h4-10,13,18-19,21H,3,11-12H2,1-2H3/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity at Dopamine receptor D1 in rat neostriatum by [3H]-SCH- 23390 displacement. |
J Med Chem 39: 4238-46 (1996)
Article DOI: 10.1021/jm960318v
BindingDB Entry DOI: 10.7270/Q2WQ04FD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50054088

((2R,3R)-2-(Methyl-propyl-amino)-3-phenyl-indan-5-o...)Show InChI InChI=1S/C19H23NO/c1-3-11-20(2)18-12-15-9-10-16(21)13-17(15)19(18)14-7-5-4-6-8-14/h4-10,13,18-19,21H,3,11-12H2,1-2H3/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity at Dopamine receptor D1 in rat neostriatum by [3H]-SCH- 23390 displacement. |
J Med Chem 39: 4238-46 (1996)
Article DOI: 10.1021/jm960318v
BindingDB Entry DOI: 10.7270/Q2WQ04FD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50595851

(CHEMBL5192735)Show SMILES CCCC[C@@H](NC(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@H](CCCNC(N)=N)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00237
BindingDB Entry DOI: 10.7270/Q21Z48F7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50584560
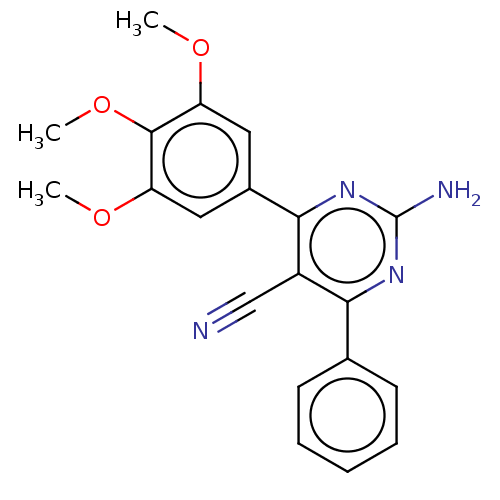
(CHEMBL5077370)Show SMILES COc1cc(cc(OC)c1OC)-c1nc(N)nc(-c2ccccc2)c1C#N | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]HZ241385 from human A2AAR expressed in HeLa cell membrane incubated for 30 mins by microbeta trilux scintillation counter analysi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317217
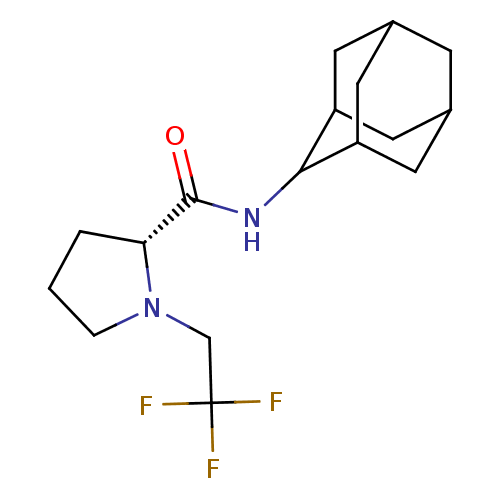
((2R)-N-(adamantan-2-yl)-1-(2,2,2-trifluoroethyl)py...)Show SMILES FC(F)(F)CN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:9.10,TLB:19:18:22:15.14.13,19:14:17.18.20:22,THB:12:13:17.18.20:22,13:14:17:20.21.22,13:21:17:15.19.14,(27.97,-20.63,;27.2,-21.96,;25.66,-21.96,;26.42,-20.62,;27.97,-23.29,;27.07,-24.54,;25.53,-24.56,;25.07,-26.02,;26.31,-26.92,;27.52,-25.95,;29.03,-26.46,;30.37,-25.71,;29,-28,;27.66,-28.74,;27.65,-30.27,;26.25,-30.63,;24.91,-30.13,;23.71,-31.41,;25.22,-30.99,;26.63,-31.56,;25.21,-29.4,;26.26,-28.16,;24.9,-28.65,)| Show InChI InChI=1S/C17H25F3N2O/c18-17(19,20)9-22-3-1-2-14(22)16(23)21-15-12-5-10-4-11(7-12)8-13(15)6-10/h10-15H,1-9H2,(H,21,23)/t10?,11?,12?,13?,14-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50375415
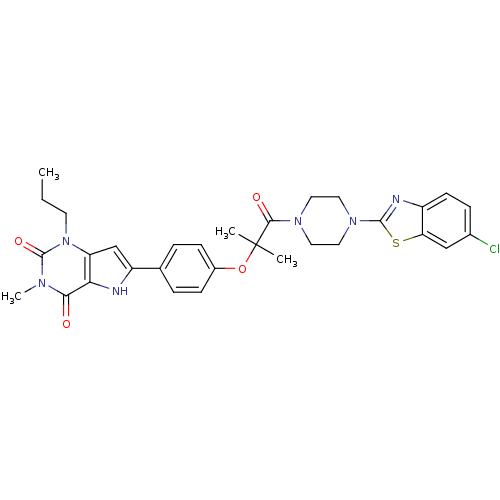
(CHEMBL259479)Show SMILES CCCn1c2cc([nH]c2c(=O)n(C)c1=O)-c1ccc(OC(C)(C)C(=O)N2CCN(CC2)c2nc3ccc(Cl)cc3s2)cc1 Show InChI InChI=1S/C31H33ClN6O4S/c1-5-12-38-24-18-23(33-26(24)27(39)35(4)30(38)41)19-6-9-21(10-7-19)42-31(2,3)28(40)36-13-15-37(16-14-36)29-34-22-11-8-20(32)17-25(22)43-29/h6-11,17-18,33H,5,12-16H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bari
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A2B receptor expressed in HEK293 cells |
Bioorg Med Chem 16: 2852-69 (2008)
Checked by Author
Article DOI: 10.1016/j.bmc.2008.01.002
BindingDB Entry DOI: 10.7270/Q2G73FMZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29864
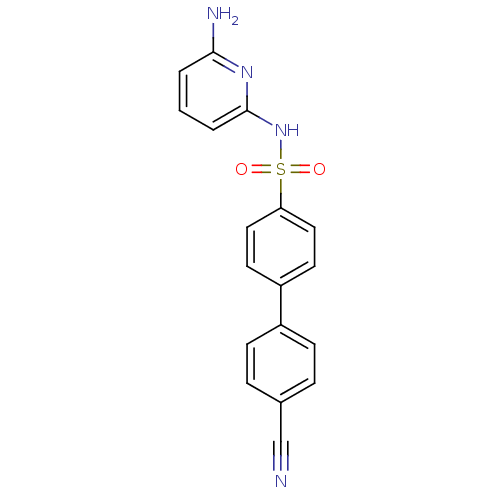
(N-(Pyridin-2-yl) arylsulfonamide, 26)Show SMILES Nc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C18H14N4O2S/c19-12-13-4-6-14(7-5-13)15-8-10-16(11-9-15)25(23,24)22-18-3-1-2-17(20)21-18/h1-11H,(H3,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317218
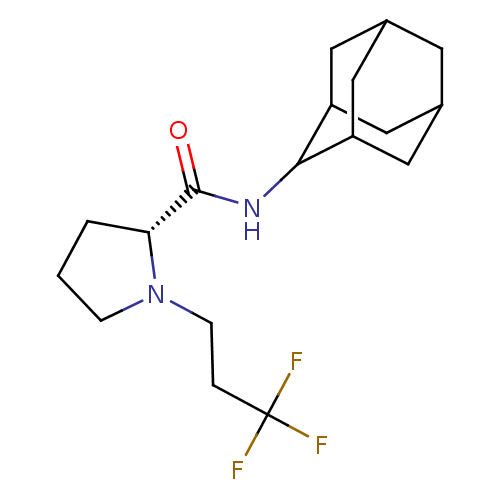
((2R)-N-(adamantan-2-yl)-1-(3,3,3-trifluoropropyl)p...)Show SMILES FC(F)(F)CCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:10.11,TLB:20:19:23:16.15.14,20:15:18.19.21:23,THB:13:14:18.19.21:23,14:15:18:21.22.23,14:22:18:16.20.15,(-5.42,-32.99,;-4.79,-34.4,;-3.25,-34.55,;-4.03,-33.05,;-5.69,-35.65,;-5.05,-37.05,;-5.95,-38.3,;-7.48,-38.31,;-7.95,-39.77,;-6.71,-40.68,;-5.5,-39.7,;-3.99,-40.21,;-2.65,-39.46,;-4.02,-41.75,;-5.36,-42.5,;-5.37,-44.03,;-6.77,-44.38,;-8.11,-43.89,;-9.31,-45.17,;-7.8,-44.74,;-6.39,-45.31,;-7.81,-43.16,;-6.76,-41.92,;-8.12,-42.4,)| Show InChI InChI=1S/C18H27F3N2O/c19-18(20,21)3-5-23-4-1-2-15(23)17(24)22-16-13-7-11-6-12(9-13)10-14(16)8-11/h11-16H,1-10H2,(H,22,24)/t11?,12?,13?,14?,15-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317221
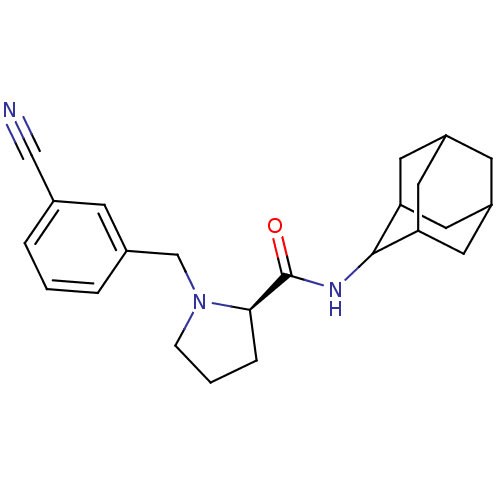
((2R)-N-(adamantan-2-yl)-1-[(3-cyanophenyl)methyl]p...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1Cc1cccc(c1)C#N |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:3:4:7:10.11.12,3:11:7:5.9.4,2:3:7.8.10:12,(29.81,-42.16,;28.46,-42.91,;28.44,-44.45,;27.09,-45.2,;27.08,-46.73,;25.68,-47.08,;24.34,-46.59,;23.15,-47.87,;24.65,-47.44,;26.06,-48.01,;24.64,-45.86,;25.69,-44.62,;24.33,-45.1,;26.95,-42.4,;25.74,-43.38,;24.5,-42.47,;24.97,-41.02,;26.5,-41,;27.4,-39.75,;26.63,-38.41,;25.1,-38.41,;24.33,-37.08,;25.11,-35.74,;26.65,-35.75,;27.41,-37.09,;27.43,-34.42,;28.2,-33.09,)| Show InChI InChI=1S/C23H29N3O/c24-13-15-3-1-4-16(7-15)14-26-6-2-5-21(26)23(27)25-22-19-9-17-8-18(11-19)12-20(22)10-17/h1,3-4,7,17-22H,2,5-6,8-12,14H2,(H,25,27)/t17?,18?,19?,20?,21-,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317213
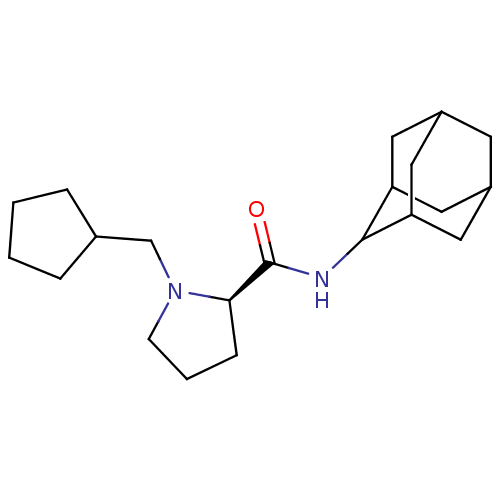
((2R)-N-(adamantan-2-yl)-1-(cyclopentylmethyl)pyrro...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(25.66,-12.64,;24.31,-13.39,;24.29,-14.93,;22.94,-15.67,;22.93,-17.2,;21.53,-17.56,;20.2,-17.06,;19,-18.34,;20.5,-17.92,;21.92,-18.49,;20.5,-16.33,;21.54,-15.09,;20.18,-15.58,;22.8,-12.88,;21.59,-13.85,;20.36,-12.95,;20.82,-11.49,;22.35,-11.47,;23.25,-10.22,;22.62,-8.82,;23.38,-7.49,;22.34,-6.35,;20.94,-6.98,;21.1,-8.51,)| Show InChI InChI=1S/C21H34N2O/c24-21(19-6-3-7-23(19)13-14-4-1-2-5-14)22-20-17-9-15-8-16(11-17)12-18(20)10-15/h14-20H,1-13H2,(H,22,24)/t15?,16?,17?,18?,19-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50317211
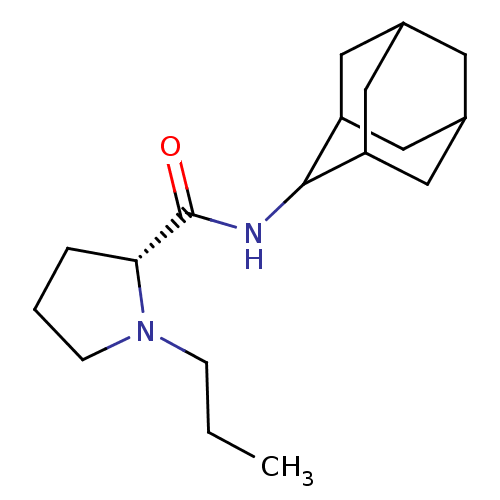
((2R)-N-(adamantan-2-yl)-1-propylpyrrolidine-2-carb...)Show SMILES CCCN1CCC[C@@H]1C(=O)NC1C2CC3CC(C2)CC1C3 |r,wU:7.8,TLB:17:16:20:13.12.11,17:12:15.16.18:20,THB:10:11:15.16.18:20,11:12:15:18.19.20,11:19:15:13.17.12,(3.7,-6.03,;2.79,-7.28,;3.42,-8.69,;2.52,-9.94,;.99,-9.95,;.52,-11.41,;1.76,-12.31,;2.97,-11.34,;4.48,-11.85,;5.83,-11.1,;4.45,-13.39,;3.11,-14.13,;3.1,-15.67,;1.7,-16.02,;.36,-15.52,;-.84,-16.81,;.67,-16.38,;2.08,-16.95,;.66,-14.8,;1.71,-13.55,;.35,-14.04,)| Show InChI InChI=1S/C18H30N2O/c1-2-5-20-6-3-4-16(20)18(21)19-17-14-8-12-7-13(10-14)11-15(17)9-12/h12-17H,2-11H2,1H3,(H,19,21)/t12?,13?,14?,15?,16-,17?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes by liquid scintillation counting based competition binding assay |
ACS Med Chem Lett 5: 1032-6 (2014)
Article DOI: 10.1021/ml500241n
BindingDB Entry DOI: 10.7270/Q23T9JS5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50207816
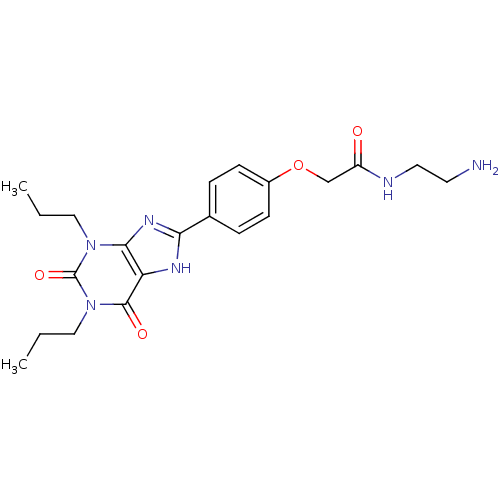
(CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCN)cc1 Show InChI InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]HZ241385 from human A2AAR expressed in HeLa cell membrane incubated for 30 mins by microbeta trilux scintillation counter analysi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50273311
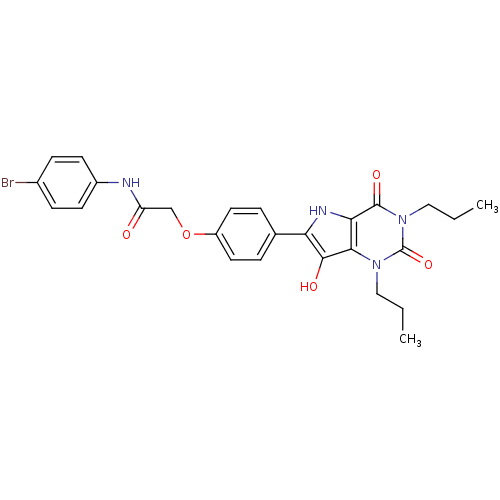
(CHEMBL456125 | N-(4-bromophenyl)-2-(4-(7-hydroxy-2...)Show SMILES CCCn1c2c(O)c([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)Nc2ccc(Br)cc2)cc1 Show InChI InChI=1S/C26H27BrN4O5/c1-3-13-30-23-22(25(34)31(14-4-2)26(30)35)29-21(24(23)33)16-5-11-19(12-6-16)36-15-20(32)28-18-9-7-17(27)8-10-18/h5-12,29,33H,3-4,13-15H2,1-2H3,(H,28,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Bari
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human cloned adenosine A2B receptor expressed in HEK293 cells |
Bioorg Med Chem 16: 9780-9 (2008)
Article DOI: 10.1016/j.bmc.2008.09.067
BindingDB Entry DOI: 10.7270/Q21Z448B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50375415

(CHEMBL259479)Show SMILES CCCn1c2cc([nH]c2c(=O)n(C)c1=O)-c1ccc(OC(C)(C)C(=O)N2CCN(CC2)c2nc3ccc(Cl)cc3s2)cc1 Show InChI InChI=1S/C31H33ClN6O4S/c1-5-12-38-24-18-23(33-26(24)27(39)35(4)30(38)41)19-6-9-21(10-7-19)42-31(2,3)28(40)36-13-15-37(16-14-36)29-34-22-11-8-20(32)17-25(22)43-29/h6-11,17-18,33H,5,12-16H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Bari
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A2B receptor expressed in HEK293 cells |
Bioorg Med Chem 16: 2852-69 (2008)
Checked by Author
Article DOI: 10.1016/j.bmc.2008.01.002
BindingDB Entry DOI: 10.7270/Q2G73FMZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317224
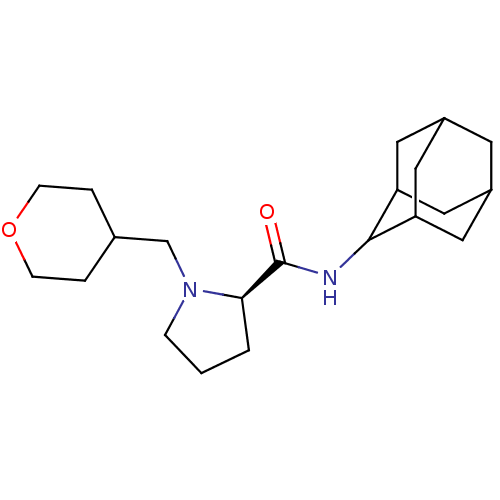
((2R)-N-(adamantan-2-yl)-1-(oxan-4-ylmethyl)pyrroli...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1CC1CCOCC1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:2:3:7.8.10:12,3:4:7:10.11.12,3:11:7:5.9.4,(16.19,2.36,;14.84,1.61,;14.82,.07,;13.47,-.68,;13.46,-2.21,;12.06,-2.57,;10.72,-2.07,;9.51,-3.36,;11.02,-2.93,;12.44,-3.5,;11.01,-1.34,;12.06,-.09,;10.7,-.58,;13.33,2.12,;12.11,1.14,;10.87,2.05,;11.33,3.52,;12.87,3.53,;13.64,4.87,;15.19,4.87,;15.96,3.52,;17.5,3.52,;18.28,4.86,;17.51,6.2,;15.96,6.21,)| Show InChI InChI=1S/C21H34N2O2/c24-21(19-2-1-5-23(19)13-14-3-6-25-7-4-14)22-20-17-9-15-8-16(11-17)12-18(20)10-15/h14-20H,1-13H2,(H,22,24)/t15?,16?,17?,18?,19-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50317223
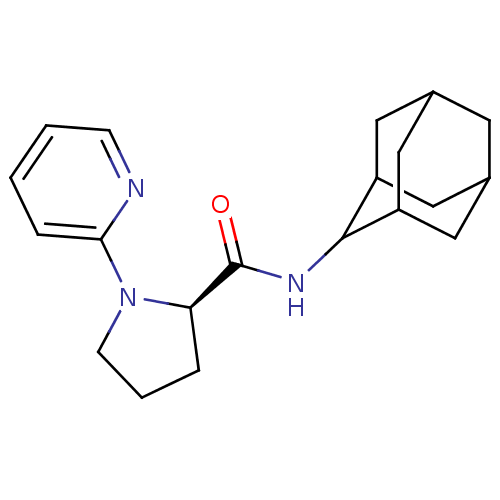
((2R)-N-(adamantan-2-yl)-1-(pyridin-2-yl)pyrrolidin...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)[C@H]1CCCN1c1ccccn1 |r,wU:13.15,TLB:9:8:12:5.4.3,9:4:7.8.10:12,THB:3:4:7:10.11.12,3:11:7:5.9.4,2:3:7.8.10:12,(6.04,2.3,;4.69,1.55,;4.66,.01,;3.32,-.73,;3.31,-2.26,;1.91,-2.62,;.57,-2.12,;-.63,-3.4,;.88,-2.98,;2.29,-3.54,;.87,-1.39,;1.92,-.15,;.56,-.63,;3.18,2.06,;1.97,1.09,;.73,1.99,;1.2,3.45,;2.72,3.47,;4.03,4.27,;4,5.82,;5.31,6.62,;6.67,5.87,;6.7,4.35,;5.39,3.54,)| Show InChI InChI=1S/C20H27N3O/c24-20(17-4-3-7-23(17)18-5-1-2-6-21-18)22-19-15-9-13-8-14(11-15)12-16(19)10-13/h1-2,5-6,13-17,19H,3-4,7-12H2,(H,22,24)/t13?,14?,15?,16?,17-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human 11-beta-HSD1 |
Bioorg Med Chem Lett 20: 2897-902 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.032
BindingDB Entry DOI: 10.7270/Q2NV9JDJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50161342

(2-Amino-4-phenyl-indeno[1,2-d]pyrimidin-5-one | 2-...)Show InChI InChI=1S/C17H11N3O/c18-17-19-14(10-6-2-1-3-7-10)13-15(20-17)11-8-4-5-9-12(11)16(13)21/h1-9H,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]SCH-58261 from A2A adenosine receptor (unknown origin) expressed in HEK cell membrane incubated for 60 mins at room temperature b... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50613231
(CHEMBL5275443)Show SMILES COc1ccc(C)cc1NC(=O)OC1CC2CCCC(C1)N2CCCCCCCCCCS(=O)(=O)c1cccc2c(cccc12)N(C)C |TLB:12:13:21:16.17.18| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50428089

(CHEMBL2323577)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@H](CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H](C)C(=O)NCC(=O)N[C@H](CC(N)=O)Cc1ccccc1)Cc1ccccc1 |r| Show InChI InChI=1S/C48H59N9O10/c1-29(53-47(66)39(49)23-33-13-17-37(58)18-14-33)45(64)51-28-44(63)56-36(22-32-11-7-4-8-12-32)26-42(61)57-40(24-34-15-19-38(59)20-16-34)48(67)54-30(2)46(65)52-27-43(62)55-35(25-41(50)60)21-31-9-5-3-6-10-31/h3-20,29-30,35-36,39-40,58-59H,21-28,49H2,1-2H3,(H2,50,60)(H,51,64)(H,52,65)(H,53,66)(H,54,67)(H,55,62)(H,56,63)(H,57,61)/t29-,30-,35+,36+,39+,40+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Sprague-Dawley rat brain membrane after 180 mins by scintillation counting analysis |
J Med Chem 56: 3419-23 (2013)
Article DOI: 10.1021/jm301456c
BindingDB Entry DOI: 10.7270/Q2X63P9H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50595850

(CHEMBL5188021)Show SMILES CCCC[C@@H](NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@H](CCCNC(N)=N)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00237
BindingDB Entry DOI: 10.7270/Q21Z48F7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50375499
(CHEMBL259319)Show SMILES O=C(Nc1nc(-c2ccccc2)c(C#N)c(n1)-c1ccc2OCOc2c1)C1CCCC1 Show InChI InChI=1S/C24H20N4O3/c25-13-18-21(15-6-2-1-3-7-15)26-24(28-23(29)16-8-4-5-9-16)27-22(18)17-10-11-19-20(12-17)31-14-30-19/h1-3,6-7,10-12,16H,4-5,8-9,14H2,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from A1 adenosine receptor (unknown origin) expressed in CHO cell membrane incubated for 60 mins at room temperature by NXT... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01636
BindingDB Entry DOI: 10.7270/Q2M61Q5K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50457149
(CHEMBL4213910)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSCc2ccccc2CSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H62N10O10S2.2C2HF3O2/c55-41(23-35-15-19-39(65)20-16-35)49(69)61-45-31-75-29-37-13-7-8-14-38(37)30-76-32-46(62-50(70)42(56)24-36-17-21-40(66)22-18-36)52(72)58-28-48(68)60-44(26-34-11-5-2-6-12-34)54(74)64-63-53(73)43(25-33-9-3-1-4-10-33)59-47(67)27-57-51(45)71;2*3-2(4,5)1(6)7/h1-22,41-46,65-66H,23-32,55-56H2,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)(H,63,73)(H,64,74);2*(H,6,7)/t41-,42-,43-,44-,45+,46+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat whole brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 858-863 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00210
BindingDB Entry DOI: 10.7270/Q2S1853J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data