

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29100 (dibenzothiazepine, 12h) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29098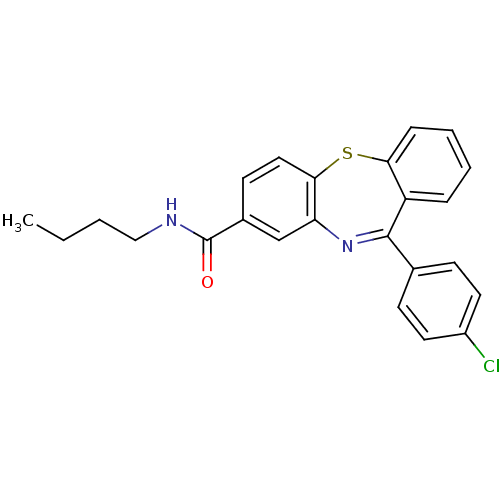 (dibenzothiazepine, 12e) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29096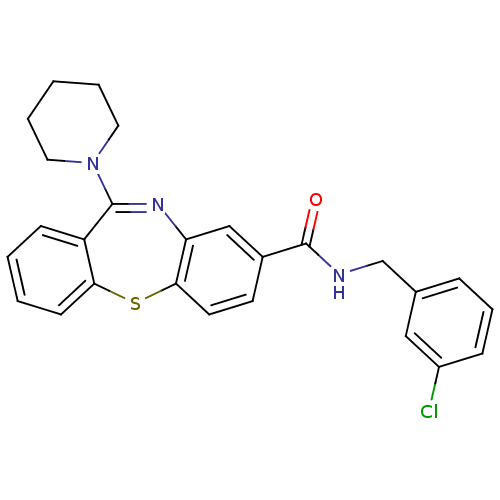 (dibenzothiazepine, 12b) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29097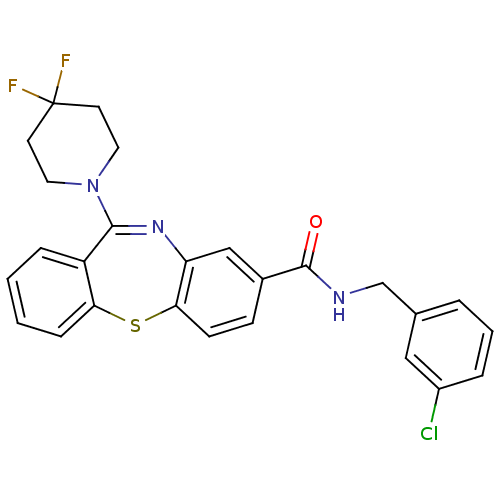 (dibenzothiazepine, 12c) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.790 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29101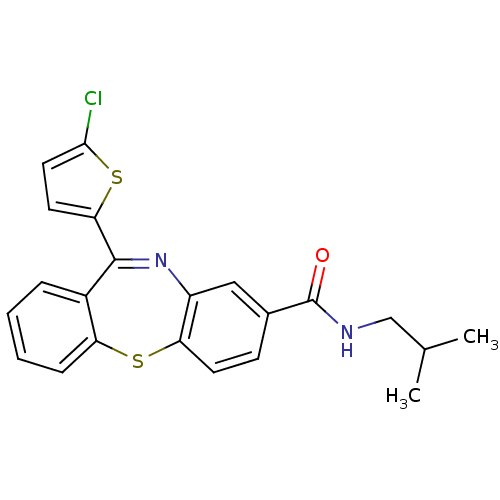 (dibenzothiazepine, 12j) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 1.20 | -50.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29102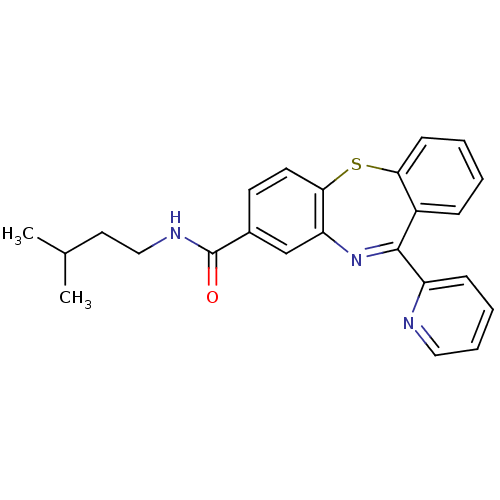 (dibenzothiazepine, 12k) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 1.60 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29095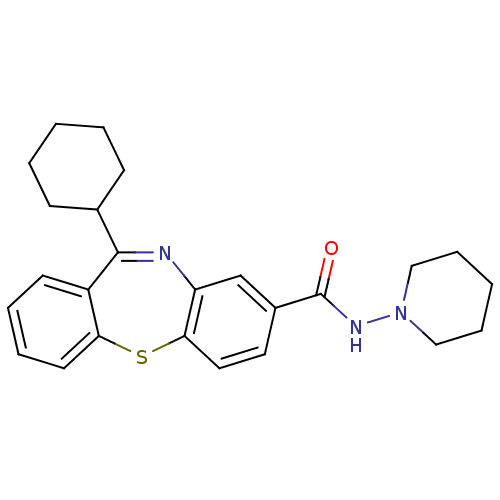 (dibenzothiazepine, 12a) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29094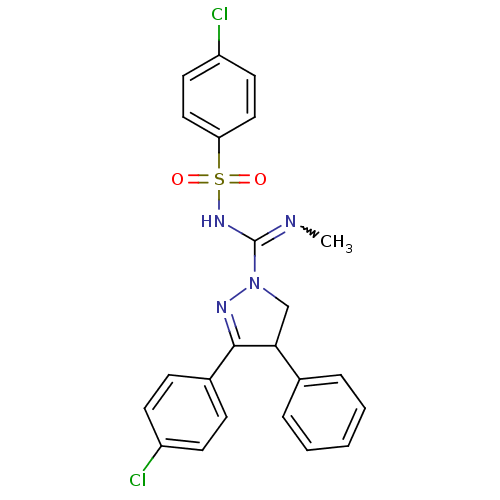 ((+/-)-SLV319 | (S)-3-(4-chlorophenyl)-N-(4-chlorop...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29099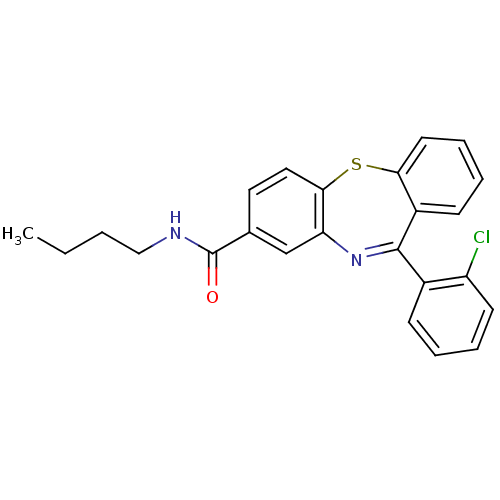 (dibenzothiazepine, 12g) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29103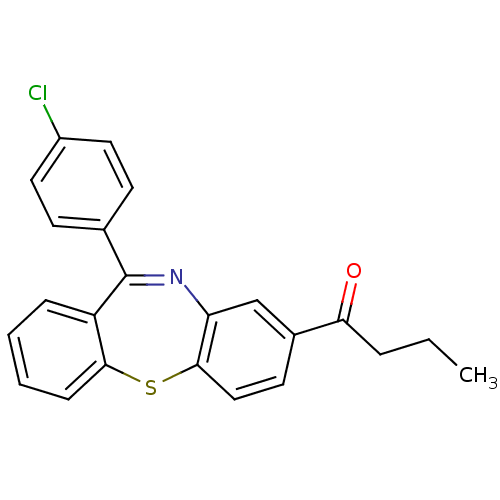 (dibenzothiazepine, 13) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | -41.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 398 | -36.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29100 (dibenzothiazepine, 12h) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | -33.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29097 (dibenzothiazepine, 12c) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.26E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29094 ((+/-)-SLV319 | (S)-3-(4-chlorophenyl)-N-(4-chlorop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29099 (dibenzothiazepine, 12g) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.01E+3 | -29.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50415093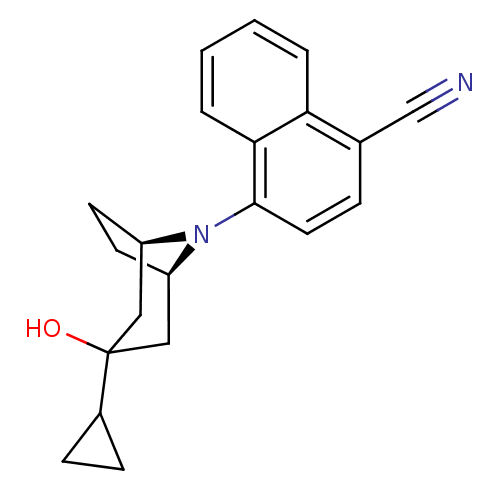 (CHEMBL570463) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50415094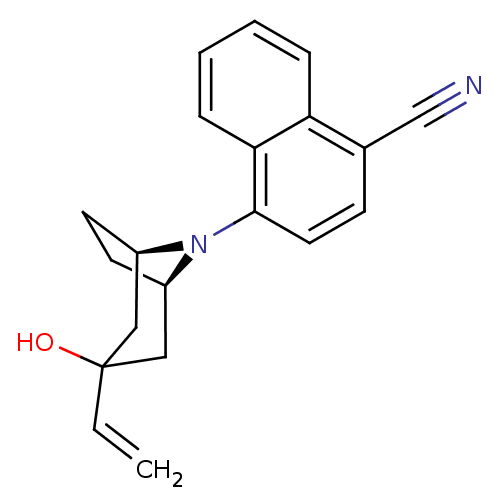 (CHEMBL578199) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50415095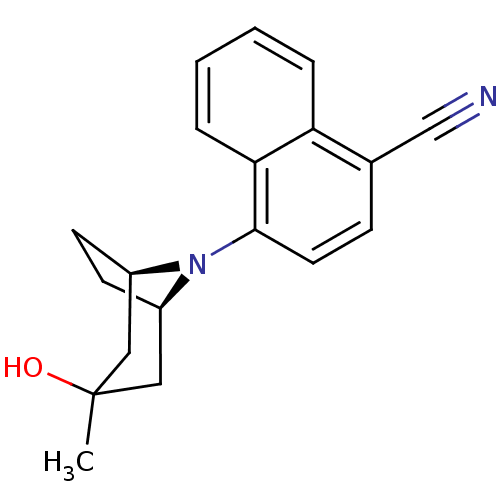 (CHEMBL568786) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50258791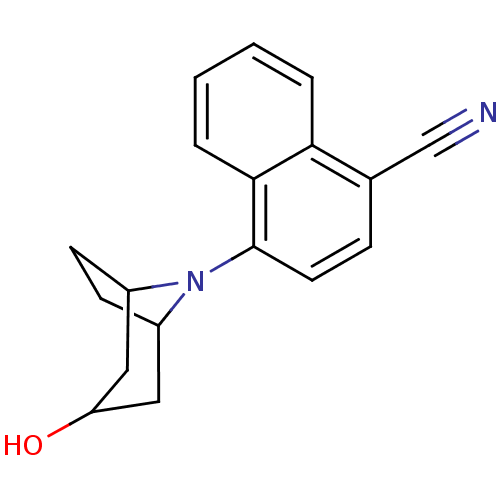 (4-(3-exo-Hydroxy-8-azabicyclo[3.2.1]oct-8-yl)napht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50415096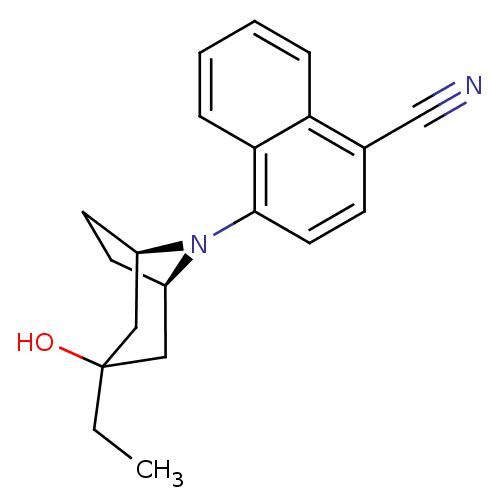 (CHEMBL568814) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50415097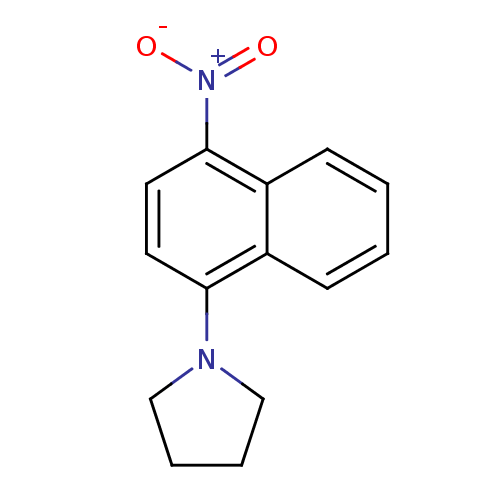 (CHEMBL570897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50415098 (CHEMBL570898) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50415099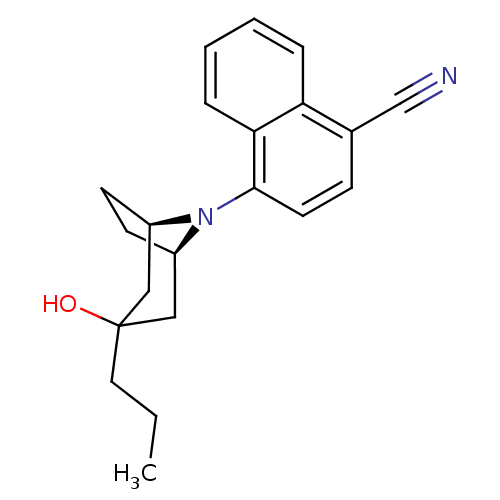 (CHEMBL569985) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50415100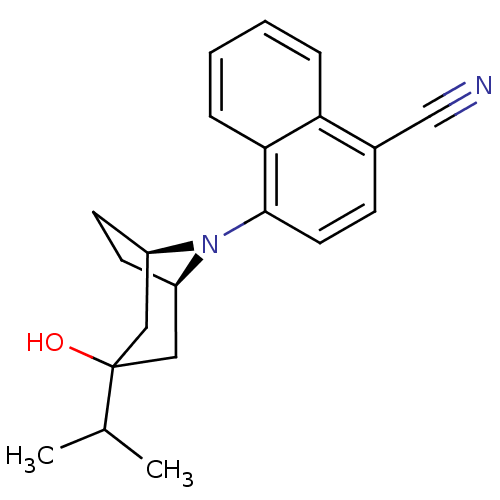 (CHEMBL570413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50415092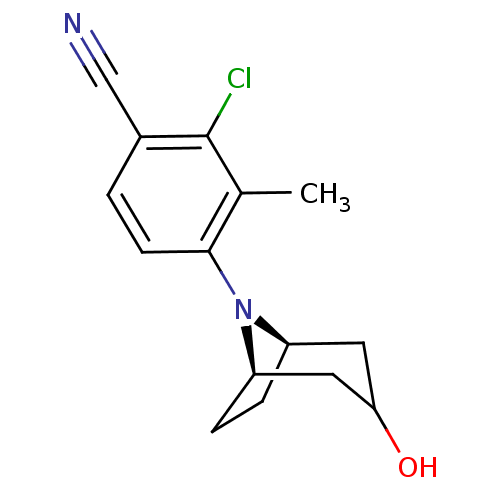 (CHEMBL570895) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50415091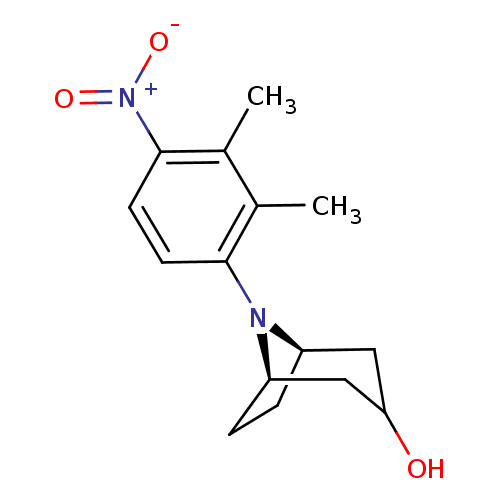 (CHEMBL576474) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Mus musculus) | BDBM8885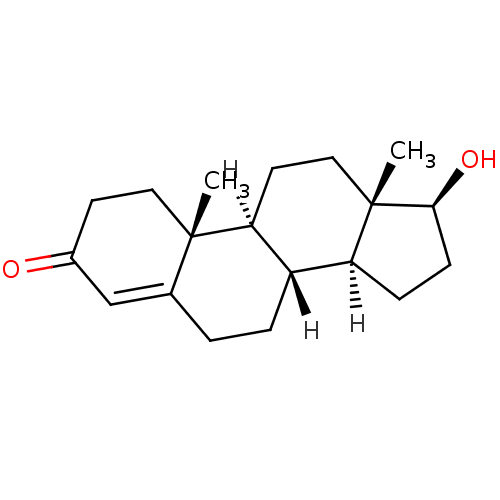 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Mus musculus) | BDBM50415090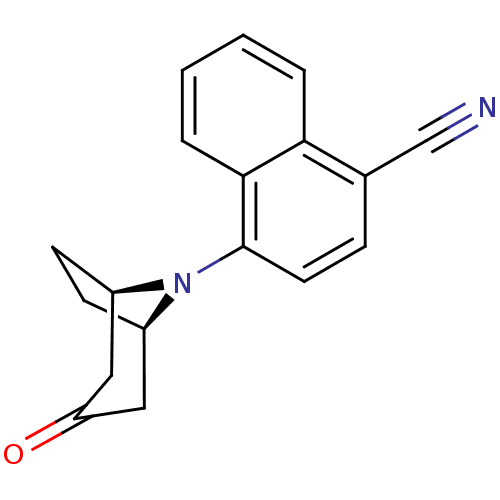 (CHEMBL576275) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50415089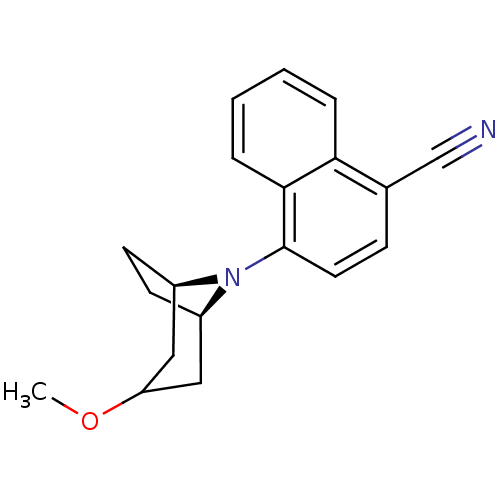 (CHEMBL570410) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50415088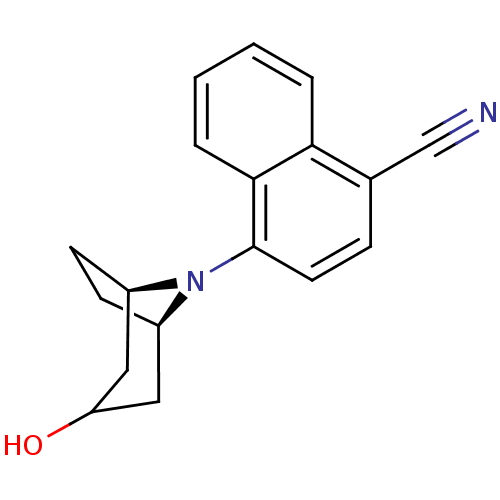 (CHEMBL577196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50415087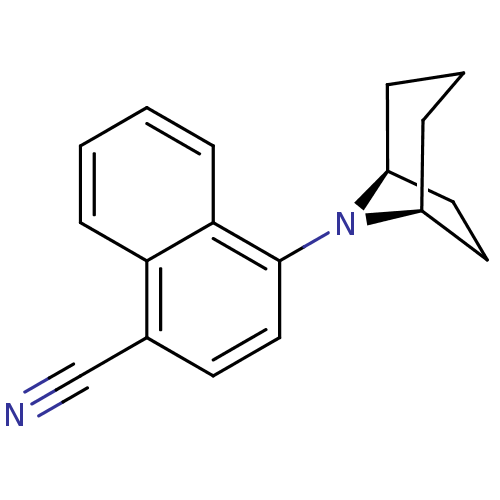 (CHEMBL570237) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50415086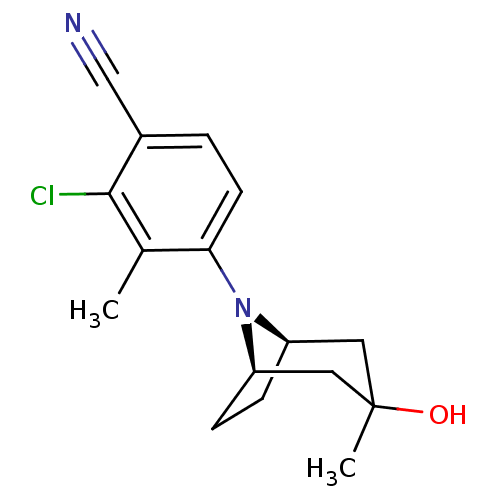 (ACP-105 | CHEMBL570435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse NIH3T3 cells transiently transfected with beta-galactosidase reporter gene assessed as cellular transf... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM8885 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-KB2 cells transfected with MMTV linked luciferase assessed as transcriptional activation by lucife... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50415086 (ACP-105 | CHEMBL570435) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-KB2 cells transfected with MMTV linked luciferase assessed as transcriptional activation by lucife... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50415088 (CHEMBL577196) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.58 | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals AB Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-KB2 cells transfected with MMTV linked luciferase assessed as transcriptional activation by lucife... | J Med Chem 52: 7186-91 (2009) Article DOI: 10.1021/jm901149c BindingDB Entry DOI: 10.7270/Q2SJ1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29096 (dibenzothiazepine, 12b) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29095 (dibenzothiazepine, 12a) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29102 (dibenzothiazepine, 12k) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29101 (dibenzothiazepine, 12j) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM29098 (dibenzothiazepine, 12e) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
ACADIA Pharmaceuticals AB | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit... | J Med Chem 52: 1975-82 (2009) Article DOI: 10.1021/jm801534c BindingDB Entry DOI: 10.7270/Q25Q4TFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||