 Found 86 hits with Last Name = 'fejzo' and Initial = 'j'
Found 86 hits with Last Name = 'fejzo' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50154137
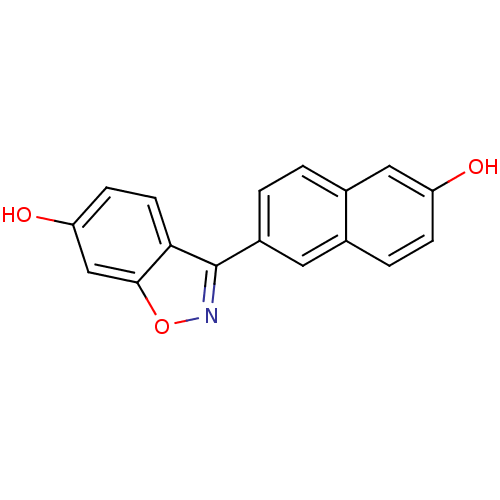
(3-(6-HYDROXY-NAPHTHALEN-2-YL)-BENZO[D]ISOOXAZOL-6-...)Show InChI InChI=1S/C17H11NO3/c19-13-4-3-10-7-12(2-1-11(10)8-13)17-15-6-5-14(20)9-16(15)21-18-17/h1-9,19-20H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50154078
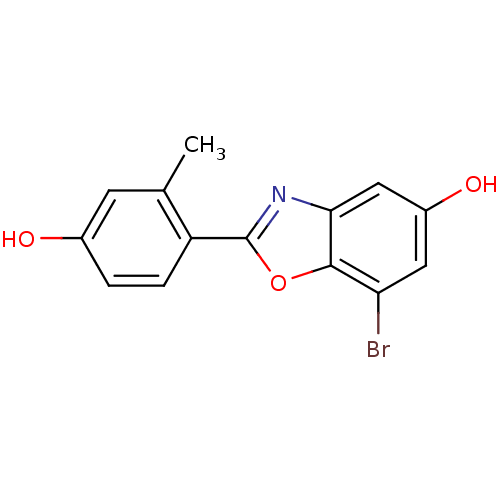
(7-Bromo-2-(4-hydroxy-2-methyl-phenyl)-benzooxazol-...)Show InChI InChI=1S/C14H10BrNO3/c1-7-4-8(17)2-3-10(7)14-16-12-6-9(18)5-11(15)13(12)19-14/h2-6,17-18H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314628
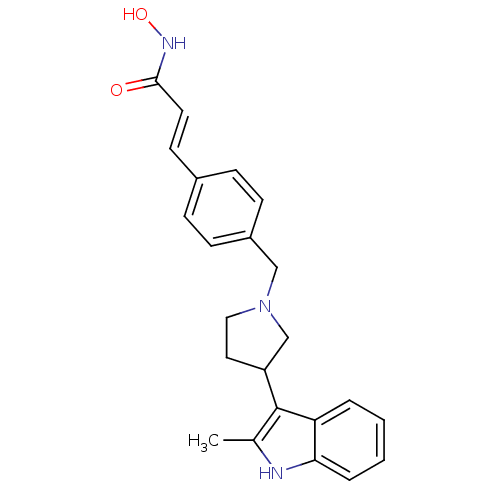
((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pyrr...)Show SMILES Cc1[nH]c2ccccc2c1C1CCN(Cc2ccc(\C=C\C(=O)NO)cc2)C1 Show InChI InChI=1S/C23H25N3O2/c1-16-23(20-4-2-3-5-21(20)24-16)19-12-13-26(15-19)14-18-8-6-17(7-9-18)10-11-22(27)25-28/h2-11,19,24,28H,12-15H2,1H3,(H,25,27)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314637

((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2)C1 Show InChI InChI=1S/C27H33N3O2/c1-27(2,3)26-25(22-8-4-5-9-23(22)28-26)21-7-6-16-30(18-21)17-20-12-10-19(11-13-20)14-15-24(31)29-32/h4-5,8-15,21,28,32H,6-7,16-18H2,1-3H3,(H,29,31)/b15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314630

((E)-N-Hydroxy-3-(4-{1-[2-(2-methyl-1H-indol-3-yl)e...)Show SMILES Cc1[nH]c2ccccc2c1CCN1CCCC1c1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C24H27N3O2/c1-17-20(21-5-2-3-6-22(21)25-17)14-16-27-15-4-7-23(27)19-11-8-18(9-12-19)10-13-24(28)26-29/h2-3,5-6,8-13,23,25,29H,4,7,14-16H2,1H3,(H,26,28)/b13-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50154062

(2-(5-HYDROXY-NAPHTHALEN-1-YL)-1,3-BENZOOXAZOL-6-OL...)Show InChI InChI=1S/C17H11NO3/c19-10-7-8-14-16(9-10)21-17(18-14)13-5-1-4-12-11(13)3-2-6-15(12)20/h1-9,19-20H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314627
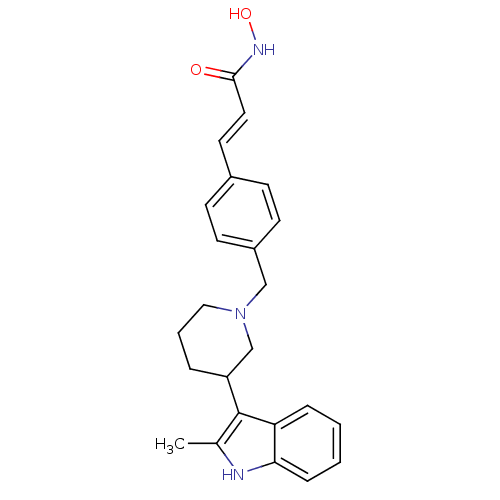
((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pipe...)Show SMILES Cc1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2)C1 Show InChI InChI=1S/C24H27N3O2/c1-17-24(21-6-2-3-7-22(21)25-17)20-5-4-14-27(16-20)15-19-10-8-18(9-11-19)12-13-23(28)26-29/h2-3,6-13,20,25,29H,4-5,14-16H2,1H3,(H,26,28)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC3 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19428
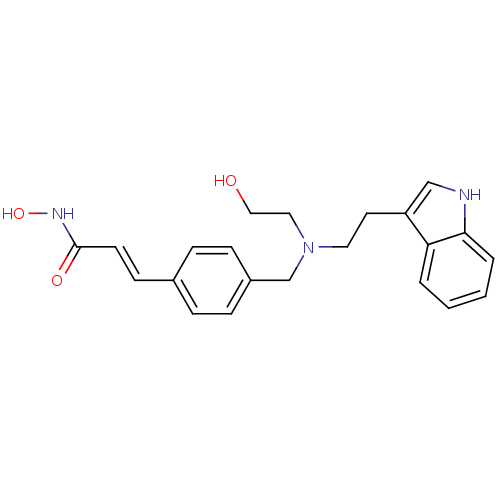
((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314640
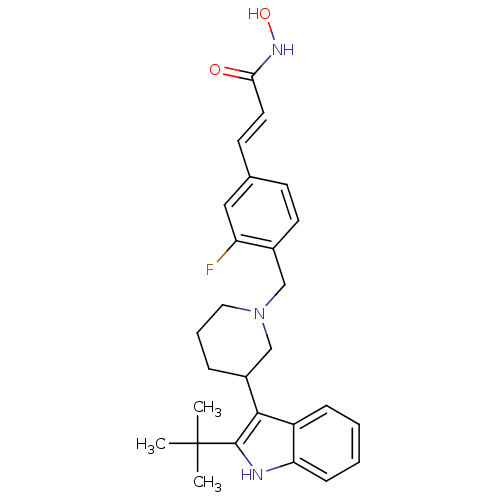
((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314635

((E)-N-Hydroxy-3-{4-[3-(1H-indol-3-yl)piperidin-1-y...)Show SMILES ONC(=O)\C=C\c1ccc(CN2CCCC(C2)c2c[nH]c3ccccc23)cc1 Show InChI InChI=1S/C23H25N3O2/c27-23(25-28)12-11-17-7-9-18(10-8-17)15-26-13-3-4-19(16-26)21-14-24-22-6-2-1-5-20(21)22/h1-2,5-12,14,19,24,28H,3-4,13,15-16H2,(H,25,27)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314636
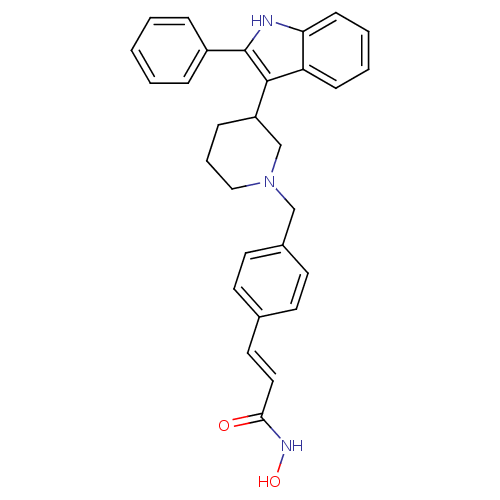
((E)-N-Hydroxy-3-{4-[3-(2-phenyl-1H-indol-3-yl)pipe...)Show SMILES ONC(=O)\C=C\c1ccc(CN2CCCC(C2)c2c([nH]c3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C29H29N3O2/c33-27(31-34)17-16-21-12-14-22(15-13-21)19-32-18-6-9-24(20-32)28-25-10-4-5-11-26(25)30-29(28)23-7-2-1-3-8-23/h1-5,7-8,10-17,24,30,34H,6,9,18-20H2,(H,31,33)/b17-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314633

(CHEMBL1093362 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...)Show SMILES Cc1[nH]c2ccccc2c1CCN1CCc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C23H25N3O2/c1-16-20(21-4-2-3-5-22(21)24-16)11-13-26-12-10-18-8-6-17(14-19(18)15-26)7-9-23(27)25-28/h2-9,14,24,28H,10-13,15H2,1H3,(H,25,27)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314643
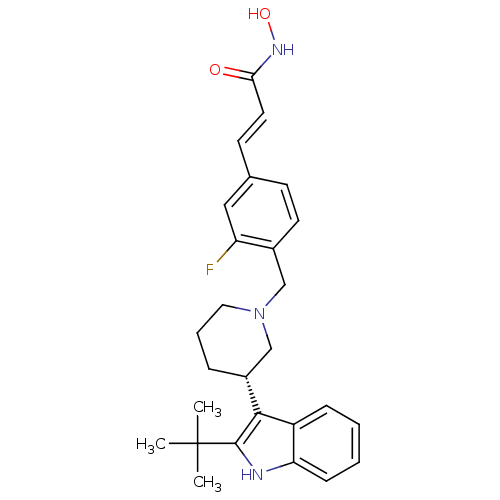
((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314639

((E)-3-{3-Fluoro-4-[3-(2-phenyl-1H-indol-3-yl)piper...)Show SMILES ONC(=O)\C=C\c1ccc(CN2CCCC(C2)c2c([nH]c3ccccc23)-c2ccccc2)c(F)c1 Show InChI InChI=1S/C29H28FN3O2/c30-25-17-20(13-15-27(34)32-35)12-14-22(25)18-33-16-6-9-23(19-33)28-24-10-4-5-11-26(24)31-29(28)21-7-2-1-3-8-21/h1-5,7-8,10-15,17,23,31,35H,6,9,16,18-19H2,(H,32,34)/b15-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314641
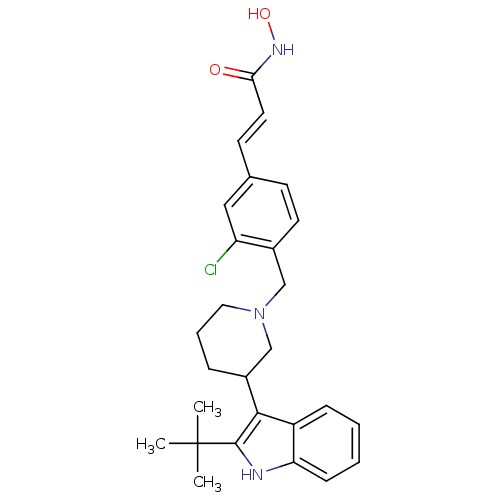
((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2Cl)C1 Show InChI InChI=1S/C27H32ClN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50314643

((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC3 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314629
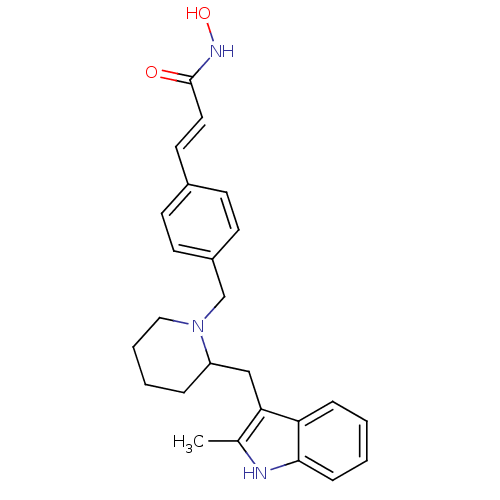
((E)-N-Hydroxy-3-{4-[2-(2-methyl-1H-indol-3-ylmethy...)Show SMILES Cc1[nH]c2ccccc2c1CC1CCCCN1Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C25H29N3O2/c1-18-23(22-7-2-3-8-24(22)26-18)16-21-6-4-5-15-28(21)17-20-11-9-19(10-12-20)13-14-25(29)27-30/h2-3,7-14,21,26,30H,4-6,15-17H2,1H3,(H,27,29)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314631

(CHEMBL1088949 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...)Show SMILES Cc1[nH]c2ccccc2c1CCN1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C22H23N3O2/c1-15-19(20-4-2-3-5-21(20)23-15)10-11-25-13-17-8-6-16(12-18(17)14-25)7-9-22(26)24-27/h2-9,12,23,27H,10-11,13-14H2,1H3,(H,24,26)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 expressed in baculovirus system by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 expressed in baculovirus system by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50314643

((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 expressed in baculovirus system by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314632
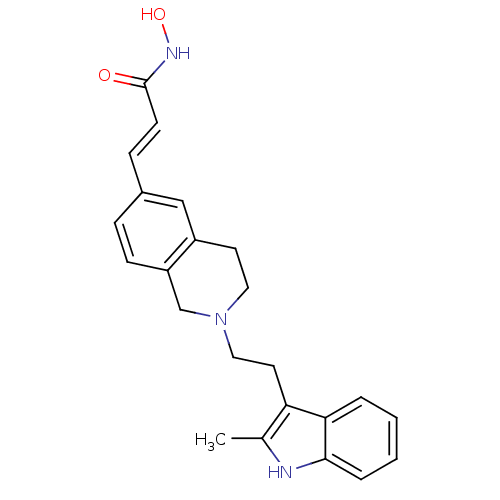
(CHEMBL1089701 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...)Show SMILES Cc1[nH]c2ccccc2c1CCN1CCc2cc(\C=C\C(=O)NO)ccc2C1 Show InChI InChI=1S/C23H25N3O2/c1-16-20(21-4-2-3-5-22(21)24-16)11-13-26-12-10-18-14-17(6-8-19(18)15-26)7-9-23(27)25-28/h2-9,14,24,28H,10-13,15H2,1H3,(H,25,27)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50314643

((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 expressed in baculovirus system by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50148169
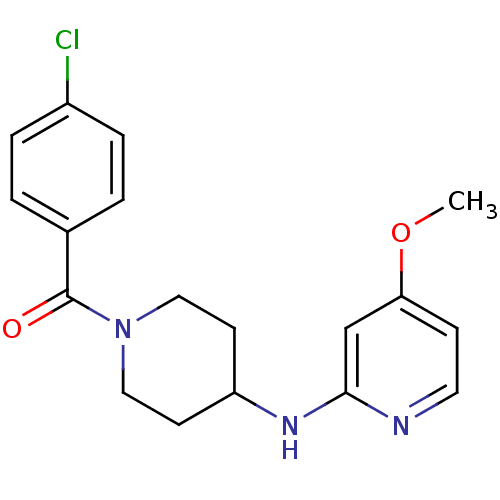
((4-Chloro-phenyl)-[4-(4-methoxy-pyridin-2-ylamino)...)Show InChI InChI=1S/C18H20ClN3O2/c1-24-16-6-9-20-17(12-16)21-15-7-10-22(11-8-15)18(23)13-2-4-14(19)5-3-13/h2-6,9,12,15H,7-8,10-11H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124525
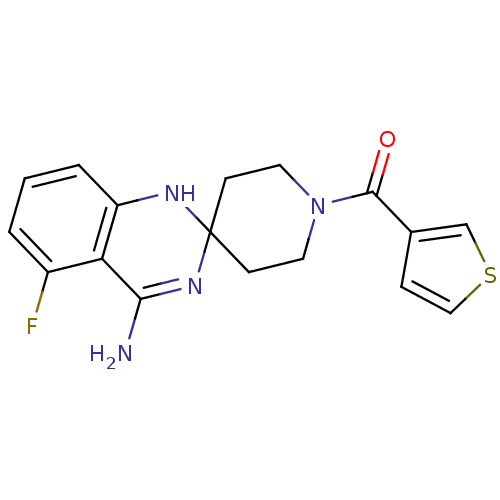
(4'-amino-5'-fluorospiro[hexahydropyridine-4,2'-(1'...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccsc2)Nc2cccc(F)c12 |t:1| Show InChI InChI=1S/C17H17FN4OS/c18-12-2-1-3-13-14(12)15(19)21-17(20-13)5-7-22(8-6-17)16(23)11-4-9-24-10-11/h1-4,9-10,20H,5-8H2,(H2,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50035206

(4-(5-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...)Show InChI InChI=1S/C17H17NO/c1-19-13-3-5-14-12(10-13)2-4-15-16(17(14)15)11-6-8-18-9-7-11/h3,5-10,15-17H,2,4H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314634
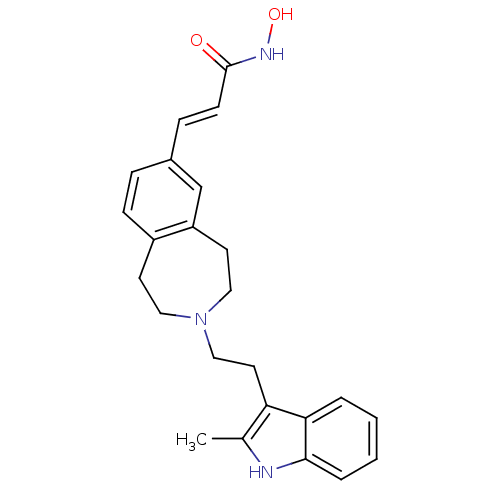
(CHEMBL1089301 | N-hydroxy-3-(3-(2-(2-methyl-1H-ind...)Show SMILES Cc1[nH]c2ccccc2c1CCN1CCc2ccc(\C=C\C(=O)NO)cc2CC1 Show InChI InChI=1S/C24H27N3O2/c1-17-21(22-4-2-3-5-23(22)25-17)12-15-27-13-10-19-8-6-18(7-9-24(28)26-29)16-20(19)11-14-27/h2-9,16,25,29H,10-15H2,1H3,(H,26,28)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM19956
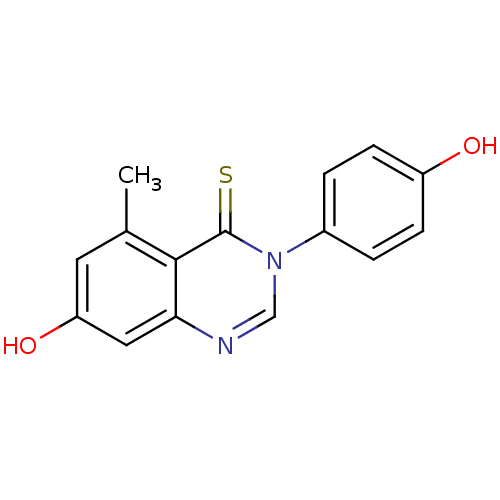
(3-arylquinazolinethione, 1bd | 7-hydroxy-3-(4-hydr...)Show InChI InChI=1S/C15H12N2O2S/c1-9-6-12(19)7-13-14(9)15(20)17(8-16-13)10-2-4-11(18)5-3-10/h2-8,18-19H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50148164
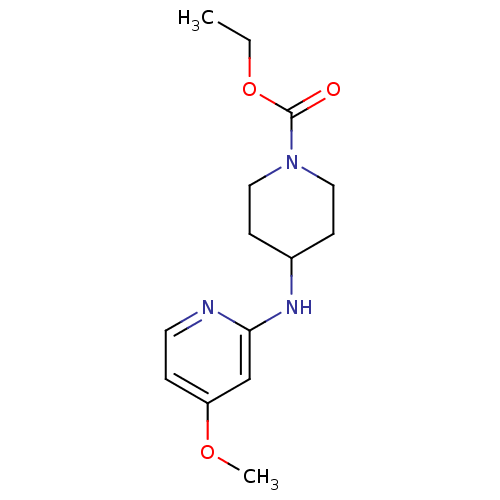
(4-(4-Methoxy-pyridin-2-ylamino)-piperidine-1-carbo...)Show InChI InChI=1S/C14H21N3O3/c1-3-20-14(18)17-8-5-11(6-9-17)16-13-10-12(19-2)4-7-15-13/h4,7,10-11H,3,5-6,8-9H2,1-2H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10009
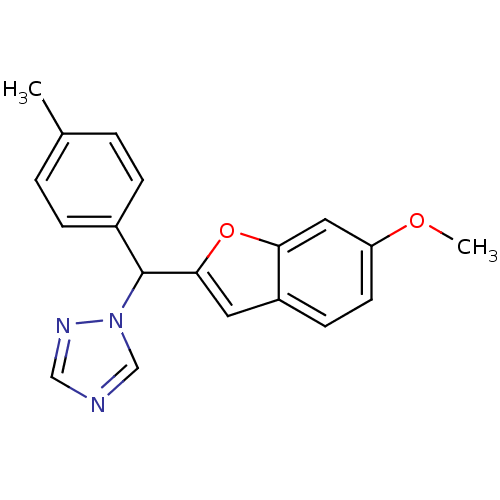
(1-[(6-Methoxybenzofuran-2-yl)-p-tolylmethyl]-1H-1,...)Show InChI InChI=1S/C19H17N3O2/c1-13-3-5-14(6-4-13)19(22-12-20-11-21-22)18-9-15-7-8-16(23-2)10-17(15)24-18/h3-12,19H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50314643

((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC6 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC6 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314638

((E)-3-{3-Fluoro-4-[3-(1H-indol-3-yl)piperidin-1-yl...)Show SMILES ONC(=O)\C=C\c1ccc(CN2CCCC(C2)c2c[nH]c3ccccc23)c(F)c1 Show InChI InChI=1S/C23H24FN3O2/c24-21-12-16(8-10-23(28)26-29)7-9-18(21)15-27-11-3-4-17(14-27)20-13-25-22-6-2-1-5-19(20)22/h1-2,5-10,12-13,17,25,29H,3-4,11,14-15H2,(H,26,28)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10002
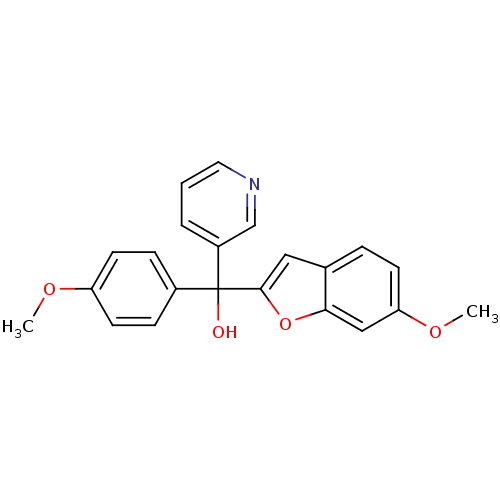
((6-Methoxybenzofuran-2-yl)-(4-methoxyphenyl)-3-pyr...)Show SMILES COc1ccc(cc1)C(O)(c1cc2ccc(OC)cc2o1)c1cccnc1 Show InChI InChI=1S/C22H19NO4/c1-25-18-9-6-16(7-10-18)22(24,17-4-3-11-23-14-17)21-12-15-5-8-19(26-2)13-20(15)27-21/h3-14,24H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 expressed in baculovirus system by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9471
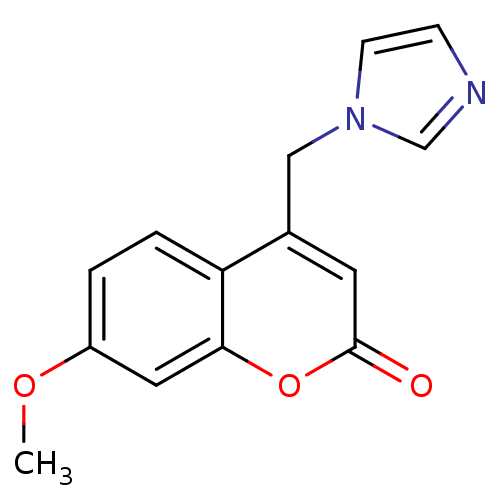
(4-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...)Show InChI InChI=1S/C14H12N2O3/c1-18-11-2-3-12-10(8-16-5-4-15-9-16)6-14(17)19-13(12)7-11/h2-7,9H,8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124528
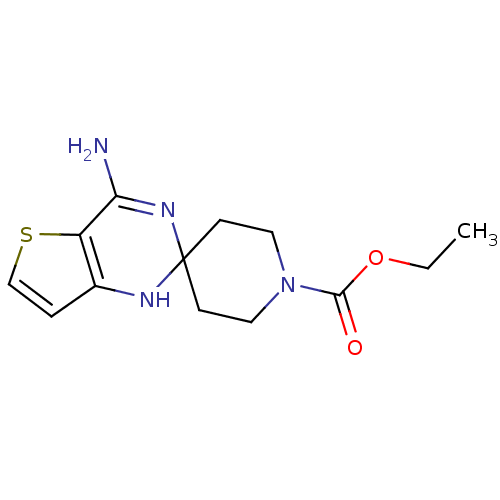
(CHEMBL170176 | ethyl 4'-amino-1H,1'H-spiro[piperid...)Show InChI InChI=1S/C13H18N4O2S/c1-2-19-12(18)17-6-4-13(5-7-17)15-9-3-8-20-10(9)11(14)16-13/h3,8,15H,2,4-7H2,1H3,(H2,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50314643

((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 364 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 expressed in baculovirus system by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50376217
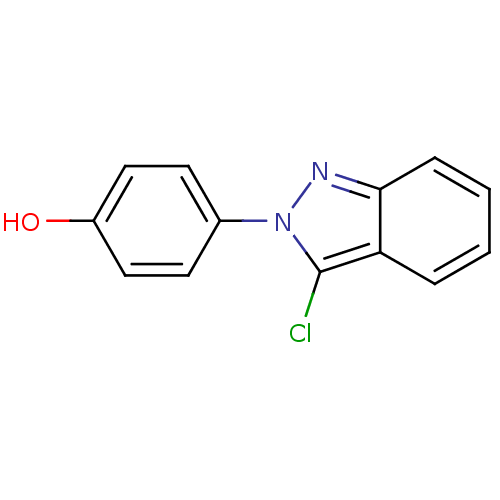
(CHEMBL362476)Show InChI InChI=1S/C13H9ClN2O/c14-13-11-3-1-2-4-12(11)15-16(13)9-5-7-10(17)8-6-9/h1-8,17H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091813
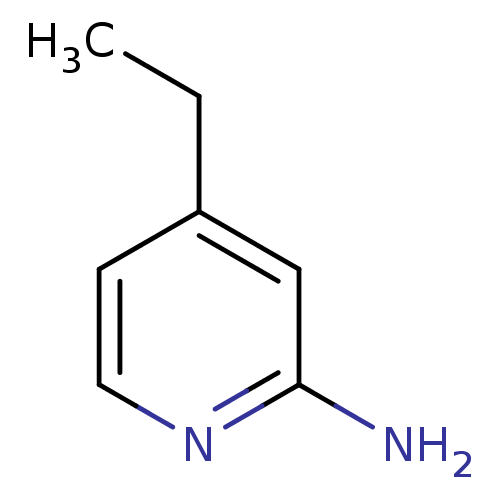
(4-Ethyl-pyridin-2-ylamine | 4-ethylpyridin-2-amine...)Show InChI InChI=1S/C7H10N2/c1-2-6-3-4-9-7(8)5-6/h3-5H,2H2,1H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50237936
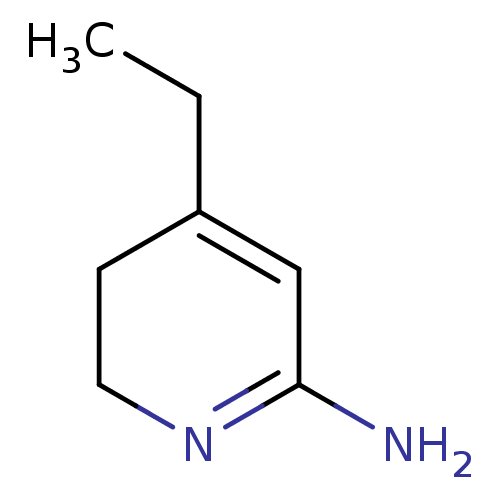
(4-Ethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneamine |...)Show InChI InChI=1S/C7H12N2/c1-2-6-3-4-9-7(8)5-6/h5H,2-4H2,1H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50376215

(CHEMBL409050)Show InChI InChI=1S/C13H17N3/c1-8-4-2-7-10-11(8)12(14)16-13(15-10)9-5-3-6-9/h2,4,7,9,13,15H,3,5-6H2,1H3,(H2,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50376221
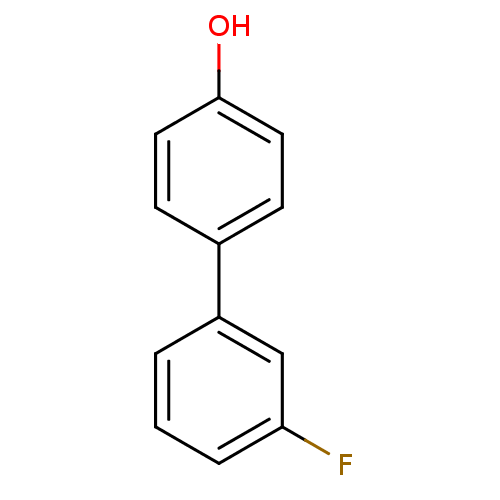
(CHEMBL261781)Show InChI InChI=1S/C12H9FO/c13-11-3-1-2-10(8-11)9-4-6-12(14)7-5-9/h1-8,14H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50117550
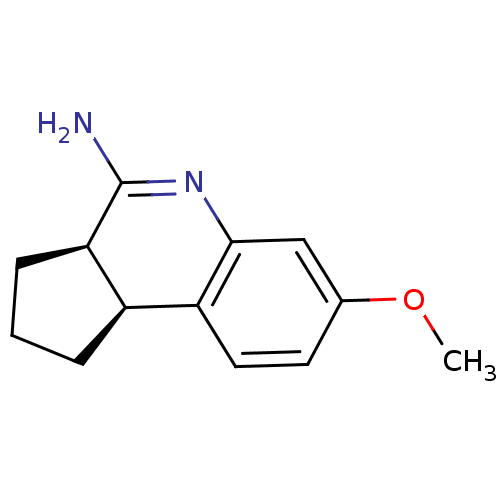
((3aR,9bS)-7-methoxy-2,3,3a,9b-tetrahydro-1H-cyclop...)Show InChI InChI=1S/C13H16N2O/c1-16-8-5-6-10-9-3-2-4-11(9)13(14)15-12(10)7-8/h5-7,9,11H,2-4H2,1H3,(H2,14,15)/t9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10025
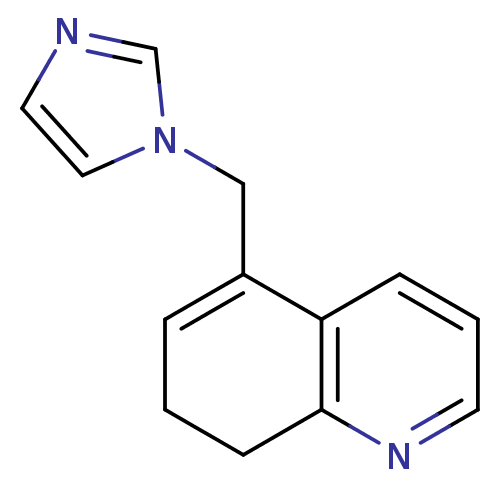
(5-(1H-imidazol-1-ylmethyl)-7,8-dihydroquinoline | ...)Show InChI InChI=1S/C13H13N3/c1-3-11(9-16-8-7-14-10-16)12-4-2-6-15-13(12)5-1/h2-4,6-8,10H,1,5,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50376223
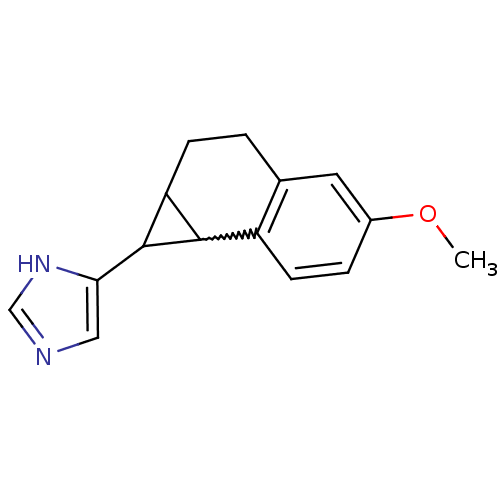
(CHEMBL438549)Show InChI InChI=1S/C15H16N2O/c1-18-10-3-5-11-9(6-10)2-4-12-14(11)15(12)13-7-16-8-17-13/h3,5-8,12,14-15H,2,4H2,1H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50376225

(CHEMBL258553)Show InChI InChI=1S/C13H9NO/c14-9-11-3-1-2-4-13(11)10-5-7-12(15)8-6-10/h1-8,15H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50168378

(2-methyl-6-o-tolylnaphthalene | 6-(2-Hydroxy-pheny...)Show InChI InChI=1S/C16H12O2/c17-14-8-7-11-9-13(6-5-12(11)10-14)15-3-1-2-4-16(15)18/h1-10,17-18H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data