

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards Wild-type kappa opioid receptor expressed in HEK cells | J Med Chem 43: 1251-2 (2001) BindingDB Entry DOI: 10.7270/Q270824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50033535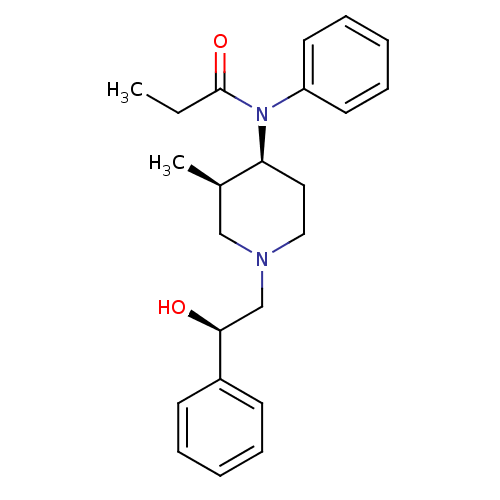 (CHEMBL331883 | N-[(3R,4S)-1-((R)-2-Hydroxy-2-pheny...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 | J Med Chem 43: 381-91 (2000) BindingDB Entry DOI: 10.7270/Q2DN45RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50021347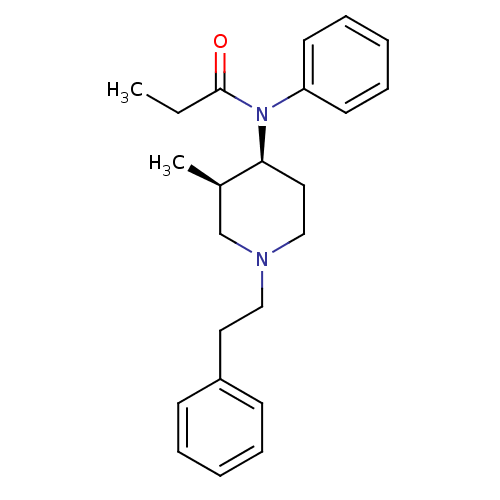 (CHEMBL12391 | N-(3-Methyl-1-phenethyl-piperidin-4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 | J Med Chem 43: 381-91 (2000) BindingDB Entry DOI: 10.7270/Q2DN45RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50012477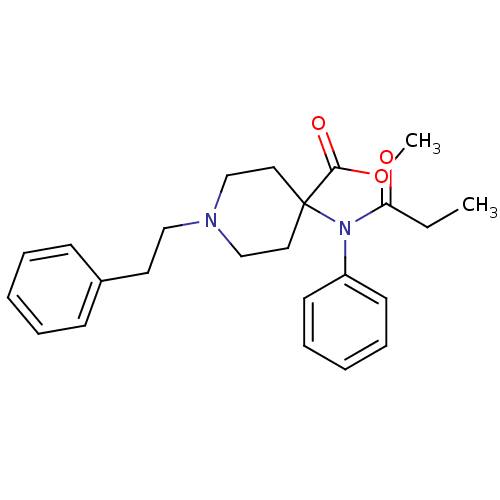 (1-Phenethyl-4-(phenyl-propionyl-amino)-piperidine-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 | J Med Chem 43: 381-91 (2000) BindingDB Entry DOI: 10.7270/Q2DN45RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071565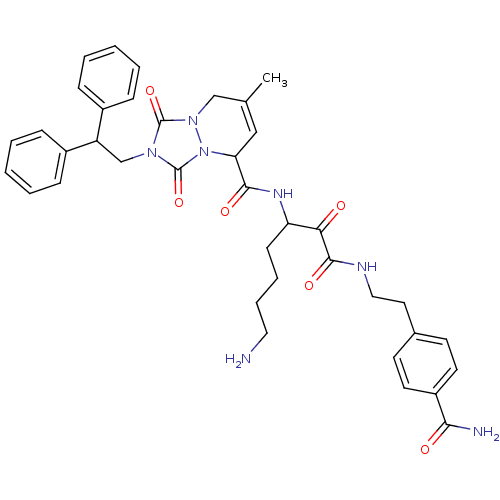 (2-(2,2-Diphenyl-ethyl)-7-methyl-1,3-dioxo-2,3,5,8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50033533 (CHEMBL121403 | N-[(3R,4R)-1-((S)-2-Hydroxy-2-pheny...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 | J Med Chem 43: 381-91 (2000) BindingDB Entry DOI: 10.7270/Q2DN45RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from opioid receptor kappa 1 expressed in HEK 293 cells | J Med Chem 44: 857-62 (2001) BindingDB Entry DOI: 10.7270/Q27H1K9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50084768 (N-(4-Methoxymethyl-1-phenethyl-piperidin-4-yl)-N-p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 | J Med Chem 43: 381-91 (2000) BindingDB Entry DOI: 10.7270/Q2DN45RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [3H]- diprenorphine from delta receptor expressed in HEK 293 cells | J Med Chem 44: 857-62 (2001) BindingDB Entry DOI: 10.7270/Q27H1K9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071575 (2,2-Dibutyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090099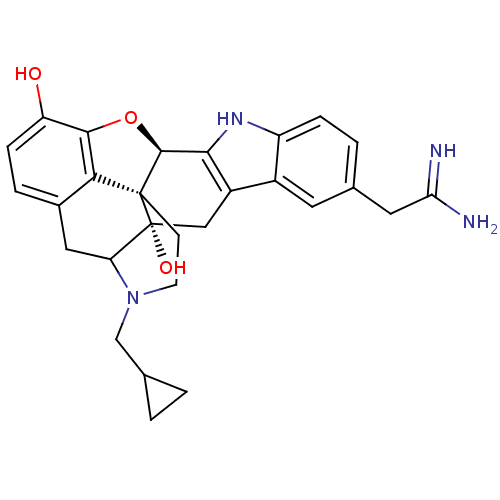 (5'-Amidinomethyl-17-cyclopropylmethyl-6,7-didehydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity on HEK-293 cells transiently transfected with plasmids encoding the rat Opioid receptor kappa 1 | J Med Chem 43: 2759-69 (2000) BindingDB Entry DOI: 10.7270/Q2JD4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090102 (5'-N-Biguanidino-17-cyclopropylmethyl-6,7-didehydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity on HEK-293 cells transiently transfected with plasmids encoding the rat Opioid receptor kappa 1 | J Med Chem 43: 2759-69 (2000) BindingDB Entry DOI: 10.7270/Q2JD4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from delta-W284E-receptor expressed in HEK 293 cells | J Med Chem 44: 857-62 (2001) BindingDB Entry DOI: 10.7270/Q27H1K9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090100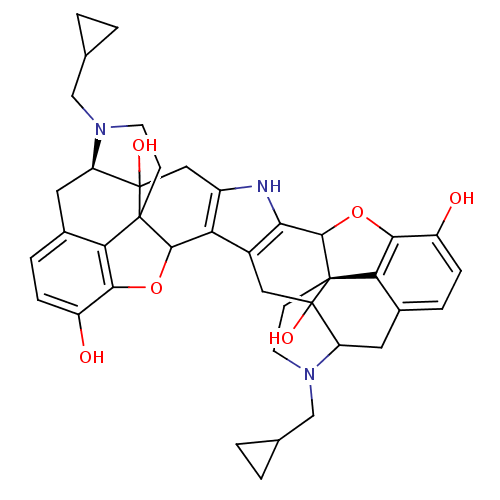 (CHEMBL319202 | Norbinaltorphimine (norBNI)) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity on HEK-293 cells transiently transfected with plasmids encoding the rat Opioid receptor kappa 1 | J Med Chem 43: 2759-69 (2000) BindingDB Entry DOI: 10.7270/Q2JD4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50071570 (8-Isobutyl-2-(4-methoxy-phenyl)-1,3-dioxo-2,3,5,8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the trypsin enzyme | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071573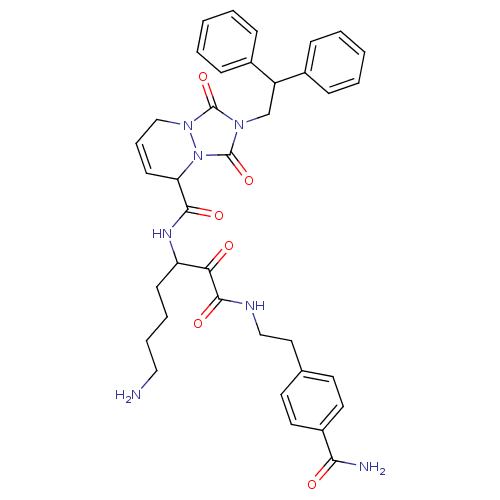 (2-(2,2-Diphenyl-ethyl)-1,3-dioxo-2,3,5,8-tetrahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090103 (5'-(Trimethylammonium)methyl-17-cyclopropylmethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.354 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity on HEK-293 cells transiently transfected with plasmids encoding the rat Opioid receptor kappa 1 | J Med Chem 43: 2759-69 (2000) BindingDB Entry DOI: 10.7270/Q2JD4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [3H]- diprenorphine from mu-W318A-receptor expressed in HEK 293 | J Med Chem 44: 857-62 (2001) BindingDB Entry DOI: 10.7270/Q27H1K9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [3H]- diprenorphine from mu-W318A-receptor expressed in HEK 293 cells | J Med Chem 44: 857-62 (2001) BindingDB Entry DOI: 10.7270/Q27H1K9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [3H]- diprenorphine from mu-W318A-receptor expressed in HEK 293 cells | J Med Chem 44: 857-62 (2001) BindingDB Entry DOI: 10.7270/Q27H1K9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50071571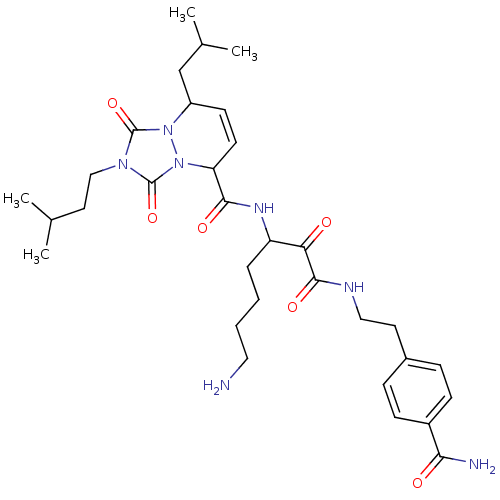 (8-Isobutyl-2-(3-methyl-butyl)-1,3-dioxo-2,3,5,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the trypsin enzyme | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090101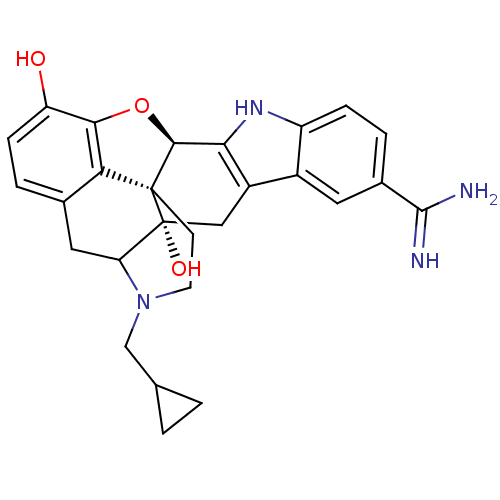 (5'-Amidino-17-cyclopropylmethyl-6,7-didehydro-4,5a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.529 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity on HEK-293 cells transiently transfected with plasmids encoding the rat Opioid receptor kappa 1 | J Med Chem 43: 2759-69 (2000) BindingDB Entry DOI: 10.7270/Q2JD4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding constant against E203Q,D204N,D206N EL-2 Opioid receptor kappa 1 using [3H]diprenorphine as radioligand expressed in HEK cells | J Med Chem 43: 1251-2 (2001) BindingDB Entry DOI: 10.7270/Q270824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding constant against wild type EL-2 Opioid receptor kappa 1 using [3H]diprenorphine as radioligand expressed in HEK cells | J Med Chem 43: 1251-2 (2001) BindingDB Entry DOI: 10.7270/Q270824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090097 (5'-(Dimethylamino)methyl-17-cyclopropylmethyl-6,7-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.823 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity on HEK-293 cells transiently transfected with plasmids encoding the rat Opioid receptor kappa 1 | J Med Chem 43: 2759-69 (2000) BindingDB Entry DOI: 10.7270/Q2JD4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding constant against E203Q,D204N,D206N,E209Q EL-2 Opioid receptor kappa 1 using [3H]diprenorphine as radioligand expressed in HEK cells | J Med Chem 43: 1251-2 (2001) BindingDB Entry DOI: 10.7270/Q270824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50100462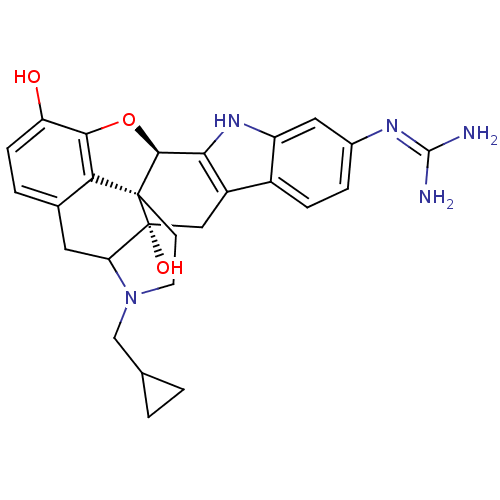 (6`-guanidino-17-(cyclopropylmethyl)-6,7-didehydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Effect on binding to wild-type opioid receptor kappa 1 using [3H]diprenorphine in transiently expressed rat HEK293 cells | J Med Chem 44: 2073-9 (2001) BindingDB Entry DOI: 10.7270/Q2416XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50100462 (6`-guanidino-17-(cyclopropylmethyl)-6,7-didehydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards rat opioid receptor kappa 1 was determined using [3H]diprenorphine radioligand | J Med Chem 44: 2073-9 (2001) BindingDB Entry DOI: 10.7270/Q2416XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from k-Y312A-receptor expressed in HEK 293 cells | J Med Chem 44: 857-62 (2001) BindingDB Entry DOI: 10.7270/Q27H1K9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from k-Y312A-receptor expressed in HEK 293 cells | J Med Chem 44: 857-62 (2001) BindingDB Entry DOI: 10.7270/Q27H1K9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071568 (2-Amino-2-benzyl-7-methyl-1,3-dioxo-2,3,5,8-tetrah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 1.82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [3H]- diprenorphine from delta-W284E-receptor expressed in HEK 293 cells | J Med Chem 44: 857-62 (2001) BindingDB Entry DOI: 10.7270/Q27H1K9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50098209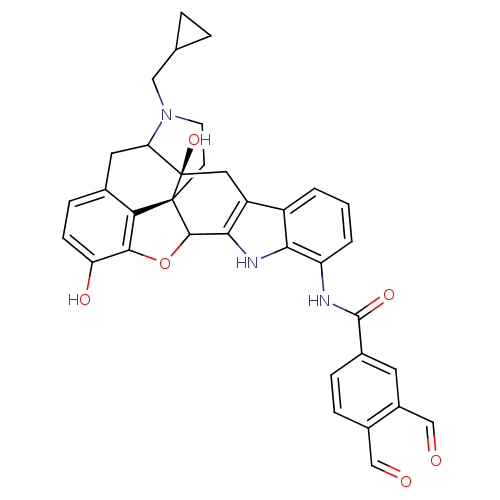 (22-cyclopropylmethyl-9-(3,4-diformylphenylcarboxam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards delta-opioid receptor from CHO cells | J Med Chem 44: 1017-20 (2001) BindingDB Entry DOI: 10.7270/Q2QF8TK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071567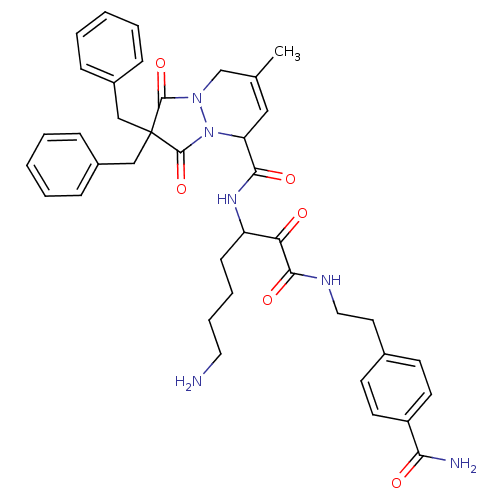 (2,2-Dibenzyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50413190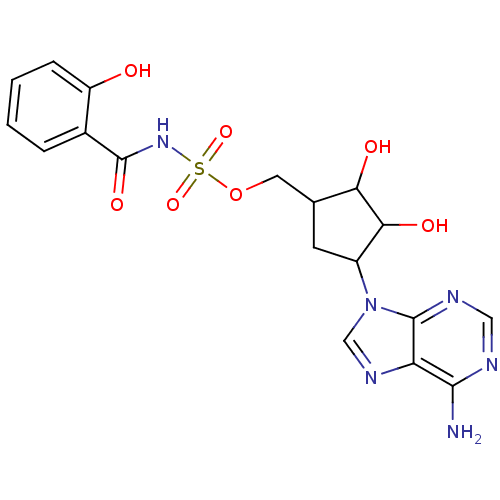 (CHEMBL609107) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis BAG RV143 MbtA expressed in Escherichia coli BL21 (DE3) assessed as ATP-[32P]PPi exchange by liquid scintill... | J Med Chem 51: 7154-60 (2009) Article DOI: 10.1021/jm800668u BindingDB Entry DOI: 10.7270/Q2ZK5HXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50100464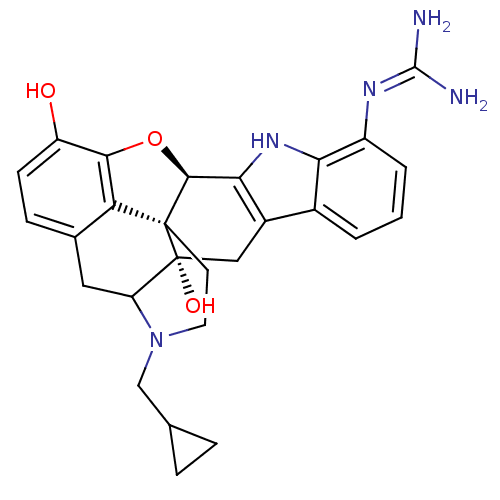 (7`-guanidino-17-(cyclopropylmethyl)-6,7-didehydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards mouse delta-opioid receptor was determined using [3H]diprenorphine as radioligand | J Med Chem 44: 2073-9 (2001) BindingDB Entry DOI: 10.7270/Q2416XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090098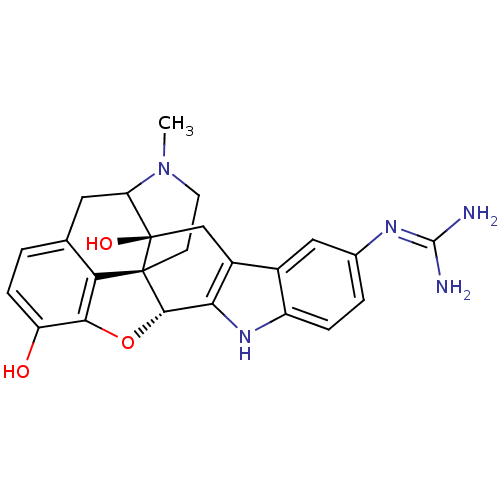 (6,7-Dihydro-4,5alpha-epoxy-5'-guanidinyl-17-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity on HEK-293 cells transiently transfected with plasmids encoding the rat Opioid receptor kappa 1 | J Med Chem 43: 2759-69 (2000) BindingDB Entry DOI: 10.7270/Q2JD4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50413192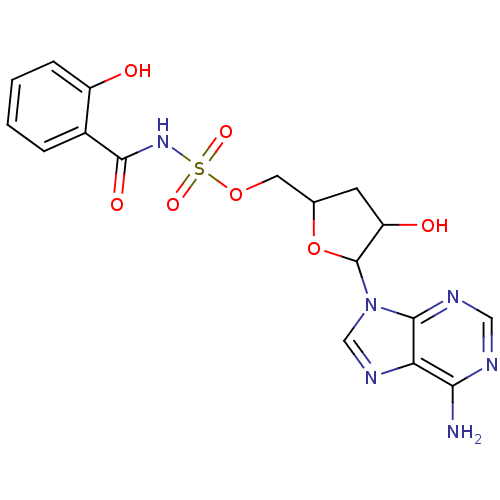 (CHEMBL611786) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis BAG RV143 MbtA expressed in Escherichia coli BL21 (DE3) assessed as ATP-[32P]PPi exchange by liquid scintill... | J Med Chem 51: 7154-60 (2009) Article DOI: 10.1021/jm800668u BindingDB Entry DOI: 10.7270/Q2ZK5HXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50012477 (1-Phenethyl-4-(phenyl-propionyl-amino)-piperidine-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor delta 1 | J Med Chem 43: 381-91 (2000) BindingDB Entry DOI: 10.7270/Q2DN45RB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding constant against D216N,D217N,E218Q EL-2 Opioid receptor kappa 1 using [3H]diprenorphine as radioligand expressed in HEK cells | J Med Chem 43: 1251-2 (2001) BindingDB Entry DOI: 10.7270/Q270824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50413191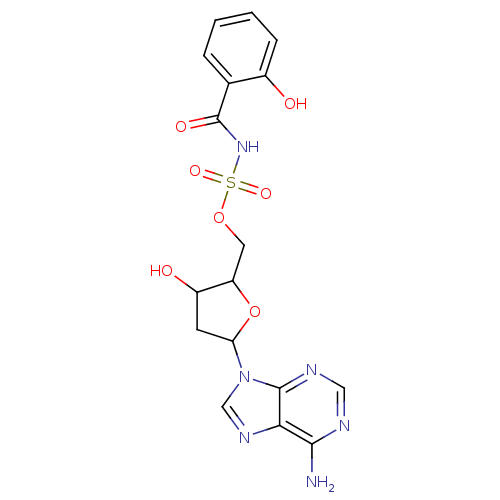 (CHEMBL609108) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis BAG RV143 MbtA expressed in Escherichia coli BL21 (DE3) assessed as ATP-[32P]PPi exchange by liquid scintill... | J Med Chem 51: 7154-60 (2009) Article DOI: 10.1021/jm800668u BindingDB Entry DOI: 10.7270/Q2ZK5HXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50413186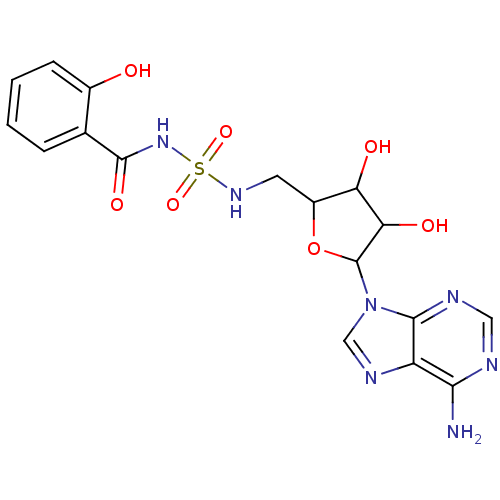 (CHEMBL609097) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis BAG RV143 MbtA expressed in Escherichia coli BL21 (DE3) assessed as ATP-[32P]PPi exchange by liquid scintill... | J Med Chem 51: 7154-60 (2009) Article DOI: 10.1021/jm800668u BindingDB Entry DOI: 10.7270/Q2ZK5HXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50297170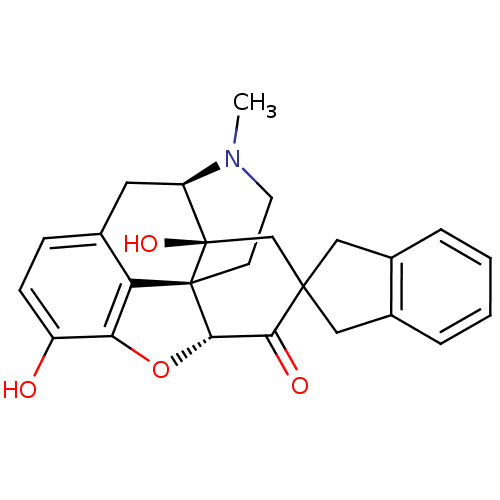 (7-(2'-spiroindanyl)oxymorphone | 7-(Spiroindano)Ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [3H]- diprenorphine from mu-W318A-receptor expressed in HEK 293 cells | J Med Chem 44: 857-62 (2001) BindingDB Entry DOI: 10.7270/Q27H1K9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008984 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 | J Med Chem 43: 381-91 (2000) BindingDB Entry DOI: 10.7270/Q2DN45RB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50297170 (7-(2'-spiroindanyl)oxymorphone | 7-(Spiroindano)Ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [3H]- diprenorphine from delta receptor expressed in HEK 293 cells | J Med Chem 44: 857-62 (2001) BindingDB Entry DOI: 10.7270/Q27H1K9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071566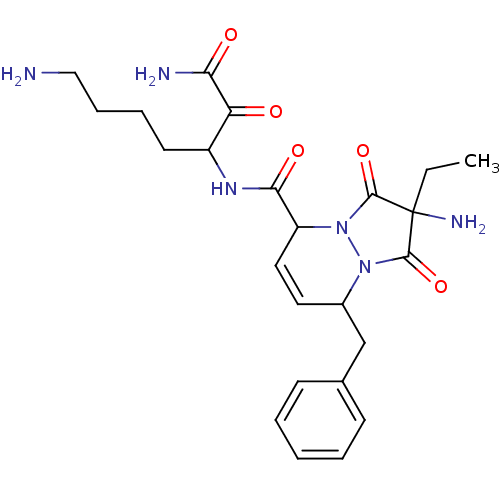 (2-Amino-8-benzyl-2-ethyl-1,3-dioxo-2,3,5,8-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50068379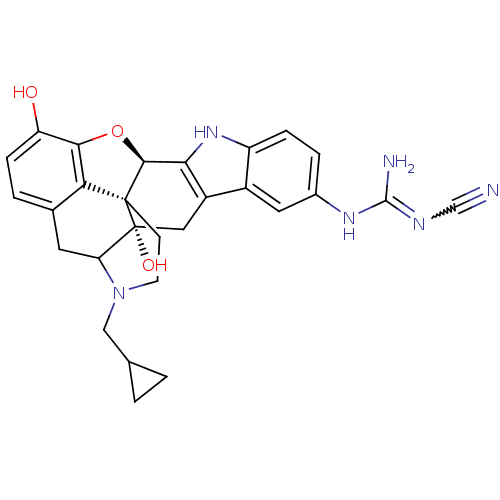 (5'-[N-(N'-Cyano)guanidinyl]-17-cyclopropylmethyl-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity on HEK-293 cells transiently transfected with plasmids encoding the rat Opioid receptor kappa 1 | J Med Chem 43: 2759-69 (2000) BindingDB Entry DOI: 10.7270/Q2JD4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50412379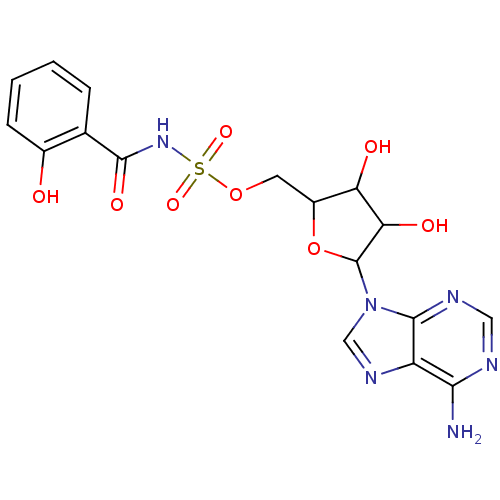 (CHEMBL608844) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis BAG RV143 MbtA expressed in Escherichia coli BL21 (DE3) assessed as ATP-[32P]PPi exchange by liquid scintill... | J Med Chem 51: 7154-60 (2009) Article DOI: 10.1021/jm800668u BindingDB Entry DOI: 10.7270/Q2ZK5HXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071574 (2,2-Diisobutyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [3H]- diprenorphine from delta-receptor expressed in HEK 293 cells | J Med Chem 44: 857-62 (2001) BindingDB Entry DOI: 10.7270/Q27H1K9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1037 total ) | Next | Last >> |