 Found 297 hits with Last Name = 'finzel' and Initial = 'b'
Found 297 hits with Last Name = 'finzel' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054629
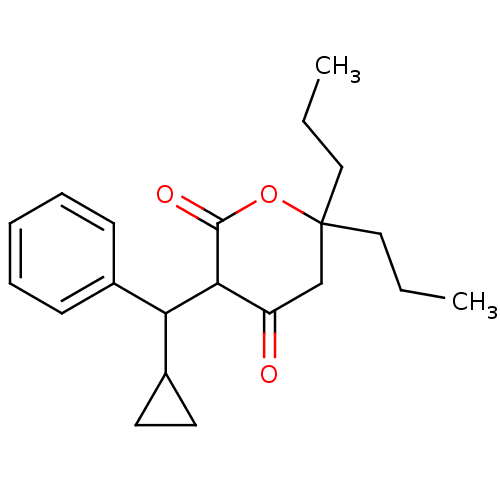
(3-(Cyclopropyl-phenyl-methyl)-4-hydroxy-6,6-diprop...)Show InChI InChI=1S/C21H28O3/c1-3-12-21(13-4-2)14-17(22)19(20(23)24-21)18(16-10-11-16)15-8-6-5-7-9-15/h5-9,16,18-19H,3-4,10-14H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn
Curated by ChEMBL
| Assay Description
HIV Protease inhibitory activity |
J Med Chem 39: 4630-42 (1996)
Article DOI: 10.1021/jm960228q
BindingDB Entry DOI: 10.7270/Q247490H |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM771
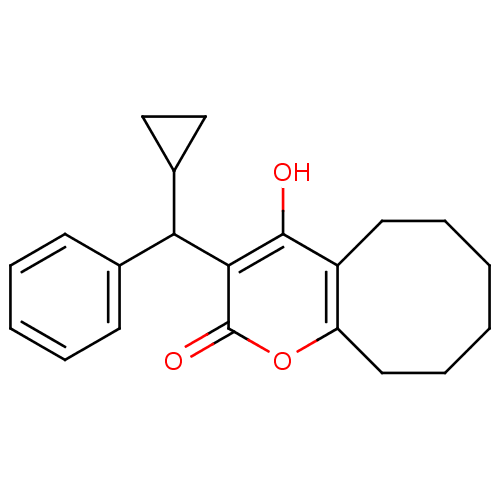
(3-[cyclopropyl(phenyl)methyl]-4-hydroxy-2H,5H,6H,7...)Show InChI InChI=1S/C21H24O3/c22-20-16-10-6-1-2-7-11-17(16)24-21(23)19(20)18(15-12-13-15)14-8-4-3-5-9-14/h3-5,8-9,15,18,22H,1-2,6-7,10-13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | -44.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn
| Assay Description
HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... |
J Med Chem 38: 1884-91 (1995)
Article DOI: 10.1021/jm00011a008
BindingDB Entry DOI: 10.7270/Q2Q81B80 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054610
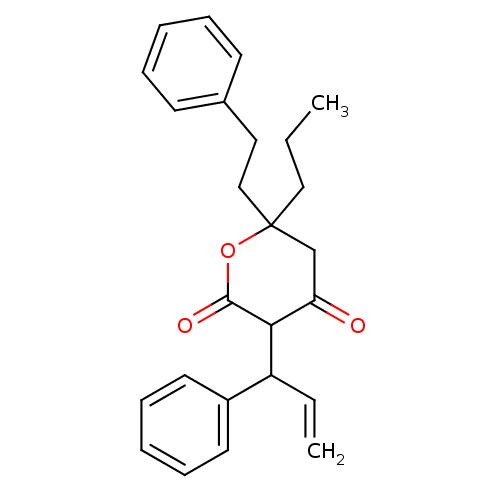
(4-Hydroxy-6-phenethyl-3-(1-phenyl-allyl)-6-propyl-...)Show SMILES CCCC1(CCc2ccccc2)CC(=O)C(C(C=C)c2ccccc2)C(=O)O1 Show InChI InChI=1S/C25H28O3/c1-3-16-25(17-15-19-11-7-5-8-12-19)18-22(26)23(24(27)28-25)21(4-2)20-13-9-6-10-14-20/h4-14,21,23H,2-3,15-18H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn
Curated by ChEMBL
| Assay Description
HIV Protease inhibitory activity |
J Med Chem 39: 4630-42 (1996)
Article DOI: 10.1021/jm960228q
BindingDB Entry DOI: 10.7270/Q247490H |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM784
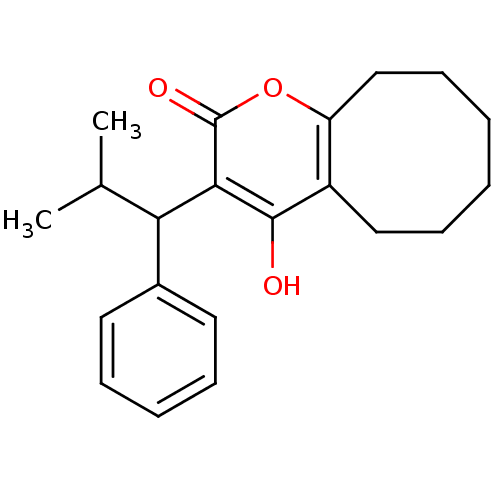
(4-hydroxy-3-(2-methyl-1-phenylpropyl)-2H,5H,6H,7H,...)Show InChI InChI=1S/C21H26O3/c1-14(2)18(15-10-6-5-7-11-15)19-20(22)16-12-8-3-4-9-13-17(16)24-21(19)23/h5-7,10-11,14,18,22H,3-4,8-9,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | -43.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn
| Assay Description
HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... |
J Med Chem 38: 1884-91 (1995)
Article DOI: 10.1021/jm00011a008
BindingDB Entry DOI: 10.7270/Q2Q81B80 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054615
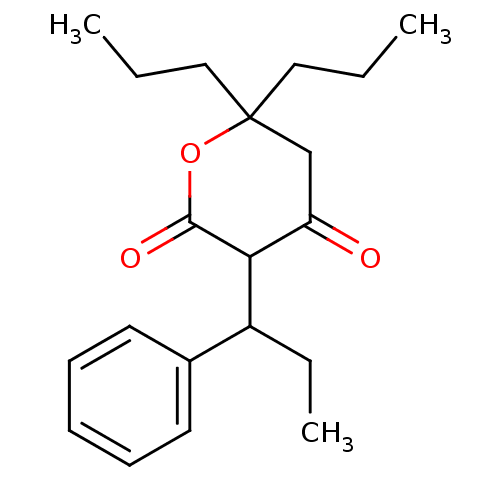
(4-Hydroxy-3-(1-phenyl-propyl)-6,6-dipropyl-5,6-dih...)Show InChI InChI=1S/C20H28O3/c1-4-12-20(13-5-2)14-17(21)18(19(22)23-20)16(6-3)15-10-8-7-9-11-15/h7-11,16,18H,4-6,12-14H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn
Curated by ChEMBL
| Assay Description
HIV Protease inhibitory activity |
J Med Chem 39: 4630-42 (1996)
Article DOI: 10.1021/jm960228q
BindingDB Entry DOI: 10.7270/Q247490H |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM783
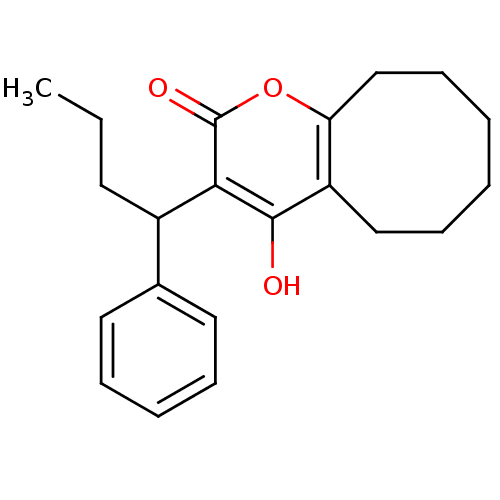
(4-hydroxy-3-(1-phenylbutyl)-2H,5H,6H,7H,8H,9H,10H-...)Show InChI InChI=1S/C21H26O3/c1-2-10-16(15-11-6-5-7-12-15)19-20(22)17-13-8-3-4-9-14-18(17)24-21(19)23/h5-7,11-12,16,22H,2-4,8-10,13-14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | -42.6 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn
| Assay Description
HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... |
J Med Chem 38: 1884-91 (1995)
Article DOI: 10.1021/jm00011a008
BindingDB Entry DOI: 10.7270/Q2Q81B80 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054616
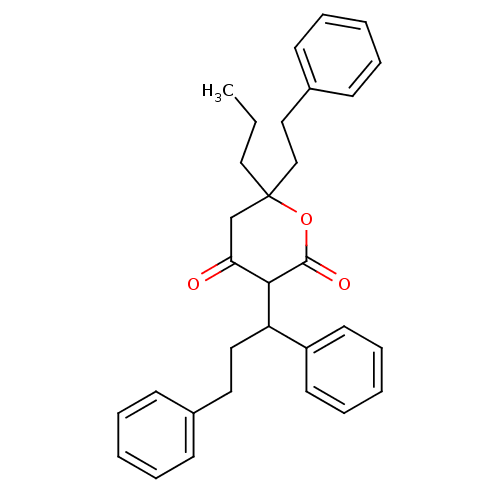
(3-(1,3-Diphenyl-propyl)-4-hydroxy-6-phenethyl-6-pr...)Show SMILES CCCC1(CCc2ccccc2)CC(=O)C(C(CCc2ccccc2)c2ccccc2)C(=O)O1 Show InChI InChI=1S/C31H34O3/c1-2-21-31(22-20-25-14-8-4-9-15-25)23-28(32)29(30(33)34-31)27(26-16-10-5-11-17-26)19-18-24-12-6-3-7-13-24/h3-17,27,29H,2,18-23H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn
Curated by ChEMBL
| Assay Description
HIV Protease inhibitory activity |
J Med Chem 39: 4630-42 (1996)
Article DOI: 10.1021/jm960228q
BindingDB Entry DOI: 10.7270/Q247490H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054619
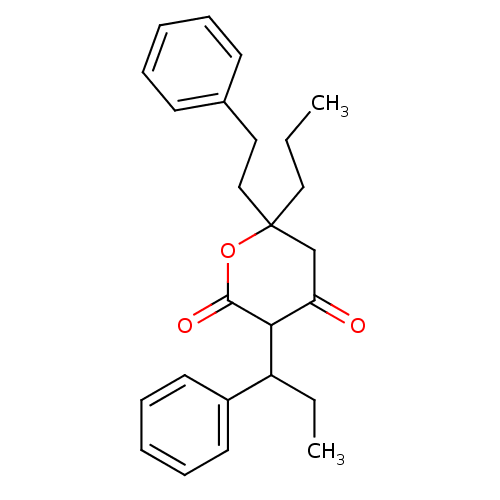
(4-Hydroxy-6-phenethyl-3-(1-phenyl-propyl)-6-propyl...)Show SMILES CCCC1(CCc2ccccc2)CC(=O)C(C(CC)c2ccccc2)C(=O)O1 Show InChI InChI=1S/C25H30O3/c1-3-16-25(17-15-19-11-7-5-8-12-19)18-22(26)23(24(27)28-25)21(4-2)20-13-9-6-10-14-20/h5-14,21,23H,3-4,15-18H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn
Curated by ChEMBL
| Assay Description
HIV Protease inhibitory activity |
J Med Chem 39: 4630-42 (1996)
Article DOI: 10.1021/jm960228q
BindingDB Entry DOI: 10.7270/Q247490H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054630
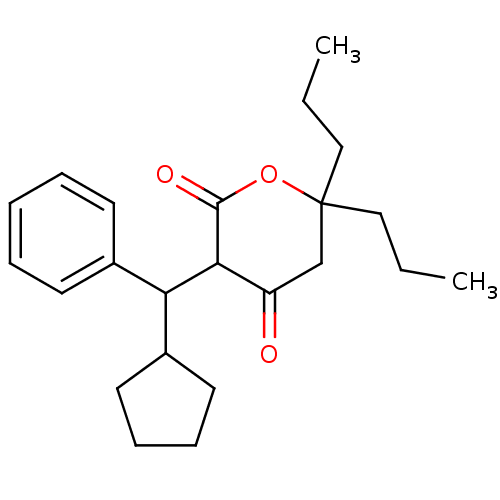
(3-(Cyclopentyl-phenyl-methyl)-4-hydroxy-6,6-diprop...)Show SMILES CCCC1(CCC)CC(=O)C(C(C2CCCC2)c2ccccc2)C(=O)O1 Show InChI InChI=1S/C23H32O3/c1-3-14-23(15-4-2)16-19(24)21(22(25)26-23)20(18-12-8-9-13-18)17-10-6-5-7-11-17/h5-7,10-11,18,20-21H,3-4,8-9,12-16H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn
Curated by ChEMBL
| Assay Description
HIV Protease inhibitory activity |
J Med Chem 39: 4630-42 (1996)
Article DOI: 10.1021/jm960228q
BindingDB Entry DOI: 10.7270/Q247490H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054614
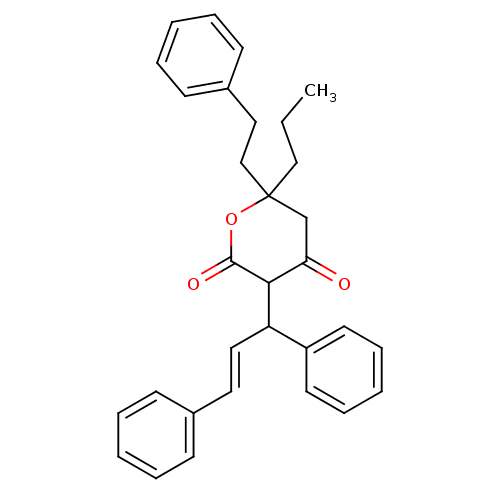
(3-((E)-1,3-Diphenyl-allyl)-4-hydroxy-6-phenethyl-6...)Show SMILES CCCC1(CCc2ccccc2)CC(=O)C(C(\C=C\c2ccccc2)c2ccccc2)C(=O)O1 Show InChI InChI=1S/C31H32O3/c1-2-21-31(22-20-25-14-8-4-9-15-25)23-28(32)29(30(33)34-31)27(26-16-10-5-11-17-26)19-18-24-12-6-3-7-13-24/h3-19,27,29H,2,20-23H2,1H3/b19-18+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn
Curated by ChEMBL
| Assay Description
HIV Protease inhibitory activity |
J Med Chem 39: 4630-42 (1996)
Article DOI: 10.1021/jm960228q
BindingDB Entry DOI: 10.7270/Q247490H |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM789
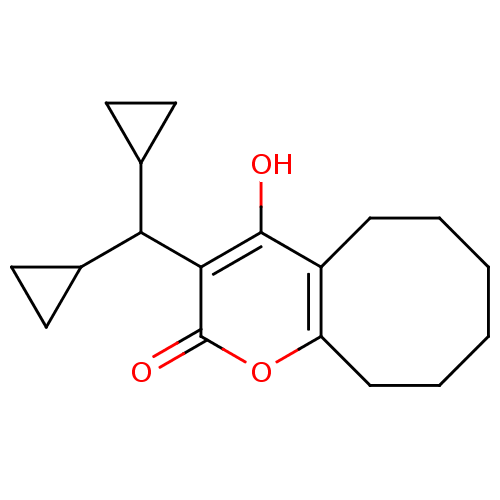
(3-(Dicyclopropylmethy1)-5,6,7,8,9,l0-hexahydro-4-d...)Show InChI InChI=1S/C18H24O3/c19-17-13-5-3-1-2-4-6-14(13)21-18(20)16(17)15(11-7-8-11)12-9-10-12/h11-12,15,19H,1-10H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | -40.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn
| Assay Description
HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... |
J Med Chem 38: 1884-91 (1995)
Article DOI: 10.1021/jm00011a008
BindingDB Entry DOI: 10.7270/Q2Q81B80 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054618
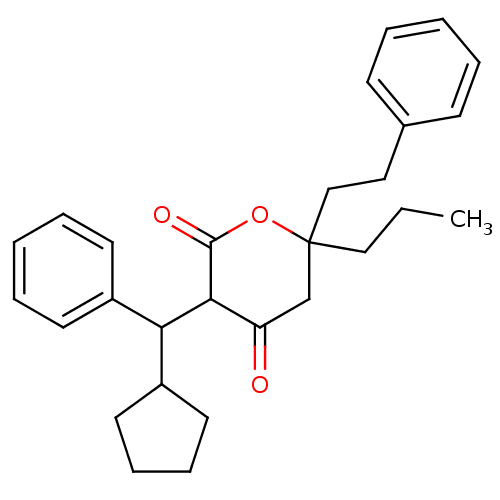
(3-(Cyclopentyl-phenyl-methyl)-4-hydroxy-6-phenethy...)Show SMILES CCCC1(CCc2ccccc2)CC(=O)C(C(C2CCCC2)c2ccccc2)C(=O)O1 Show InChI InChI=1S/C28H34O3/c1-2-18-28(19-17-21-11-5-3-6-12-21)20-24(29)26(27(30)31-28)25(23-15-9-10-16-23)22-13-7-4-8-14-22/h3-8,11-14,23,25-26H,2,9-10,15-20H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn
Curated by ChEMBL
| Assay Description
HIV Protease inhibitory activity |
J Med Chem 39: 4630-42 (1996)
Article DOI: 10.1021/jm960228q
BindingDB Entry DOI: 10.7270/Q247490H |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM786
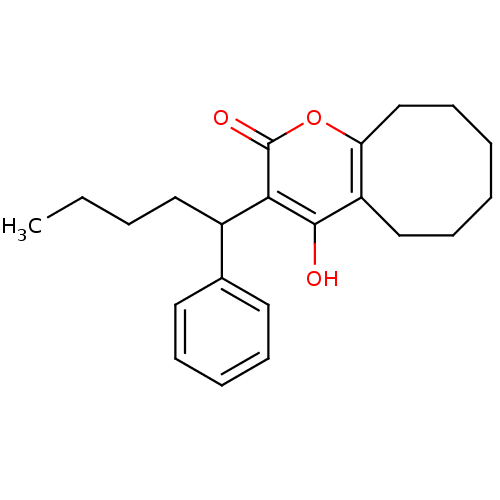
(4-hydroxy-3-(1-phenylpentyl)-2H,5H,6H,7H,8H,9H,10H...)Show InChI InChI=1S/C22H28O3/c1-2-3-13-17(16-11-7-6-8-12-16)20-21(23)18-14-9-4-5-10-15-19(18)25-22(20)24/h6-8,11-12,17,23H,2-5,9-10,13-15H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | -40.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn
| Assay Description
HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... |
J Med Chem 38: 1884-91 (1995)
Article DOI: 10.1021/jm00011a008
BindingDB Entry DOI: 10.7270/Q2Q81B80 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054628
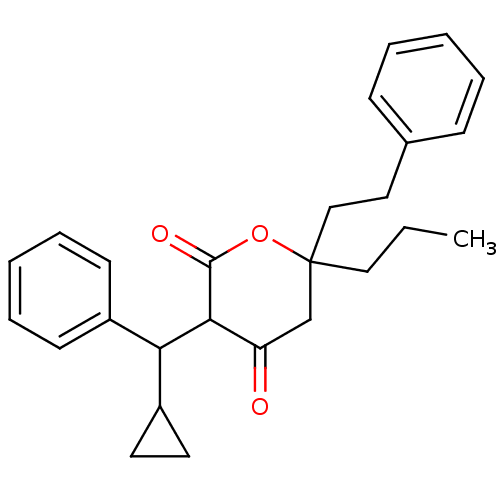
(3-(Cyclopropyl-phenyl-methyl)-4-hydroxy-6-phenethy...)Show SMILES CCCC1(CCc2ccccc2)CC(=O)C(C(C2CC2)c2ccccc2)C(=O)O1 Show InChI InChI=1S/C26H30O3/c1-2-16-26(17-15-19-9-5-3-6-10-19)18-22(27)24(25(28)29-26)23(21-13-14-21)20-11-7-4-8-12-20/h3-12,21,23-24H,2,13-18H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn
Curated by ChEMBL
| Assay Description
HIV Protease inhibitory activity |
J Med Chem 39: 4630-42 (1996)
Article DOI: 10.1021/jm960228q
BindingDB Entry DOI: 10.7270/Q247490H |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM782
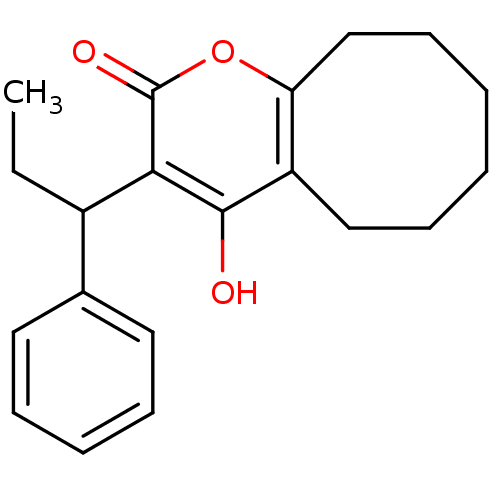
(4-hydroxy-3-(1-phenylpropyl)-2H,5H,6H,7H,8H,9H,10H...)Show InChI InChI=1S/C20H24O3/c1-2-15(14-10-6-5-7-11-14)18-19(21)16-12-8-3-4-9-13-17(16)23-20(18)22/h5-7,10-11,15,21H,2-4,8-9,12-13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 75 | -40.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn
| Assay Description
HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... |
J Med Chem 38: 1884-91 (1995)
Article DOI: 10.1021/jm00011a008
BindingDB Entry DOI: 10.7270/Q2Q81B80 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM787
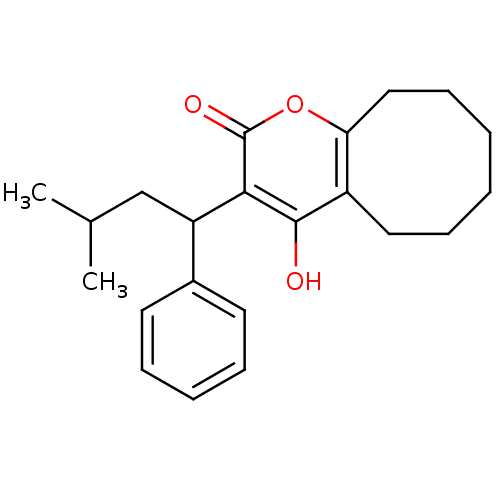
(4-hydroxy-3-(3-methyl-1-phenylbutyl)-2H,5H,6H,7H,8...)Show InChI InChI=1S/C22H28O3/c1-15(2)14-18(16-10-6-5-7-11-16)20-21(23)17-12-8-3-4-9-13-19(17)25-22(20)24/h5-7,10-11,15,18,23H,3-4,8-9,12-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | -39.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn
| Assay Description
HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... |
J Med Chem 38: 1884-91 (1995)
Article DOI: 10.1021/jm00011a008
BindingDB Entry DOI: 10.7270/Q2Q81B80 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054608
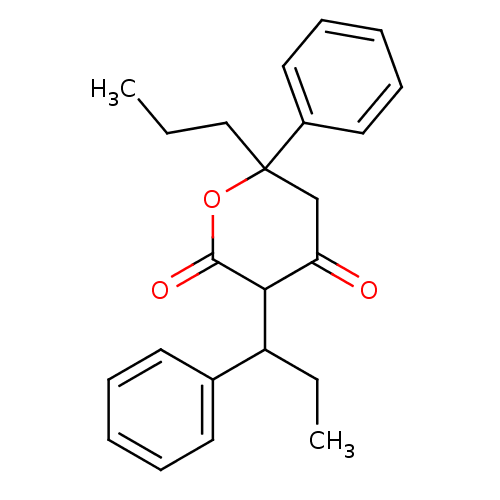
(4-Hydroxy-6-phenyl-3-(1-phenyl-propyl)-6-propyl-5,...)Show SMILES CCCC1(CC(=O)C(C(CC)c2ccccc2)C(=O)O1)c1ccccc1 Show InChI InChI=1S/C23H26O3/c1-3-15-23(18-13-9-6-10-14-18)16-20(24)21(22(25)26-23)19(4-2)17-11-7-5-8-12-17/h5-14,19,21H,3-4,15-16H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn
Curated by ChEMBL
| Assay Description
HIV Protease inhibitory activity |
J Med Chem 39: 4630-42 (1996)
Article DOI: 10.1021/jm960228q
BindingDB Entry DOI: 10.7270/Q247490H |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054609
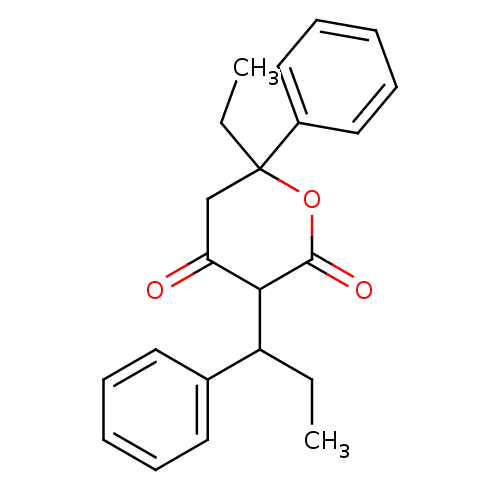
(6-Ethyl-4-hydroxy-6-phenyl-3-(1-phenyl-propyl)-5,6...)Show InChI InChI=1S/C22H24O3/c1-3-18(16-11-7-5-8-12-16)20-19(23)15-22(4-2,25-21(20)24)17-13-9-6-10-14-17/h5-14,18,20H,3-4,15H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn
Curated by ChEMBL
| Assay Description
HIV Protease inhibitory activity |
J Med Chem 39: 4630-42 (1996)
Article DOI: 10.1021/jm960228q
BindingDB Entry DOI: 10.7270/Q247490H |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM788
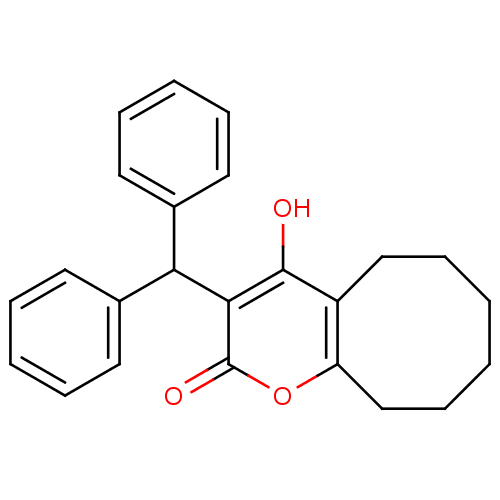
(3-(Diphenylmethyl)-5,6,7,8,9,1O-hexahydro-4-hydrox...)Show InChI InChI=1S/C24H24O3/c25-23-19-15-9-1-2-10-16-20(19)27-24(26)22(23)21(17-11-5-3-6-12-17)18-13-7-4-8-14-18/h3-8,11-14,21,25H,1-2,9-10,15-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | -38.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn
| Assay Description
HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... |
J Med Chem 38: 1884-91 (1995)
Article DOI: 10.1021/jm00011a008
BindingDB Entry DOI: 10.7270/Q2Q81B80 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50054624
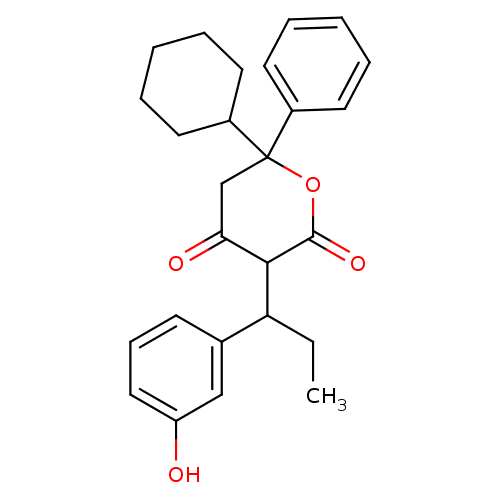
(6-Cyclohexyl-4-hydroxy-3-[1-(3-hydroxy-phenyl)-pro...)Show SMILES CCC(C1C(=O)CC(OC1=O)(C1CCCCC1)c1ccccc1)c1cccc(O)c1 Show InChI InChI=1S/C26H30O4/c1-2-22(18-10-9-15-21(27)16-18)24-23(28)17-26(30-25(24)29,19-11-5-3-6-12-19)20-13-7-4-8-14-20/h3,5-6,9-12,15-16,20,22,24,27H,2,4,7-8,13-14,17H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn
Curated by ChEMBL
| Assay Description
HIV Protease inhibitory activity |
J Med Chem 39: 4630-42 (1996)
Article DOI: 10.1021/jm960228q
BindingDB Entry DOI: 10.7270/Q247490H |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382321
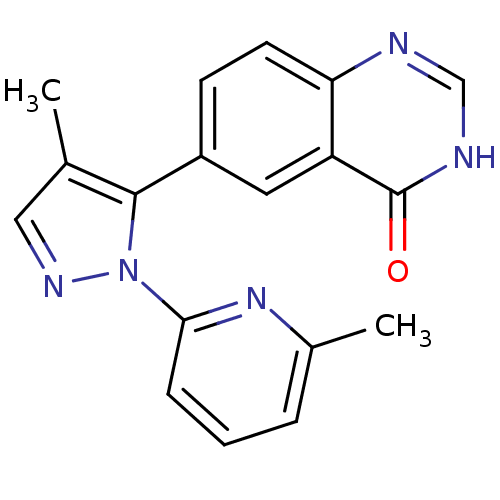
(CHEMBL2024688)Show SMILES Cc1cnn(c1-c1ccc2nc[nH]c(=O)c2c1)-c1cccc(C)n1 Show InChI InChI=1S/C18H15N5O/c1-11-9-21-23(16-5-3-4-12(2)22-16)17(11)13-6-7-15-14(8-13)18(24)20-10-19-15/h3-10H,1-2H3,(H,19,20,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382322
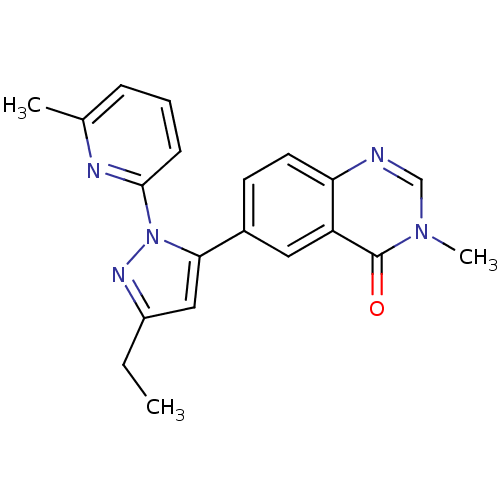
(CHEMBL2024689)Show SMILES CCc1cc(-c2ccc3ncn(C)c(=O)c3c2)n(n1)-c1cccc(C)n1 Show InChI InChI=1S/C20H19N5O/c1-4-15-11-18(25(23-15)19-7-5-6-13(2)22-19)14-8-9-17-16(10-14)20(26)24(3)12-21-17/h5-12H,4H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215294

(2-(N-(6-chloro-7-methyl-2,3-dioxo-1,2,3,4-tetrahyd...)Show SMILES Cc1cc2[nH]c(=O)c(=O)[nH]c2c(N(CC(O)=O)S(C)(=O)=O)c1Cl Show InChI InChI=1S/C12H12ClN3O6S/c1-5-3-6-9(15-12(20)11(19)14-6)10(8(5)13)16(4-7(17)18)23(2,21)22/h3H,4H2,1-2H3,(H,14,19)(H,15,20)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215284

(2-(N-(7-chloro-6-methyl-2,3-dioxo-1,2,3,4-tetrahyd...)Show SMILES Cc1c(Cl)cc2[nH]c(=O)c(=O)[nH]c2c1N(CC(O)=O)S(C)(=O)=O Show InChI InChI=1S/C12H12ClN3O6S/c1-5-6(13)3-7-9(15-12(20)11(19)14-7)10(5)16(4-8(17)18)23(2,21)22/h3H,4H2,1-2H3,(H,14,19)(H,15,20)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382324

(CHEMBL2024691)Show SMILES CCc1cnn(c1-c1ccc2nc[nH]c(=O)c2c1)-c1cccc(C)n1 Show InChI InChI=1S/C19H17N5O/c1-3-13-10-22-24(17-6-4-5-12(2)23-17)18(13)14-7-8-16-15(9-14)19(25)21-11-20-16/h4-11H,3H2,1-2H3,(H,20,21,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215283
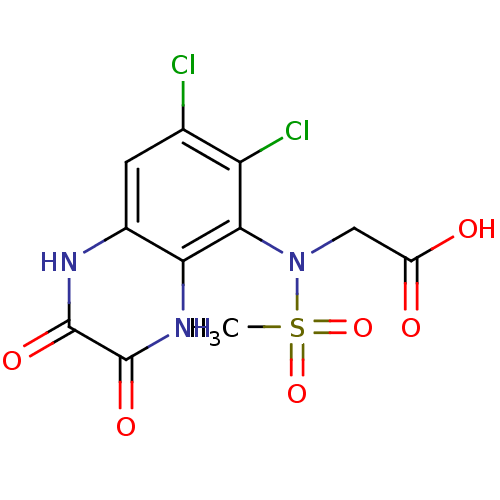
(2-(N-(6,7-dichloro-2,3-dioxo-1,2,3,4-tetrahydroqui...)Show SMILES CS(=O)(=O)N(CC(O)=O)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C11H9Cl2N3O6S/c1-23(21,22)16(3-6(17)18)9-7(13)4(12)2-5-8(9)15-11(20)10(19)14-5/h2H,3H2,1H3,(H,14,19)(H,15,20)(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382320
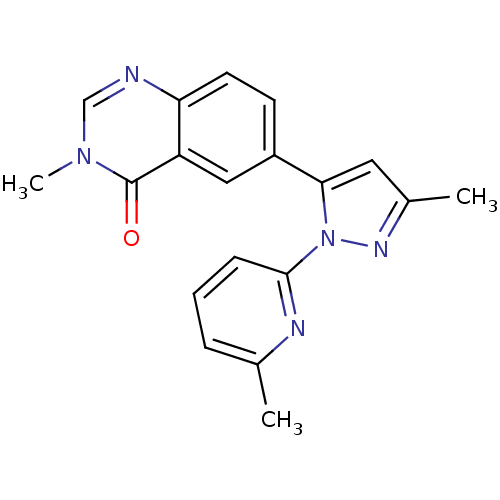
(CHEMBL2024687)Show SMILES Cc1cc(-c2ccc3ncn(C)c(=O)c3c2)n(n1)-c1cccc(C)n1 Show InChI InChI=1S/C19H17N5O/c1-12-5-4-6-18(21-12)24-17(9-13(2)22-24)14-7-8-16-15(10-14)19(25)23(3)11-20-16/h4-11H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382318

(CHEMBL2024685)Show SMILES Cc1cccc(n1)-n1nccc1-c1ccc2ncn(C=C)c(=O)c2c1 Show InChI InChI=1S/C19H15N5O/c1-3-23-12-20-16-8-7-14(11-15(16)19(23)25)17-9-10-21-24(17)18-6-4-5-13(2)22-18/h3-12H,1H2,2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215282
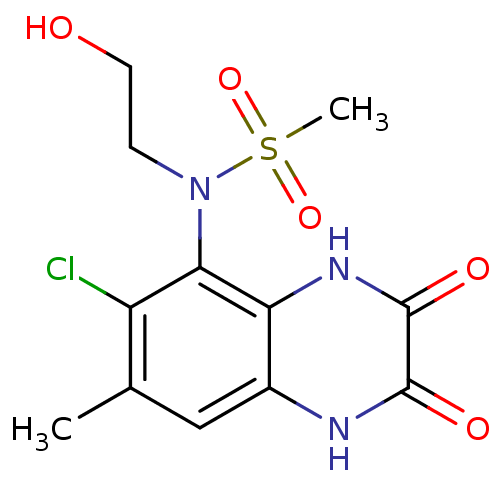
(CHEMBL429296 | N-(6-chloro-7-methyl-2,3-dioxo-1,2,...)Show SMILES Cc1cc2[nH]c(=O)c(=O)[nH]c2c(N(CCO)S(C)(=O)=O)c1Cl Show InChI InChI=1S/C12H14ClN3O5S/c1-6-5-7-9(15-12(19)11(18)14-7)10(8(6)13)16(3-4-17)22(2,20)21/h5,17H,3-4H2,1-2H3,(H,14,18)(H,15,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382319

(CHEMBL2024686)Show SMILES Cc1cnn(c1-c1ccc2ncn(C)c(=O)c2c1)-c1cccc(C)n1 Show InChI InChI=1S/C19H17N5O/c1-12-10-21-24(17-6-4-5-13(2)22-17)18(12)14-7-8-16-15(9-14)19(25)23(3)11-20-16/h4-11H,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382325

(CHEMBL2024693)Show SMILES Cc1cccc(n1)-n1nc2CCCc2c1-c1ccc2nc[nH]c(=O)c2c1 Show InChI InChI=1S/C20H17N5O/c1-12-4-2-7-18(23-12)25-19(14-5-3-6-17(14)24-25)13-8-9-16-15(10-13)20(26)22-11-21-16/h2,4,7-11H,3,5-6H2,1H3,(H,21,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382323
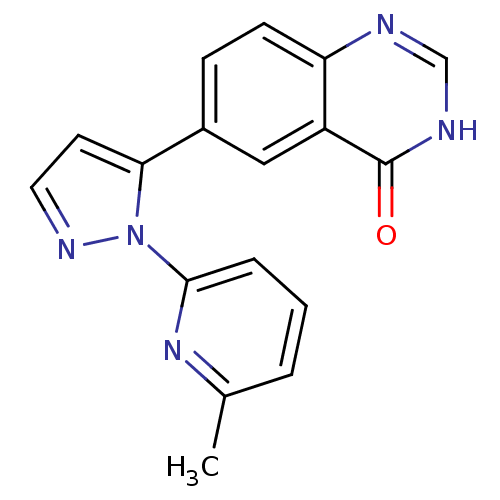
(CHEMBL2024690)Show InChI InChI=1S/C17H13N5O/c1-11-3-2-4-16(21-11)22-15(7-8-20-22)12-5-6-14-13(9-12)17(23)19-10-18-14/h2-10H,1H3,(H,18,19,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215286
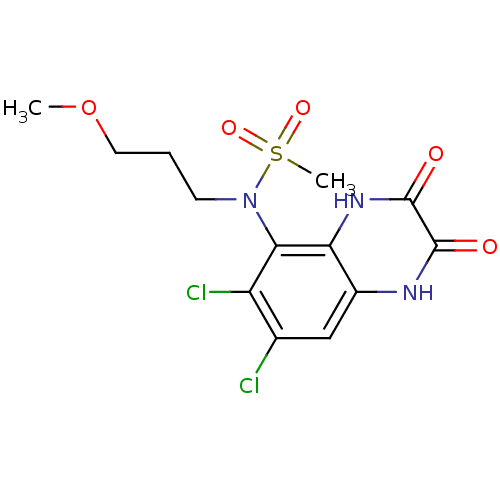
(CHEMBL399275 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES COCCCN(c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12)S(C)(=O)=O Show InChI InChI=1S/C13H15Cl2N3O5S/c1-23-5-3-4-18(24(2,21)22)11-9(15)7(14)6-8-10(11)17-13(20)12(19)16-8/h6H,3-5H2,1-2H3,(H,16,19)(H,17,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215286

(CHEMBL399275 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES COCCCN(c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12)S(C)(=O)=O Show InChI InChI=1S/C13H15Cl2N3O5S/c1-23-5-3-4-18(24(2,21)22)11-9(15)7(14)6-8-10(11)17-13(20)12(19)16-8/h6H,3-5H2,1-2H3,(H,16,19)(H,17,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215281
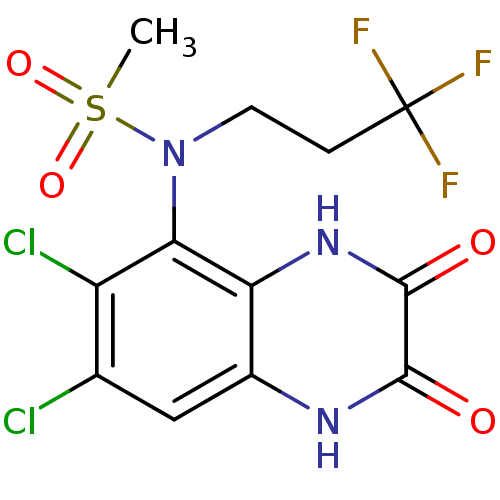
(CHEMBL248443 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CS(=O)(=O)N(CCC(F)(F)F)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C12H10Cl2F3N3O4S/c1-25(23,24)20(3-2-12(15,16)17)9-7(14)5(13)4-6-8(9)19-11(22)10(21)18-6/h4H,2-3H2,1H3,(H,18,21)(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215287

(CHEMBL247035 | N-(3-methoxybenzyl)-N-(6,7-dichloro...)Show SMILES COc1cccc(CN(c2c(Cl)c(Cl)cc3[nH]c(=O)c(=O)[nH]c23)S(C)(=O)=O)c1 Show InChI InChI=1S/C17H15Cl2N3O5S/c1-27-10-5-3-4-9(6-10)8-22(28(2,25)26)15-13(19)11(18)7-12-14(15)21-17(24)16(23)20-12/h3-7H,8H2,1-2H3,(H,20,23)(H,21,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382317
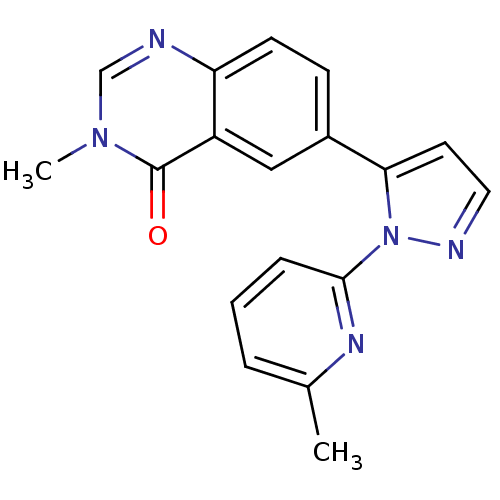
(CHEMBL2024684)Show InChI InChI=1S/C18H15N5O/c1-12-4-3-5-17(21-12)23-16(8-9-20-23)13-6-7-15-14(10-13)18(24)22(2)11-19-15/h3-11H,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215296

(CHEMBL401849 | N-(7-chloro-6-methyl-2,3-dioxo-1,2,...)Show SMILES Cc1c(Cl)cc2[nH]c(=O)c(=O)[nH]c2c1N(CCO)S(C)(=O)=O Show InChI InChI=1S/C12H14ClN3O5S/c1-6-7(13)5-8-9(15-12(19)11(18)14-8)10(6)16(3-4-17)22(2,20)21/h5,17H,3-4H2,1-2H3,(H,14,18)(H,15,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215285

(CHEMBL400508 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CCOCCCN(c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12)S(C)(=O)=O Show InChI InChI=1S/C14H17Cl2N3O5S/c1-3-24-6-4-5-19(25(2,22)23)12-10(16)8(15)7-9-11(12)18-14(21)13(20)17-9/h7H,3-6H2,1-2H3,(H,17,20)(H,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215276

(CHEMBL399519 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CCS(=O)(=O)N(CCC(F)(F)F)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C13H12Cl2F3N3O4S/c1-2-26(24,25)21(4-3-13(16,17)18)10-8(15)6(14)5-7-9(10)20-12(23)11(22)19-7/h5H,2-4H2,1H3,(H,19,22)(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215281

(CHEMBL248443 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CS(=O)(=O)N(CCC(F)(F)F)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C12H10Cl2F3N3O4S/c1-25(23,24)20(3-2-12(15,16)17)9-7(14)5(13)4-6-8(9)19-11(22)10(21)18-6/h4H,2-3H2,1H3,(H,18,21)(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215281

(CHEMBL248443 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CS(=O)(=O)N(CCC(F)(F)F)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C12H10Cl2F3N3O4S/c1-25(23,24)20(3-2-12(15,16)17)9-7(14)5(13)4-6-8(9)19-11(22)10(21)18-6/h4H,2-3H2,1H3,(H,18,21)(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50166407

((Z)-2-Benzoylamino-3-[4-(2-bromo-6-fluoro-phenoxy)...)Show SMILES OC(=O)C(\NC(=O)c1ccccc1)=C\c1ccc(Oc2c(F)cccc2Br)cc1 Show InChI InChI=1S/C22H15BrFNO4/c23-17-7-4-8-18(24)20(17)29-16-11-9-14(10-12-16)13-19(22(27)28)25-21(26)15-5-2-1-3-6-15/h1-13H,(H,25,26)(H,27,28)/b19-13- | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Hepatitis C virus NS5B polymerase |
Bioorg Med Chem Lett 15: 2481-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.066
BindingDB Entry DOI: 10.7270/Q2VH5NC7 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50166407

((Z)-2-Benzoylamino-3-[4-(2-bromo-6-fluoro-phenoxy)...)Show SMILES OC(=O)C(\NC(=O)c1ccccc1)=C\c1ccc(Oc2c(F)cccc2Br)cc1 Show InChI InChI=1S/C22H15BrFNO4/c23-17-7-4-8-18(24)20(17)29-16-11-9-14(10-12-16)13-19(22(27)28)25-21(26)15-5-2-1-3-6-15/h1-13H,(H,25,26)(H,27,28)/b19-13- | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against RNA dependent RNA polymerase Nonstructural protein 5B (HCV NS5B polymerase) in Hepatitis C virus |
Bioorg Med Chem Lett 15: 2812-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.106
BindingDB Entry DOI: 10.7270/Q26M36C7 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215279
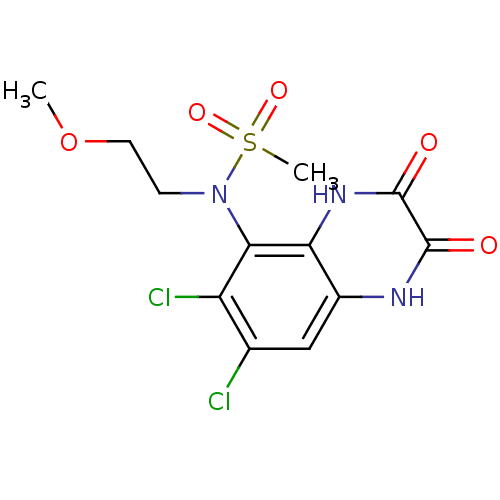
(CHEMBL248439 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES COCCN(c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12)S(C)(=O)=O Show InChI InChI=1S/C12H13Cl2N3O5S/c1-22-4-3-17(23(2,20)21)10-8(14)6(13)5-7-9(10)16-12(19)11(18)15-7/h5H,3-4H2,1-2H3,(H,15,18)(H,16,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215298

(CHEMBL399075 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CS(=O)(=O)N(CC1CCCCO1)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 |w:6.5| Show InChI InChI=1S/C15H17Cl2N3O5S/c1-26(23,24)20(7-8-4-2-3-5-25-8)13-11(17)9(16)6-10-12(13)19-15(22)14(21)18-10/h6,8H,2-5,7H2,1H3,(H,18,21)(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50166360
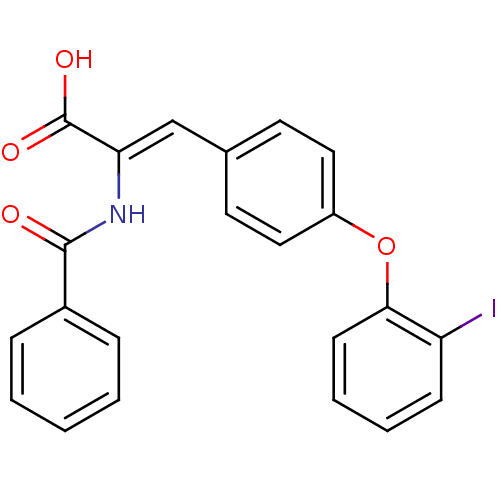
((Z)-2-Benzoylamino-3-[4-(2-iodo-phenoxy)-phenyl]-a...)Show SMILES OC(=O)C(\NC(=O)c1ccccc1)=C\c1ccc(Oc2ccccc2I)cc1 Show InChI InChI=1S/C22H16INO4/c23-18-8-4-5-9-20(18)28-17-12-10-15(11-13-17)14-19(22(26)27)24-21(25)16-6-2-1-3-7-16/h1-14H,(H,24,25)(H,26,27)/b19-14- | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Hepatitis C virus NS5B polymerase |
Bioorg Med Chem Lett 15: 2481-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.066
BindingDB Entry DOI: 10.7270/Q2VH5NC7 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215297
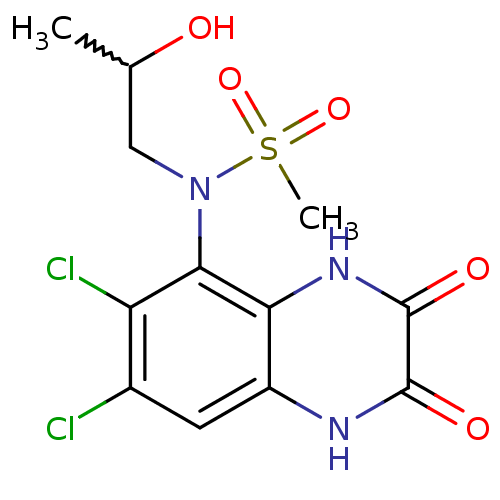
(CHEMBL247034 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CC(O)CN(c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12)S(C)(=O)=O |w:1.0| Show InChI InChI=1S/C12H13Cl2N3O5S/c1-5(18)4-17(23(2,21)22)10-8(14)6(13)3-7-9(10)16-12(20)11(19)15-7/h3,5,18H,4H2,1-2H3,(H,15,19)(H,16,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50215292

(CHEMBL401100 | N-(6,7-dichloro-2,3-dioxo-1,2,3,4-t...)Show SMILES CS(=O)(=O)N(CCCO)c1c(Cl)c(Cl)cc2[nH]c(=O)c(=O)[nH]c12 Show InChI InChI=1S/C12H13Cl2N3O5S/c1-23(21,22)17(3-2-4-18)10-8(14)6(13)5-7-9(10)16-12(20)11(19)15-7/h5,18H,2-4H2,1H3,(H,15,19)(H,16,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human NMDA NR1 receptor |
Bioorg Med Chem Lett 17: 4599-603 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.083
BindingDB Entry DOI: 10.7270/Q2QR4WTR |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Mus musculus) | BDBM50382318

(CHEMBL2024685)Show SMILES Cc1cccc(n1)-n1nccc1-c1ccc2ncn(C=C)c(=O)c2c1 Show InChI InChI=1S/C19H15N5O/c1-3-23-12-20-16-8-7-14(11-15(16)19(23)25)17-9-10-21-24(17)18-6-4-5-13(2)22-18/h3-12H,1H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 in mouse NIH/3T3 cells by smad binding element reporter based assay |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data