 Found 624 hits with Last Name = 'franzini' and Initial = 'm'
Found 624 hits with Last Name = 'franzini' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sEH using 14,15-epoxy-5Z,8Z,11Z-eicosatrienoic acid as substrate assessed as formation of 14,15-dihydroxy-5Z,8Z,11Zei... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sEH using 14,15-epoxy-5Z,8Z,11Z-eicosatrienoic acid as substrate assessed as formation of 14,15-dihydroxy-5Z,8Z,11Zei... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50519649

(CHEMBL4439913)Show SMILES CNC(=O)c1ccc2n(cnc2c1)-c1ccc(CC(=O)OC(C)(C)C)cc1 Show InChI InChI=1S/C21H23N3O3/c1-21(2,3)27-19(25)11-14-5-8-16(9-6-14)24-13-23-17-12-15(20(26)22-4)7-10-18(17)24/h5-10,12-13H,11H2,1-4H3,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant RIP3 (2 to 328 residues) expressed in baculovirus by ADP-glo assay |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50519649

(CHEMBL4439913)Show SMILES CNC(=O)c1ccc2n(cnc2c1)-c1ccc(CC(=O)OC(C)(C)C)cc1 Show InChI InChI=1S/C21H23N3O3/c1-21(2,3)27-19(25)11-14-5-8-16(9-6-14)24-13-23-17-12-15(20(26)22-4)7-10-18(17)24/h5-10,12-13H,11H2,1-4H3,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant RIP3 (2 to 328 residues) expressed in baculovirus by ADP-glo assay |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM282619
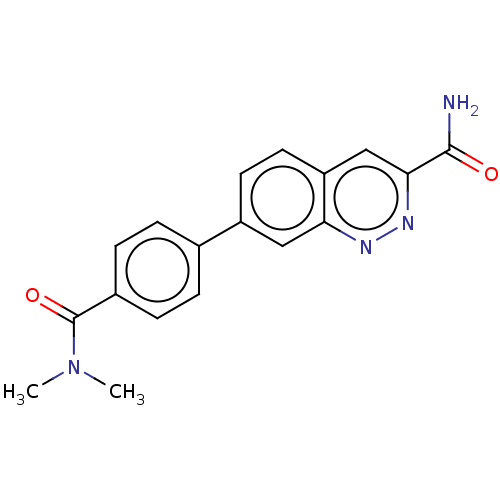
(US9884828, 2-114)Show SMILES CN(C)C(=O)c1ccc(cc1)-c1ccc2cc(nnc2c1)C(N)=O Show InChI InChI=1S/C18H16N4O2/c1-22(2)18(24)12-5-3-11(4-6-12)13-7-8-14-10-16(17(19)23)21-20-15(14)9-13/h3-10H,1-2H3,(H2,19,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... |
US Patent US9884828 (2018)
BindingDB Entry DOI: 10.7270/Q2NG4SNW |
More data for this
Ligand-Target Pair | |
2',5'-phosphodiesterase 12
(Homo sapiens) | BDBM50532283
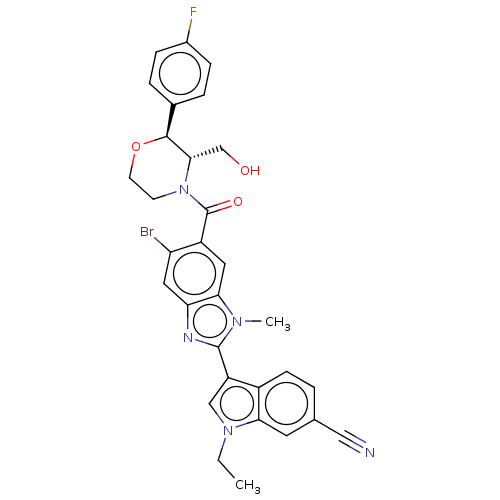
(CHEMBL4556129)Show SMILES CCn1cc(-c2nc3cc(Br)c(cc3n2C)C(=O)N2CCO[C@H]([C@@H]2CO)c2ccc(F)cc2)c2ccc(cc12)C#N |r| Show InChI InChI=1S/C31H27BrFN5O3/c1-3-37-16-23(21-9-4-18(15-34)12-26(21)37)30-35-25-14-24(32)22(13-27(25)36(30)2)31(40)38-10-11-41-29(28(38)17-39)19-5-7-20(33)8-6-19/h4-9,12-14,16,28-29,39H,3,10-11,17H2,1-2H3/t28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human PDE12 (17 to 609 residues) expresssed in Escherichia coli BL21(DE3) cells using 2-5A as substrate assessed as AMP monomers and AT... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
2',5'-phosphodiesterase 12
(Homo sapiens) | BDBM50532283

(CHEMBL4556129)Show SMILES CCn1cc(-c2nc3cc(Br)c(cc3n2C)C(=O)N2CCO[C@H]([C@@H]2CO)c2ccc(F)cc2)c2ccc(cc12)C#N |r| Show InChI InChI=1S/C31H27BrFN5O3/c1-3-37-16-23(21-9-4-18(15-34)12-26(21)37)30-35-25-14-24(32)22(13-27(25)36(30)2)31(40)38-10-11-41-29(28(38)17-39)19-5-7-20(33)8-6-19/h4-9,12-14,16,28-29,39H,3,10-11,17H2,1-2H3/t28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human PDE12 (17 to 609 residues) expresssed in Escherichia coli BL21(DE3) cells using 2-5A as substrate assessed as AMP monomers and AT... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM282670
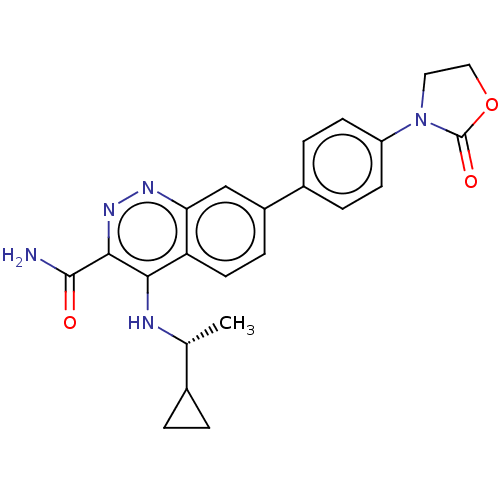
(US9884828, 9-213)Show SMILES C[C@@H](Nc1c(nnc2cc(ccc12)-c1ccc(cc1)N1CCOC1=O)C(N)=O)C1CC1 Show InChI InChI=1S/C23H23N5O3/c1-13(14-2-3-14)25-20-18-9-6-16(12-19(18)26-27-21(20)22(24)29)15-4-7-17(8-5-15)28-10-11-31-23(28)30/h4-9,12-14H,2-3,10-11H2,1H3,(H2,24,29)(H,25,26)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... |
US Patent US9884828 (2018)
BindingDB Entry DOI: 10.7270/Q2NG4SNW |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50428716

(CHEMBL2333128 | US9884828, 2-41)Show SMILES C[C@@H](Nc1c(nnc2cc(ccc12)-c1ccc(cc1)S(C)(=O)=O)C(N)=O)C1CC1 |r| Show InChI InChI=1S/C21H22N4O3S/c1-12(13-3-4-13)23-19-17-10-7-15(11-18(17)24-25-20(19)21(22)26)14-5-8-16(9-6-14)29(2,27)28/h5-13H,3-4H2,1-2H3,(H2,22,26)(H,23,24)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory... |
Bioorg Med Chem Lett 23: 71-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.11.021
BindingDB Entry DOI: 10.7270/Q2930VHH |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50428717
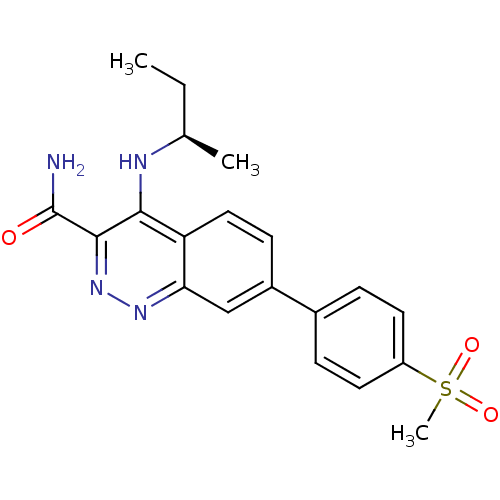
(CHEMBL2333127 | US9884828, 2-37)Show SMILES CC[C@@H](C)Nc1c(nnc2cc(ccc12)-c1ccc(cc1)S(C)(=O)=O)C(N)=O |r| Show InChI InChI=1S/C20H22N4O3S/c1-4-12(2)22-18-16-10-7-14(11-17(16)23-24-19(18)20(21)25)13-5-8-15(9-6-13)28(3,26)27/h5-12H,4H2,1-3H3,(H2,21,25)(H,22,23)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory... |
Bioorg Med Chem Lett 23: 71-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.11.021
BindingDB Entry DOI: 10.7270/Q2930VHH |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM282606
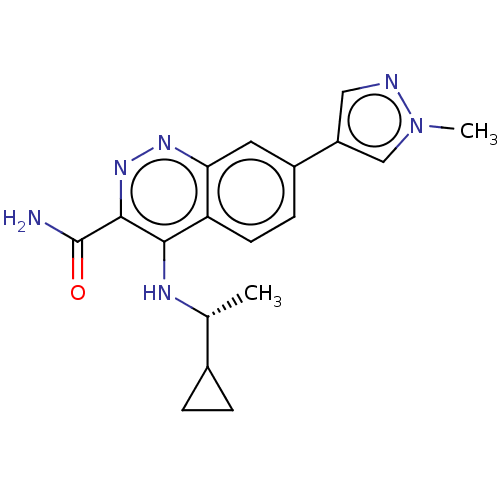
(US9884828, 2-101)Show SMILES C[C@@H](Nc1c(nnc2cc(ccc12)-c1cnn(C)c1)C(N)=O)C1CC1 Show InChI InChI=1S/C18H20N6O/c1-10(11-3-4-11)21-16-14-6-5-12(13-8-20-24(2)9-13)7-15(14)22-23-17(16)18(19)25/h5-11H,3-4H2,1-2H3,(H2,19,25)(H,21,22)/t10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... |
US Patent US9884828 (2018)
BindingDB Entry DOI: 10.7270/Q2NG4SNW |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM282616
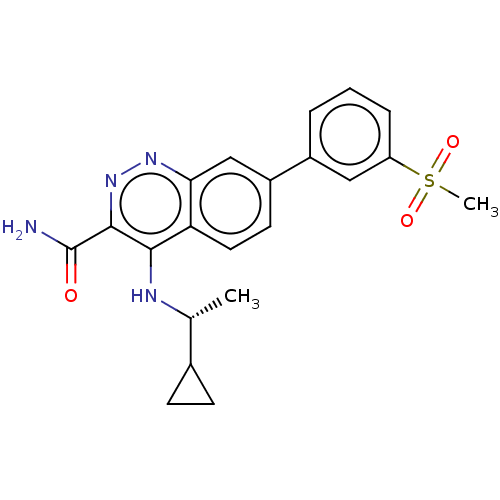
(US9884828, 2-111)Show SMILES C[C@@H](Nc1c(nnc2cc(ccc12)-c1cccc(c1)S(C)(=O)=O)C(N)=O)C1CC1 Show InChI InChI=1S/C21H22N4O3S/c1-12(13-6-7-13)23-19-17-9-8-15(11-18(17)24-25-20(19)21(22)26)14-4-3-5-16(10-14)29(2,27)28/h3-5,8-13H,6-7H2,1-2H3,(H2,22,26)(H,23,24)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... |
US Patent US9884828 (2018)
BindingDB Entry DOI: 10.7270/Q2NG4SNW |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM282625
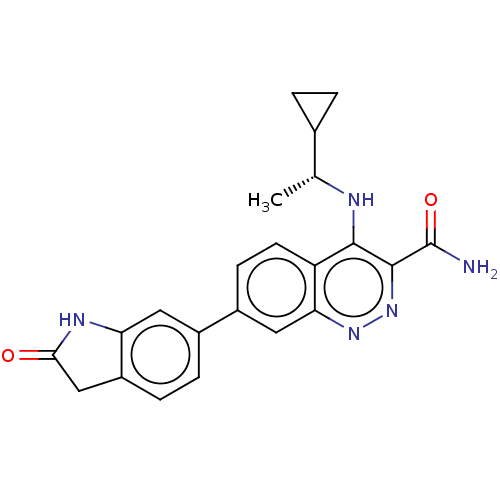
(US9884828, 2-120)Show SMILES C[C@@H](Nc1c(nnc2cc(ccc12)-c1ccc2CC(=O)Nc2c1)C(N)=O)C1CC1 Show InChI InChI=1S/C22H21N5O2/c1-11(12-2-3-12)24-20-16-7-6-14(9-18(16)26-27-21(20)22(23)29)13-4-5-15-10-19(28)25-17(15)8-13/h4-9,11-12H,2-3,10H2,1H3,(H2,23,29)(H,24,26)(H,25,28)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... |
US Patent US9884828 (2018)
BindingDB Entry DOI: 10.7270/Q2NG4SNW |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50428701

(CHEMBL2333115 | US9884828, 2-127)Show InChI InChI=1S/C15H16N6O/c1-8(2)19-13-11-5-9(10-6-17-18-7-10)3-4-12(11)20-21-14(13)15(16)22/h3-8H,1-2H3,(H2,16,22)(H,17,18)(H,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory... |
Bioorg Med Chem Lett 23: 71-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.11.021
BindingDB Entry DOI: 10.7270/Q2930VHH |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380930
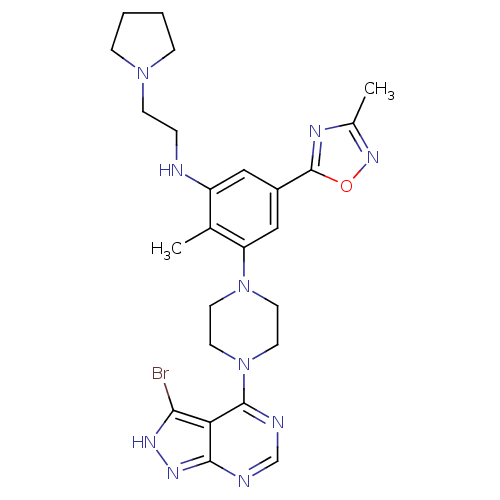
(CHEMBL2016890)Show SMILES Cc1noc(n1)-c1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C25H31BrN10O/c1-16-19(27-5-8-34-6-3-4-7-34)13-18(25-30-17(2)33-37-25)14-20(16)35-9-11-36(12-10-35)24-21-22(26)31-32-23(21)28-15-29-24/h13-15,27H,3-12H2,1-2H3,(H,28,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380931
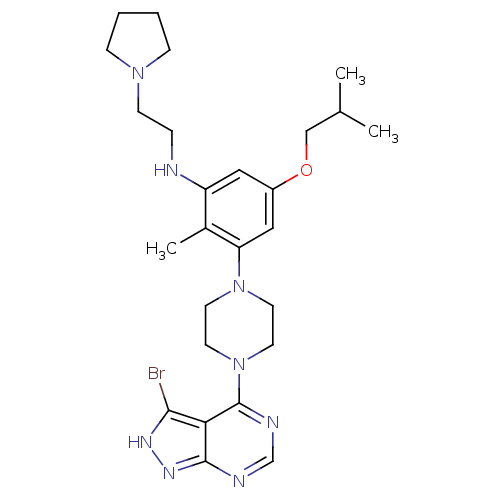
(CHEMBL2016886)Show SMILES CC(C)COc1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C26H37BrN8O/c1-18(2)16-36-20-14-21(28-6-9-33-7-4-5-8-33)19(3)22(15-20)34-10-12-35(13-11-34)26-23-24(27)31-32-25(23)29-17-30-26/h14-15,17-18,28H,4-13,16H2,1-3H3,(H,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380935
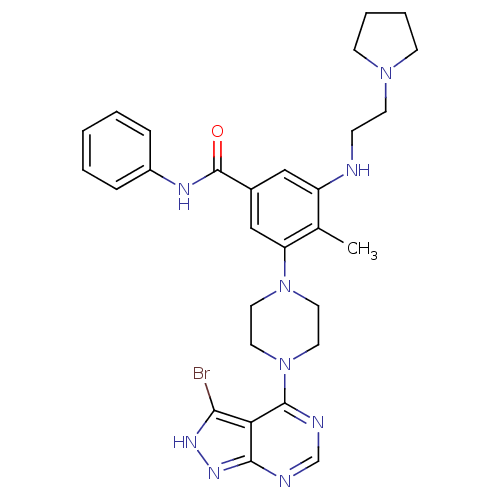
(CHEMBL2016887)Show SMILES Cc1c(NCCN2CCCC2)cc(cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12)C(=O)Nc1ccccc1 Show InChI InChI=1S/C29H34BrN9O/c1-20-23(31-9-12-37-10-5-6-11-37)17-21(29(40)34-22-7-3-2-4-8-22)18-24(20)38-13-15-39(16-14-38)28-25-26(30)35-36-27(25)32-19-33-28/h2-4,7-8,17-19,31H,5-6,9-16H2,1H3,(H,34,40)(H,32,33,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380929
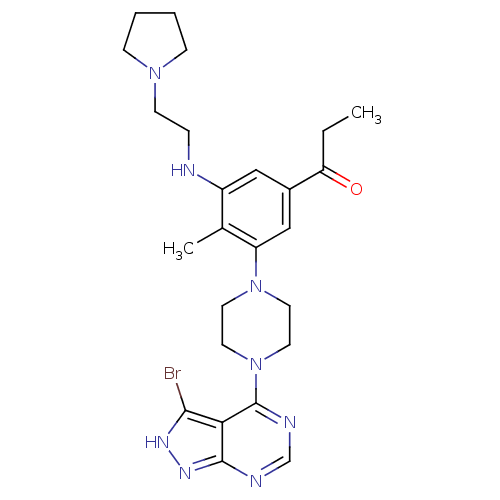
(CHEMBL2016892)Show SMILES CCC(=O)c1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C25H33BrN8O/c1-3-21(35)18-14-19(27-6-9-32-7-4-5-8-32)17(2)20(15-18)33-10-12-34(13-11-33)25-22-23(26)30-31-24(22)28-16-29-25/h14-16,27H,3-13H2,1-2H3,(H,28,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50380939
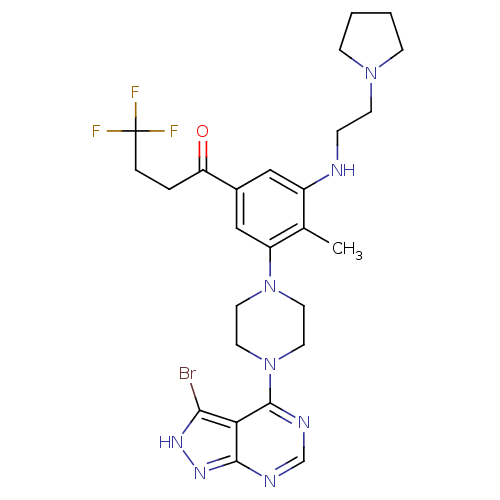
(CHEMBL2016893)Show SMILES Cc1c(NCCN2CCCC2)cc(cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12)C(=O)CCC(F)(F)F Show InChI InChI=1S/C26H32BrF3N8O/c1-17-19(31-6-9-36-7-2-3-8-36)14-18(21(39)4-5-26(28,29)30)15-20(17)37-10-12-38(13-11-37)25-22-23(27)34-35-24(22)32-16-33-25/h14-16,31H,2-13H2,1H3,(H,32,33,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50272192
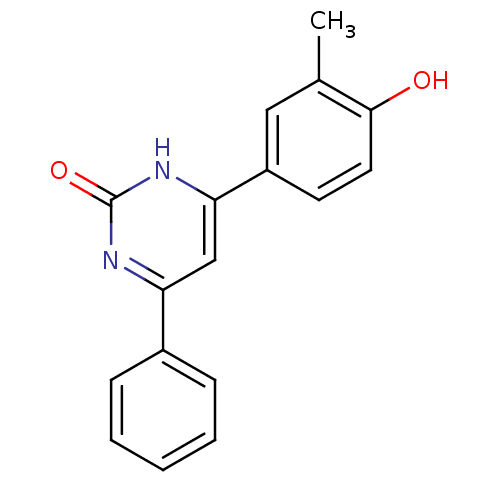
(4-(4-hydroxy-3-methylphenyl)-6-phenylpyrimidin-2(1...)Show InChI InChI=1S/C17H14N2O2/c1-11-9-13(7-8-16(11)20)15-10-14(18-17(21)19-15)12-5-3-2-4-6-12/h2-10,20H,1H3,(H,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50532278
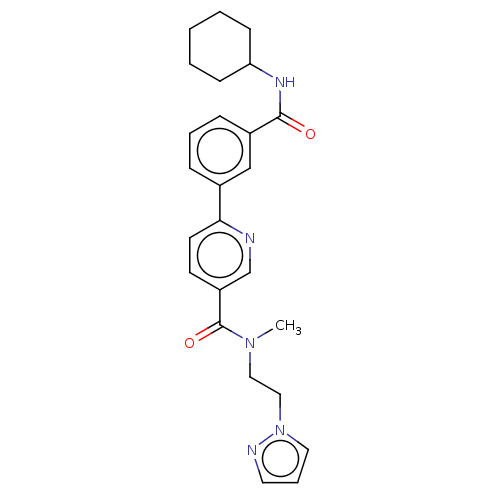
(CHEMBL4452411)Show SMILES CN(CCn1cccn1)C(=O)c1ccc(nc1)-c1cccc(c1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C25H29N5O2/c1-29(15-16-30-14-6-13-27-30)25(32)21-11-12-23(26-18-21)19-7-5-8-20(17-19)24(31)28-22-9-3-2-4-10-22/h5-8,11-14,17-18,22H,2-4,9-10,15-16H2,1H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His-tagged human recombinant sEH expressed in insect Sf21 cells using Epoxy Fluor 7 as substrate preincubated fo... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50532278

(CHEMBL4452411)Show SMILES CN(CCn1cccn1)C(=O)c1ccc(nc1)-c1cccc(c1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C25H29N5O2/c1-29(15-16-30-14-6-13-27-30)25(32)21-11-12-23(26-18-21)19-7-5-8-20(17-19)24(31)28-22-9-3-2-4-10-22/h5-8,11-14,17-18,22H,2-4,9-10,15-16H2,1H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His-tagged human recombinant sEH expressed in insect Sf21 cells using Epoxy Fluor 7 as substrate preincubated fo... |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50428703

(CHEMBL2333113 | US9884828, 2-100)Show SMILES C[C@@H](Nc1c(nnc2cc(ccc12)-c1ccncc1)C(N)=O)C1CC1 |r| Show InChI InChI=1S/C19H19N5O/c1-11(12-2-3-12)22-17-15-5-4-14(13-6-8-21-9-7-13)10-16(15)23-24-18(17)19(20)25/h4-12H,2-3H2,1H3,(H2,20,25)(H,22,23)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type GST-tagged LRRK2 (970 to 2527 amino acid residues) (unknown origin) assessed as inhibition of biotinylated-LRRKtide phosphory... |
Bioorg Med Chem Lett 23: 71-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.11.021
BindingDB Entry DOI: 10.7270/Q2930VHH |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM282626
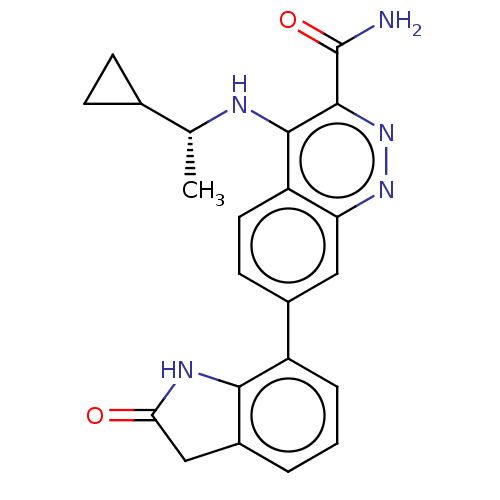
(US9884828, 2-121)Show SMILES C[C@@H](Nc1c(nnc2cc(ccc12)-c1cccc2CC(=O)Nc12)C(N)=O)C1CC1 Show InChI InChI=1S/C22H21N5O2/c1-11(12-5-6-12)24-20-16-8-7-13(9-17(16)26-27-21(20)22(23)29)15-4-2-3-14-10-18(28)25-19(14)15/h2-4,7-9,11-12H,5-6,10H2,1H3,(H2,23,29)(H,24,26)(H,25,28)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Imago Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds as described herein (compounds of Formula I, e.g., compounds of the above Examples) are tested for their in vitro kinase activities using v... |
US Patent US9884828 (2018)
BindingDB Entry DOI: 10.7270/Q2NG4SNW |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380932
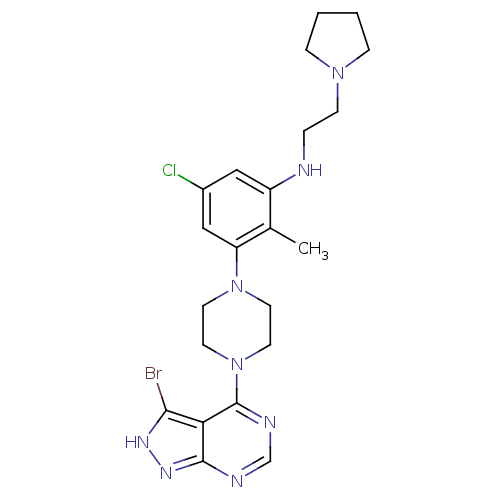
(CHEMBL2016771)Show SMILES Cc1c(NCCN2CCCC2)cc(Cl)cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C22H28BrClN8/c1-15-17(25-4-7-30-5-2-3-6-30)12-16(24)13-18(15)31-8-10-32(11-9-31)22-19-20(23)28-29-21(19)26-14-27-22/h12-14,25H,2-11H2,1H3,(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50379531
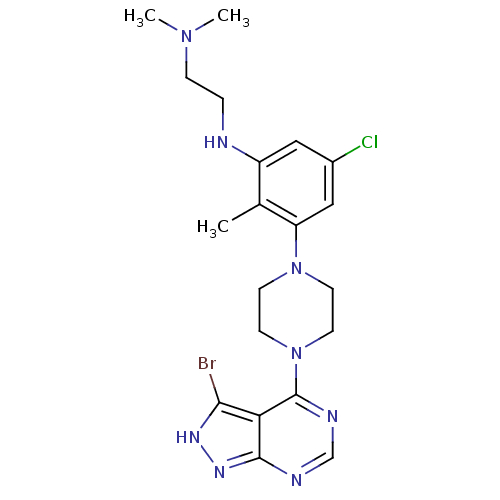
(CHEMBL2012702)Show SMILES CN(C)CCNc1cc(Cl)cc(N2CCN(CC2)c2ncnc3n[nH]c(Br)c23)c1C Show InChI InChI=1S/C20H26BrClN8/c1-13-15(23-4-5-28(2)3)10-14(22)11-16(13)29-6-8-30(9-7-29)20-17-18(21)26-27-19(17)24-12-25-20/h10-12,23H,4-9H2,1-3H3,(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380936
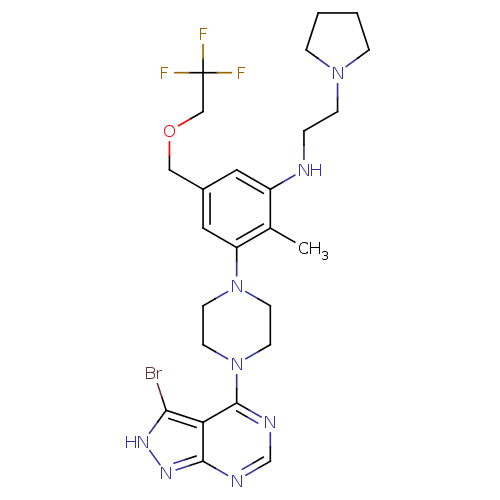
(CHEMBL2016888)Show SMILES Cc1c(NCCN2CCCC2)cc(COCC(F)(F)F)cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C25H32BrF3N8O/c1-17-19(30-4-7-35-5-2-3-6-35)12-18(14-38-15-25(27,28)29)13-20(17)36-8-10-37(11-9-36)24-21-22(26)33-34-23(21)31-16-32-24/h12-13,16,30H,2-11,14-15H2,1H3,(H,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380937
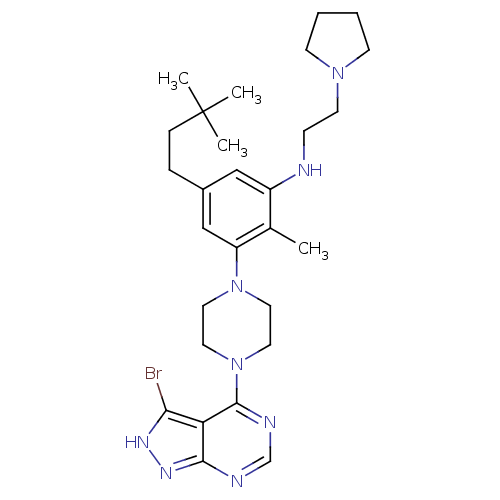
(CHEMBL2016889)Show SMILES Cc1c(NCCN2CCCC2)cc(CCC(C)(C)C)cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C28H41BrN8/c1-20-22(30-9-12-35-10-5-6-11-35)17-21(7-8-28(2,3)4)18-23(20)36-13-15-37(16-14-36)27-24-25(29)33-34-26(24)31-19-32-27/h17-19,30H,5-16H2,1-4H3,(H,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380938
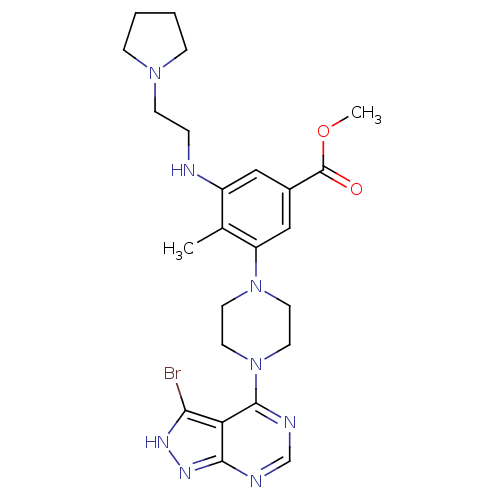
(CHEMBL2016891)Show SMILES COC(=O)c1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C24H31BrN8O2/c1-16-18(26-5-8-31-6-3-4-7-31)13-17(24(34)35-2)14-19(16)32-9-11-33(12-10-32)23-20-21(25)29-30-22(20)27-15-28-23/h13-15,26H,3-12H2,1-2H3,(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380939

(CHEMBL2016893)Show SMILES Cc1c(NCCN2CCCC2)cc(cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12)C(=O)CCC(F)(F)F Show InChI InChI=1S/C26H32BrF3N8O/c1-17-19(31-6-9-36-7-2-3-8-36)14-18(21(39)4-5-26(28,29)30)15-20(17)37-10-12-38(13-11-37)25-22-23(27)34-35-24(22)32-16-33-25/h14-16,31H,2-13H2,1H3,(H,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50379530
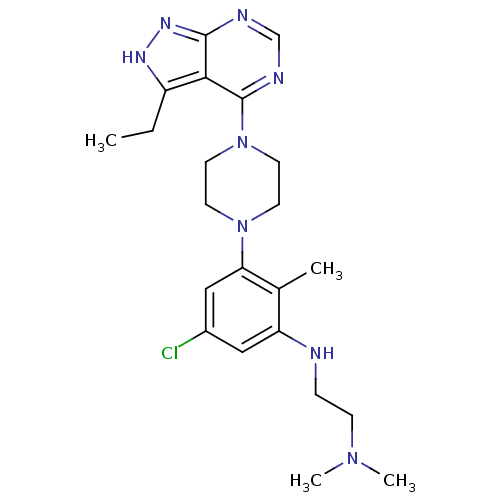
(CHEMBL2012701)Show SMILES CCc1[nH]nc2ncnc(N3CCN(CC3)c3cc(Cl)cc(NCCN(C)C)c3C)c12 Show InChI InChI=1S/C22H31ClN8/c1-5-17-20-21(28-27-17)25-14-26-22(20)31-10-8-30(9-11-31)19-13-16(23)12-18(15(19)2)24-6-7-29(3)4/h12-14,24H,5-11H2,1-4H3,(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380934
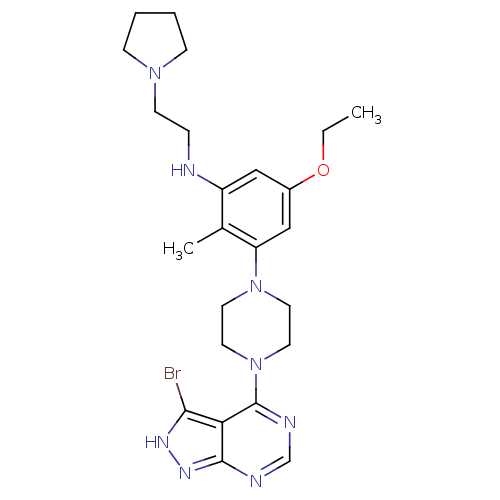
(CHEMBL2016885)Show SMILES CCOc1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C24H33BrN8O/c1-3-34-18-14-19(26-6-9-31-7-4-5-8-31)17(2)20(15-18)32-10-12-33(13-11-32)24-21-22(25)29-30-23(21)27-16-28-24/h14-16,26H,3-13H2,1-2H3,(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385084

(CHEMBL2035636)Show SMILES CN1C[C@@H]2CC[C@H]1CN2Cc1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 |r| Show InChI InChI=1S/C18H19BrN4O2/c1-22-7-12-4-3-11(22)8-23(12)9-15-20-16-13-6-10(19)2-5-14(13)25-17(16)18(24)21-15/h2,5-6,11-12H,3-4,7-9H2,1H3,(H,20,21,24)/t11-,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385078
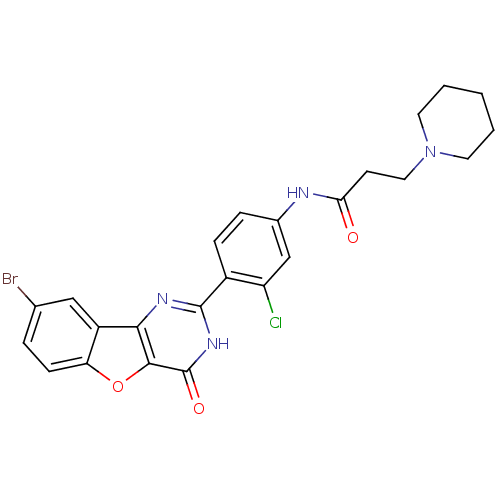
(CHEMBL2035629)Show SMILES Clc1cc(NC(=O)CCN2CCCCC2)ccc1-c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 Show InChI InChI=1S/C24H22BrClN4O3/c25-14-4-7-19-17(12-14)21-22(33-19)24(32)29-23(28-21)16-6-5-15(13-18(16)26)27-20(31)8-11-30-9-2-1-3-10-30/h4-7,12-13H,1-3,8-11H2,(H,27,31)(H,28,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385075

(CHEMBL2035626)Show SMILES NCc1ccc(c(Cl)c1)-c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 Show InChI InChI=1S/C17H11BrClN3O2/c18-9-2-4-13-11(6-9)14-15(24-13)17(23)22-16(21-14)10-3-1-8(7-20)5-12(10)19/h1-6H,7,20H2,(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380933

(CHEMBL2012699)Show SMILES CCc1[nH]nc2ncnc(N3CCN(CC3)c3cc(Cl)cc(NCCN4CCCC4)c3C)c12 Show InChI InChI=1S/C24H33ClN8/c1-3-19-22-23(30-29-19)27-16-28-24(22)33-12-10-32(11-13-33)21-15-18(25)14-20(17(21)2)26-6-9-31-7-4-5-8-31/h14-16,26H,3-13H2,1-2H3,(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383714
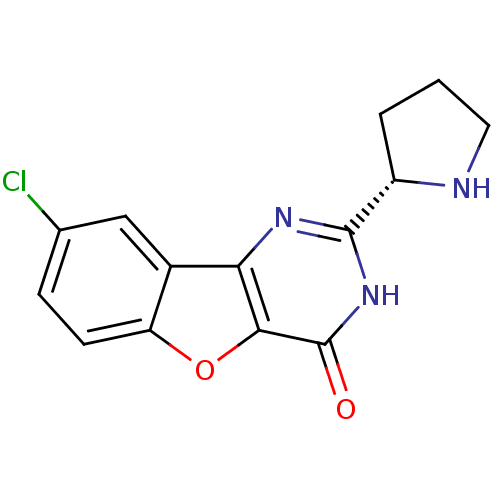
(CHEMBL2030402)Show SMILES Clc1ccc2oc3c(nc([nH]c3=O)[C@@H]3CCCN3)c2c1 |r| Show InChI InChI=1S/C14H12ClN3O2/c15-7-3-4-10-8(6-7)11-12(20-10)14(19)18-13(17-11)9-2-1-5-16-9/h3-4,6,9,16H,1-2,5H2,(H,17,18,19)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383736
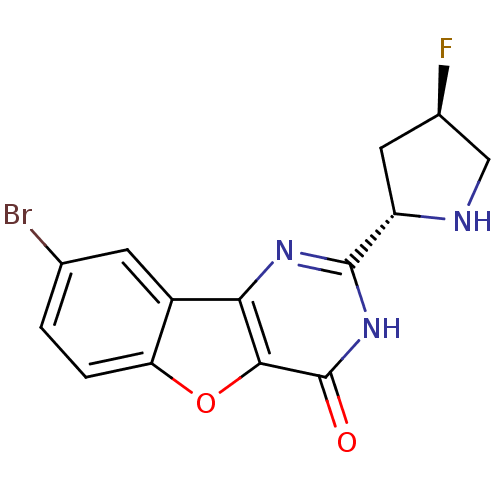
(CHEMBL2030400)Show SMILES F[C@H]1CN[C@@H](C1)c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 |r| Show InChI InChI=1S/C14H11BrFN3O2/c15-6-1-2-10-8(3-6)11-12(21-10)14(20)19-13(18-11)9-4-7(16)5-17-9/h1-3,7,9,17H,4-5H2,(H,18,19,20)/t7-,9+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383727
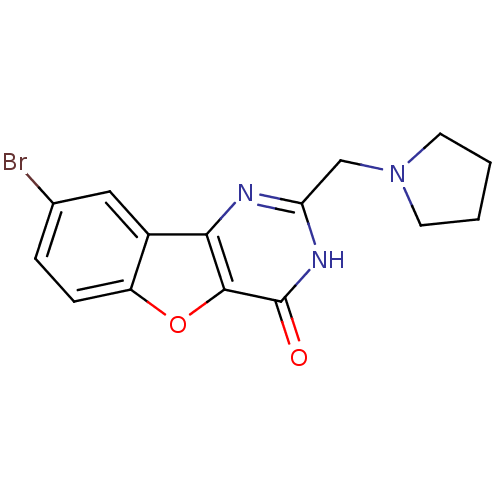
(CHEMBL2030389)Show InChI InChI=1S/C15H14BrN3O2/c16-9-3-4-11-10(7-9)13-14(21-11)15(20)18-12(17-13)8-19-5-1-2-6-19/h3-4,7H,1-2,5-6,8H2,(H,17,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50383711
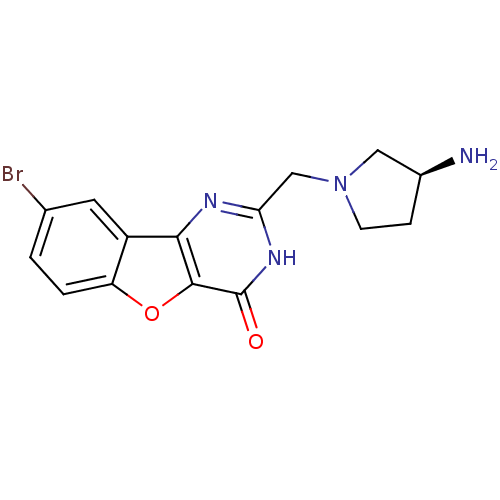
(CHEMBL2030387)Show SMILES N[C@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H15BrN4O2/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)19-12(18-13)7-20-4-3-9(17)6-20/h1-2,5,9H,3-4,6-7,17H2,(H,18,19,21)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by chemiluminescence assay |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383740
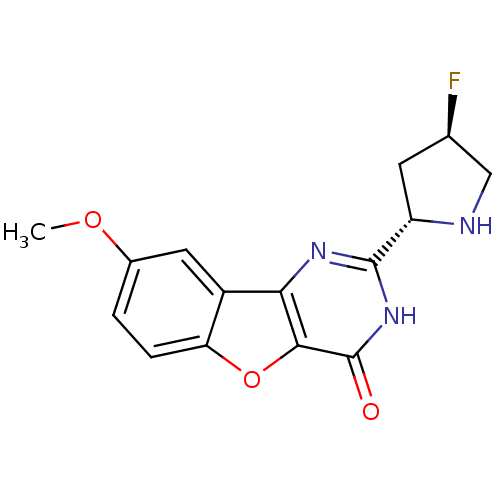
(CHEMBL2030405)Show SMILES COc1ccc2oc3c(nc([nH]c3=O)[C@@H]3C[C@@H](F)CN3)c2c1 |r| Show InChI InChI=1S/C15H14FN3O3/c1-21-8-2-3-11-9(5-8)12-13(22-11)15(20)19-14(18-12)10-4-7(16)6-17-10/h2-3,5,7,10,17H,4,6H2,1H3,(H,18,19,20)/t7-,10+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Gallus gallus (Chicken)) | BDBM50532280

(CHEMBL4465847)Show SMILES [H][C@]1(CCNC(=O)[C@H](CCCNC(=O)c2cnccn2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC2CCCCC2)NC(=O)\C=C/C(=O)N1)C(O)=O |r,c:48| Show InChI InChI=1S/C36H46N8O8/c45-30-13-14-31(46)42-27(20-23-8-3-1-4-9-23)34(49)44-28(21-24-10-5-2-6-11-24)35(50)43-25(32(47)40-17-15-26(41-30)36(51)52)12-7-16-39-33(48)29-22-37-18-19-38-29/h2,5-6,10-11,13-14,18-19,22-23,25-28H,1,3-4,7-9,12,15-17,20-21H2,(H,39,48)(H,40,47)(H,41,45)(H,42,46)(H,43,50)(H,44,49)(H,51,52)/b14-13-/t25-,26-,27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of chicken Src kinase domain using AEEEIYGEFAKKK as substrate by continuous spectrophotometric assay |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Gallus gallus (Chicken)) | BDBM50532280

(CHEMBL4465847)Show SMILES [H][C@]1(CCNC(=O)[C@H](CCCNC(=O)c2cnccn2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC2CCCCC2)NC(=O)\C=C/C(=O)N1)C(O)=O |r,c:48| Show InChI InChI=1S/C36H46N8O8/c45-30-13-14-31(46)42-27(20-23-8-3-1-4-9-23)34(49)44-28(21-24-10-5-2-6-11-24)35(50)43-25(32(47)40-17-15-26(41-30)36(51)52)12-7-16-39-33(48)29-22-37-18-19-38-29/h2,5-6,10-11,13-14,18-19,22-23,25-28H,1,3-4,7-9,12,15-17,20-21H2,(H,39,48)(H,40,47)(H,41,45)(H,42,46)(H,43,50)(H,44,49)(H,51,52)/b14-13-/t25-,26-,27-,28-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of chicken Src kinase domain using AEEEIYGEFAKKK as substrate by continuous spectrophotometric assay |
J Med Chem 59: 6629-44 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01874
BindingDB Entry DOI: 10.7270/Q2N58QV5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50383711

(CHEMBL2030387)Show SMILES N[C@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H15BrN4O2/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)19-12(18-13)7-20-4-3-9(17)6-20/h1-2,5,9H,3-4,6-7,17H2,(H,18,19,21)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50383725
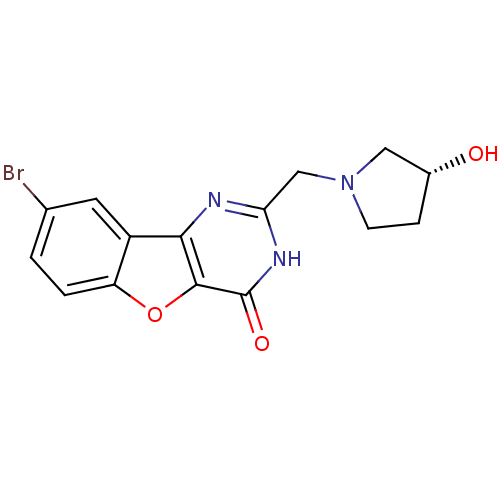
(CHEMBL2030386)Show SMILES O[C@@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H14BrN3O3/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)18-12(17-13)7-19-4-3-9(20)6-19/h1-2,5,9,20H,3-4,6-7H2,(H,17,18,21)/t9-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM3 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383739
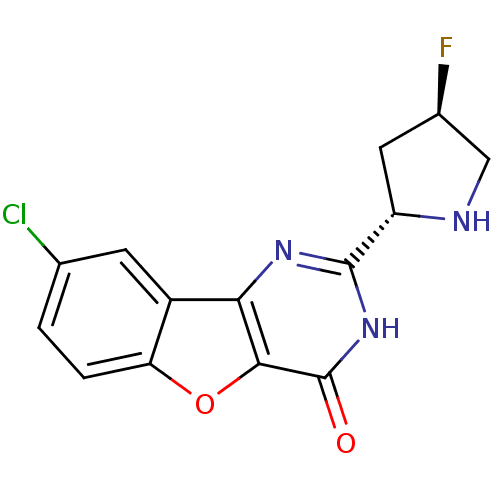
(CHEMBL2030404)Show SMILES F[C@H]1CN[C@@H](C1)c1nc2c3cc(Cl)ccc3oc2c(=O)[nH]1 |r| Show InChI InChI=1S/C14H11ClFN3O2/c15-6-1-2-10-8(3-6)11-12(21-10)14(20)19-13(18-11)9-4-7(16)5-17-9/h1-3,7,9,17H,4-5H2,(H,18,19,20)/t7-,9+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385076

(CHEMBL2035627)Show SMILES Clc1cc(ccc1-c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1)C(=O)NCCN1CCCCC1 Show InChI InChI=1S/C24H22BrClN4O3/c25-15-5-7-19-17(13-15)20-21(33-19)24(32)29-22(28-20)16-6-4-14(12-18(16)26)23(31)27-8-11-30-9-2-1-3-10-30/h4-7,12-13H,1-3,8-11H2,(H,27,31)(H,28,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385077

(CHEMBL2035628)Show SMILES Clc1cc(CNC2CCNCC2)ccc1-c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 Show InChI InChI=1S/C22H20BrClN4O2/c23-13-2-4-18-16(10-13)19-20(30-18)22(29)28-21(27-19)15-3-1-12(9-17(15)24)11-26-14-5-7-25-8-6-14/h1-4,9-10,14,25-26H,5-8,11H2,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383730
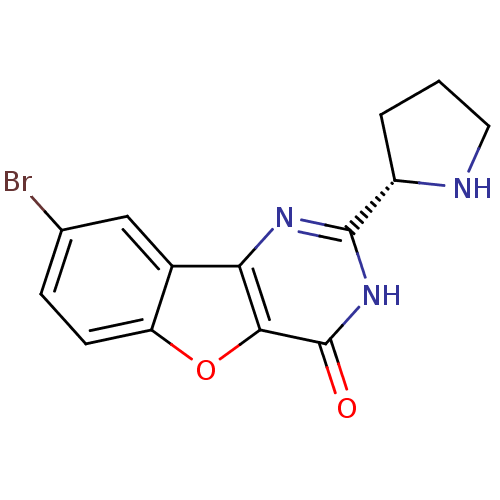
(CHEMBL2030393)Show SMILES Brc1ccc2oc3c(nc([nH]c3=O)[C@@H]3CCCN3)c2c1 |r| Show InChI InChI=1S/C14H12BrN3O2/c15-7-3-4-10-8(6-7)11-12(20-10)14(19)18-13(17-11)9-2-1-5-16-9/h3-4,6,9,16H,1-2,5H2,(H,17,18,19)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50383725

(CHEMBL2030386)Show SMILES O[C@@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H14BrN3O3/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)18-12(17-13)7-19-4-3-9(20)6-19/h1-2,5,9,20H,3-4,6-7H2,(H,17,18,21)/t9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data