

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM126826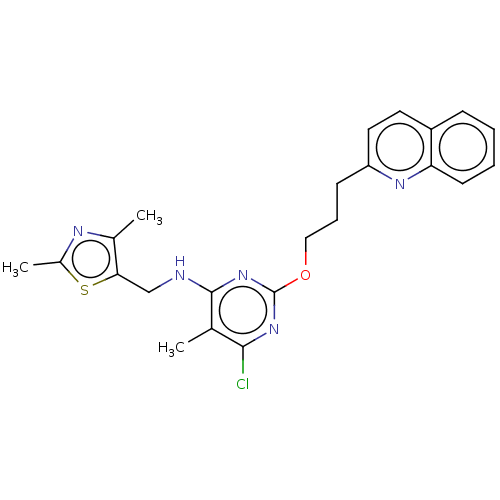 (US8785467, 1-29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay | J Med Chem 58: 7888-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b00983 BindingDB Entry DOI: 10.7270/Q26Q202C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM126825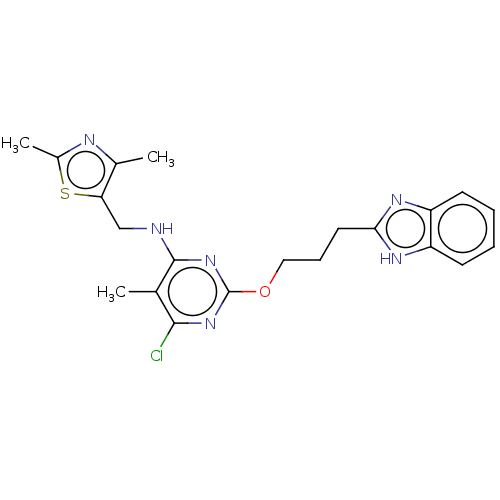 (US8785467, 1-27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay | J Med Chem 58: 7888-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b00983 BindingDB Entry DOI: 10.7270/Q26Q202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM126827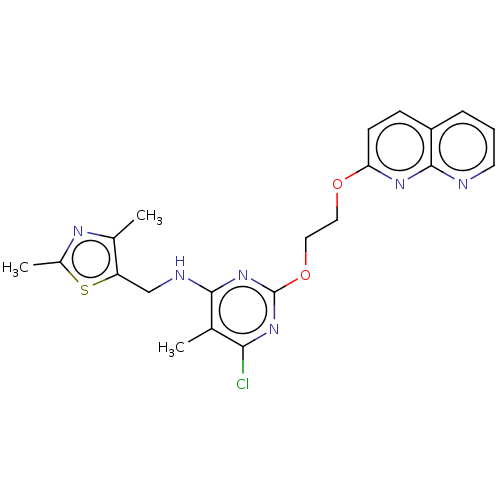 (US8785467, 1-32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay | J Med Chem 58: 7888-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b00983 BindingDB Entry DOI: 10.7270/Q26Q202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50124648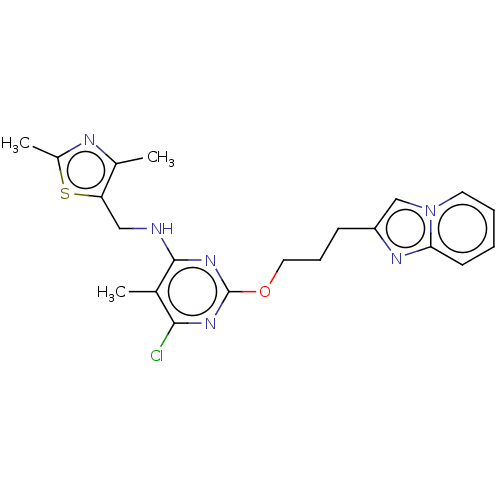 (CHEMBL3622901) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay | J Med Chem 58: 7888-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b00983 BindingDB Entry DOI: 10.7270/Q26Q202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50124649 (CHEMBL3622902) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay | J Med Chem 58: 7888-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b00983 BindingDB Entry DOI: 10.7270/Q26Q202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50463758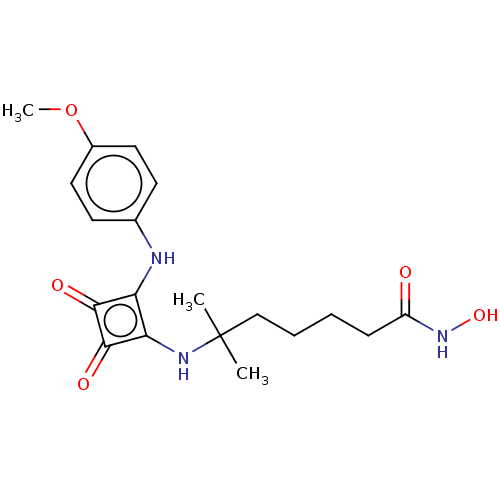 (CHEMBL4250302) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50463741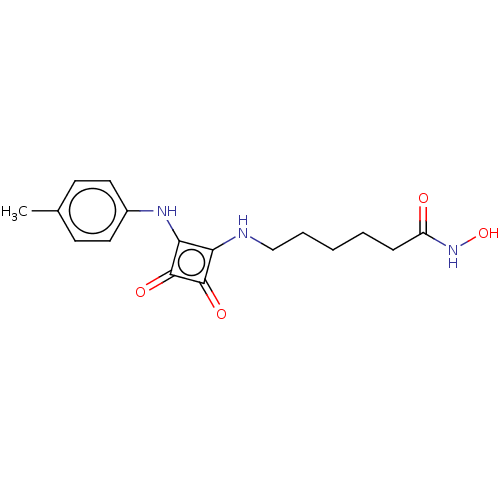 (CHEMBL4239232) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50463759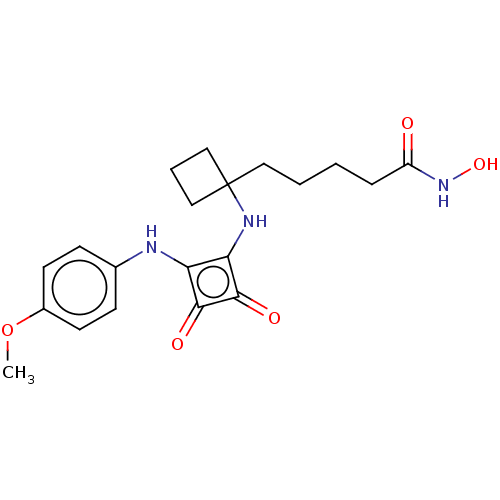 (CHEMBL4237636) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50463739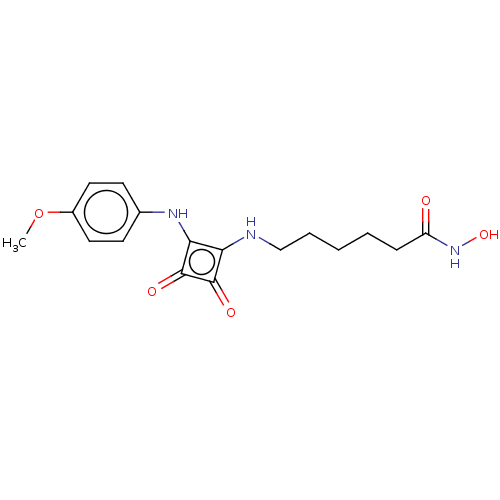 (CHEMBL4237803) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50463736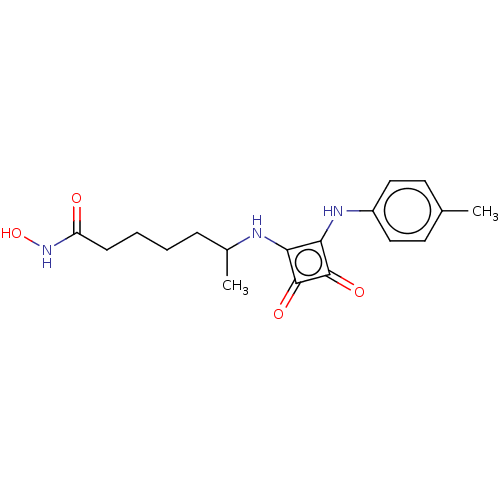 (CHEMBL4251203) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50124650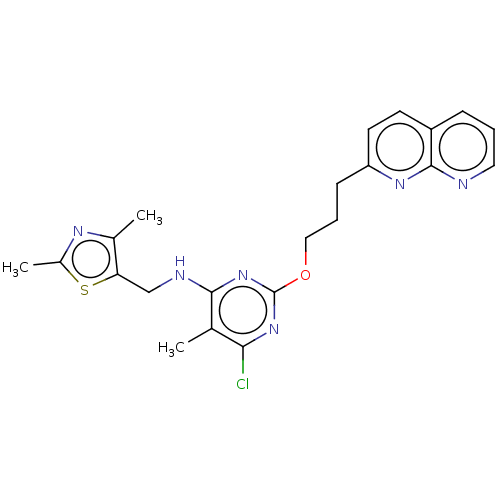 (CHEMBL3622903) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay | J Med Chem 58: 7888-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b00983 BindingDB Entry DOI: 10.7270/Q26Q202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077581 ((R)-2-(Benzylamino-methyl)-3,4,7,9-tetrahydro-2H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro high binding affinity towards Dopamine receptor D2 by the displacement of [3H]-quinpirole radioligand in rat striatal membranes | J Med Chem 42: 2007-20 (1999) Article DOI: 10.1021/jm990023s BindingDB Entry DOI: 10.7270/Q25D8R1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077577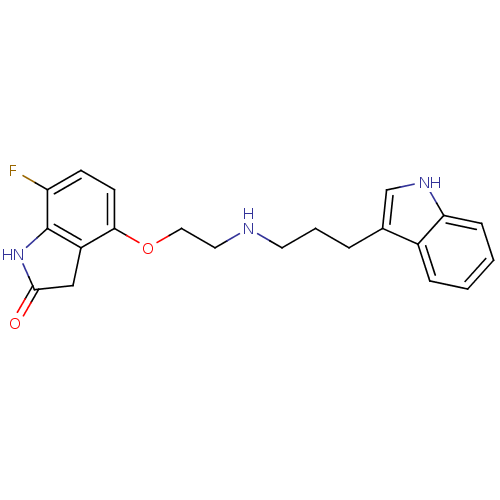 (7-Fluoro-4-{2-[3-(1H-indol-3-yl)-propylamino]-etho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro high binding affinity towards Dopamine receptor D2 by the displacement of [3H]-quinpirole radioligand in rat striatal membranes | J Med Chem 42: 2007-20 (1999) Article DOI: 10.1021/jm990023s BindingDB Entry DOI: 10.7270/Q25D8R1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50463756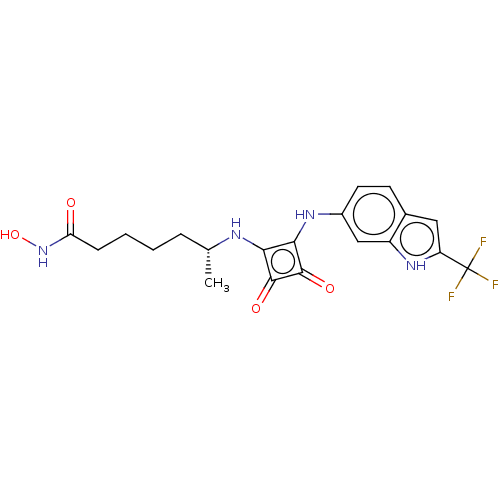 (CHEMBL4246561) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061669 ((R)-2-(Benzylamino-methyl)-chroman-7-ol; oxalic ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Antagonistic activity towards dopamine D2 receptor using radioligand [3H]-spiperone in rat striatal membranes | Bioorg Med Chem Lett 8: 295-300 (1999) BindingDB Entry DOI: 10.7270/Q28W3CG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318697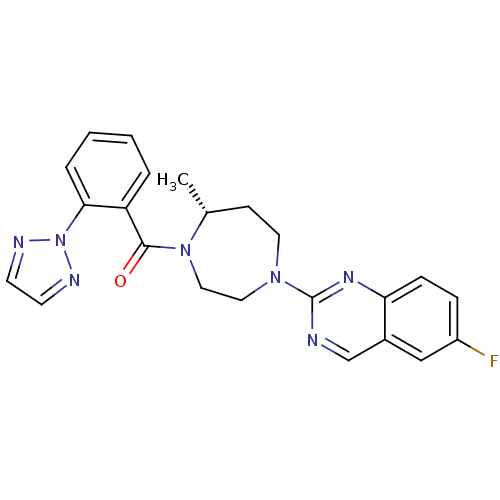 (6-Fluoro-2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061669 ((R)-2-(Benzylamino-methyl)-chroman-7-ol; oxalic ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro high binding affinity towards Dopamine receptor D2 by the displacement of [3H]-quinpirole radioligand in rat striatal membranes | J Med Chem 42: 2007-20 (1999) Article DOI: 10.1021/jm990023s BindingDB Entry DOI: 10.7270/Q25D8R1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50314676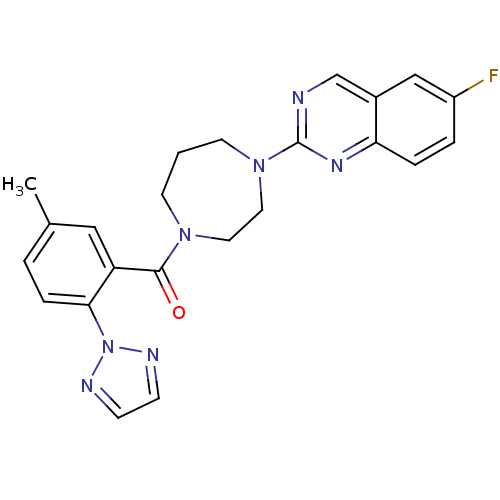 ((4-(6-fluoroquinazolin-2-yl)-1,4-diazepan-1-yl)(5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX2 receptor by radioligand displacement assay | Bioorg Med Chem Lett 20: 2311-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.138 BindingDB Entry DOI: 10.7270/Q2MK6D26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077578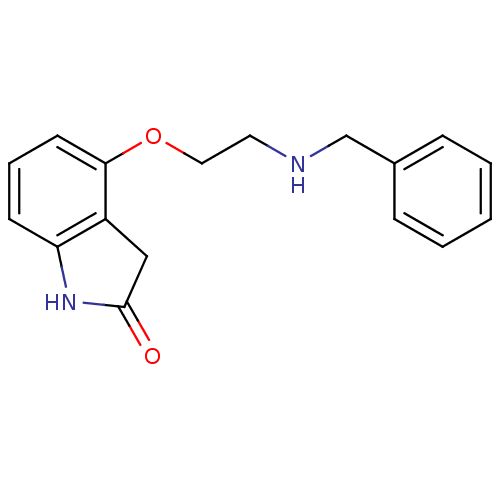 (4-(2-Benzylamino-ethoxy)-1,3-dihydro-indol-2-one |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro high binding affinity towards Dopamine receptor D2 by the displacement of [3H]-quinpirole radioligand in rat striatal membranes | J Med Chem 42: 2007-20 (1999) Article DOI: 10.1021/jm990023s BindingDB Entry DOI: 10.7270/Q25D8R1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077602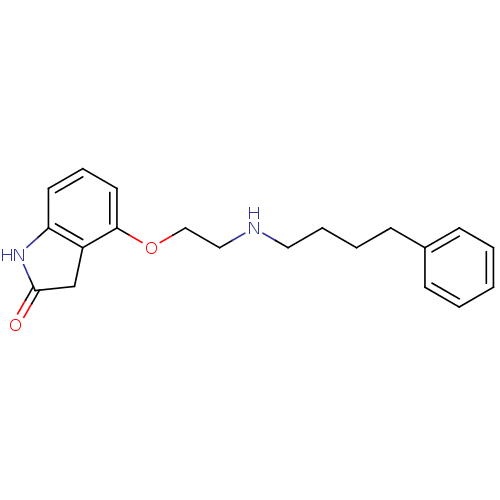 (4-[2-(4-Phenyl-butylamino)-ethoxy]-1,3-dihydro-ind...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro high binding affinity towards Dopamine receptor D2 by the displacement of [3H]-quinpirole radioligand in rat striatal membranes | J Med Chem 42: 2007-20 (1999) Article DOI: 10.1021/jm990023s BindingDB Entry DOI: 10.7270/Q25D8R1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50463750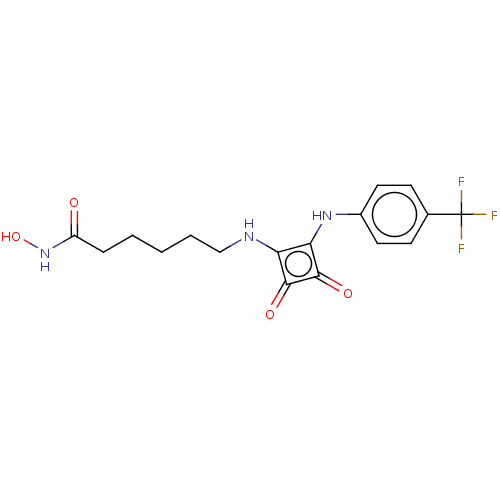 (CHEMBL4241807) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077580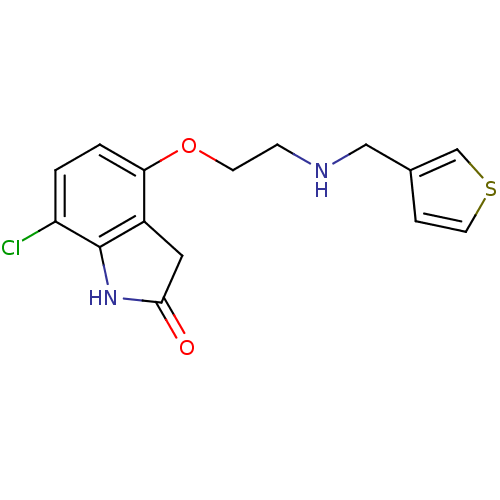 (7-Chloro-4-{2-[(thiophen-3-ylmethyl)-amino]-ethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro high binding affinity towards Dopamine receptor D2 by the displacement of [3H]-quinpirole radioligand in rat striatal membranes | J Med Chem 42: 2007-20 (1999) Article DOI: 10.1021/jm990023s BindingDB Entry DOI: 10.7270/Q25D8R1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318698 (6,7-Fluoro-2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50463743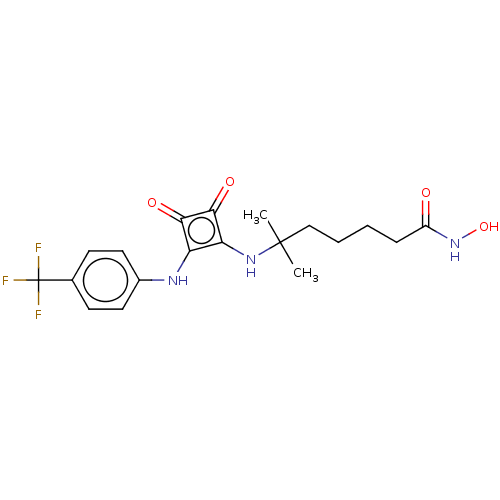 (CHEMBL4241370) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50463753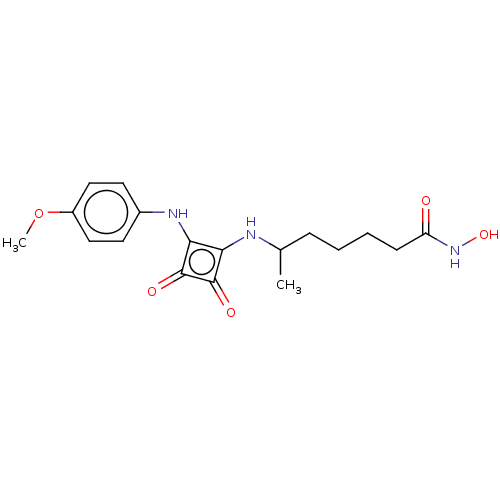 (CHEMBL4250739) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50463752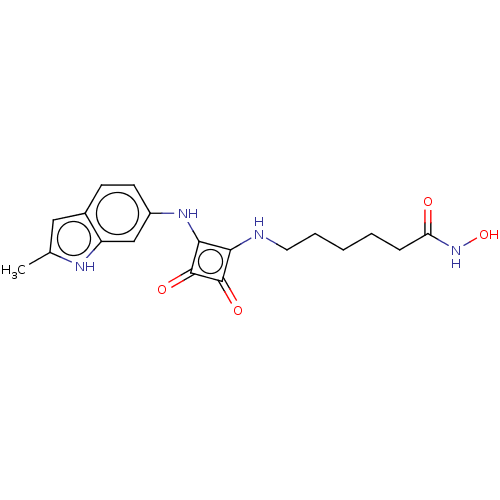 (CHEMBL4247370) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318699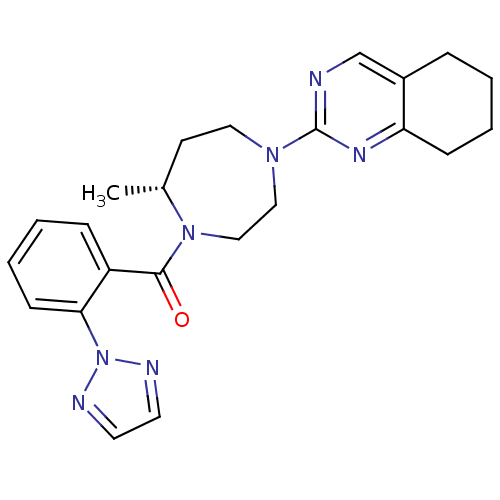 (2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol-2-yl)benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50463754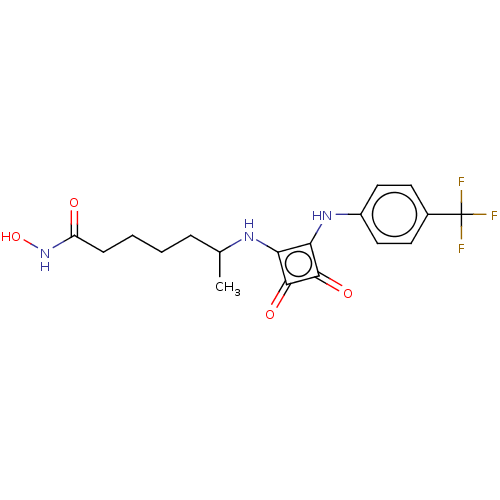 (CHEMBL4240635) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50463740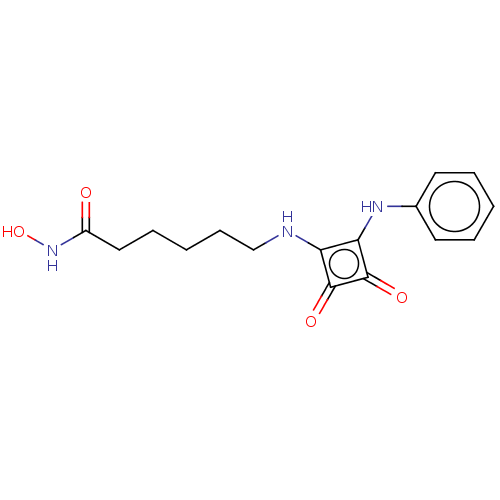 (CHEMBL4251365) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50463739 (CHEMBL4237803) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using fluorogenic HDAC substrate after 15 mins by fluorimetrc method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318701 (CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061642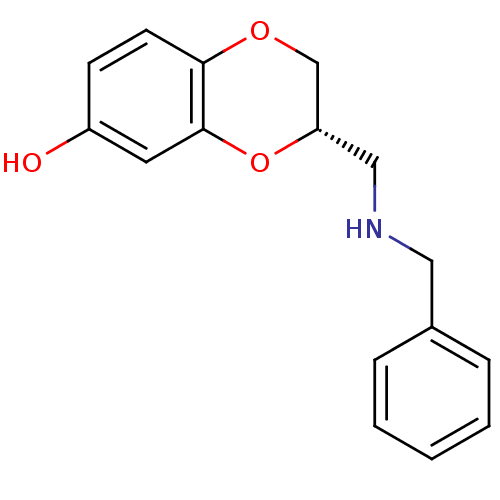 ((S)-3-(Benzylamino-methyl)-2,3-dihydro-benzo[1,4]d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Agonistic activity towards dopamine D2 receptor using radioligand [3H]-quinpirole in rat striatal membranes | Bioorg Med Chem Lett 8: 295-300 (1999) BindingDB Entry DOI: 10.7270/Q28W3CG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318695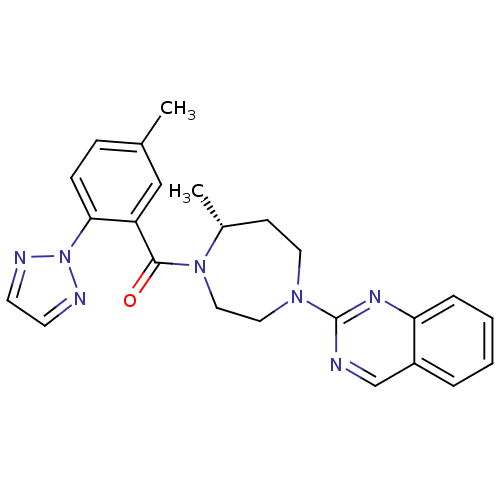 (2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50124651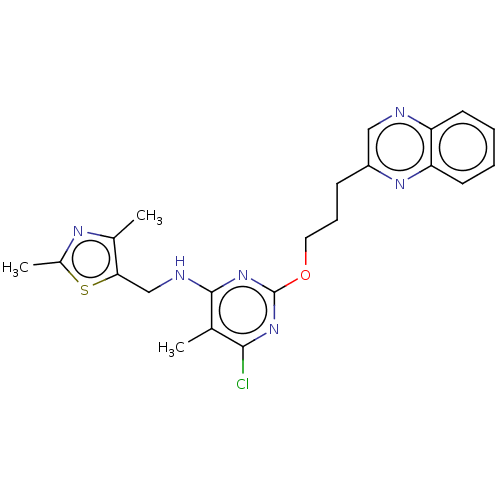 (CHEMBL3622904) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay | J Med Chem 58: 7888-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b00983 BindingDB Entry DOI: 10.7270/Q26Q202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318695 (2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50463763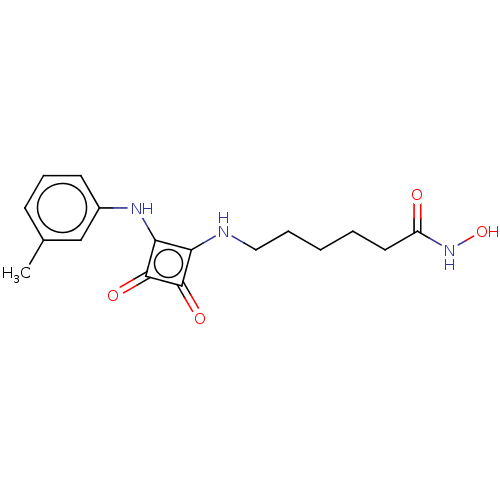 (CHEMBL4248374) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50318695 (2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50077569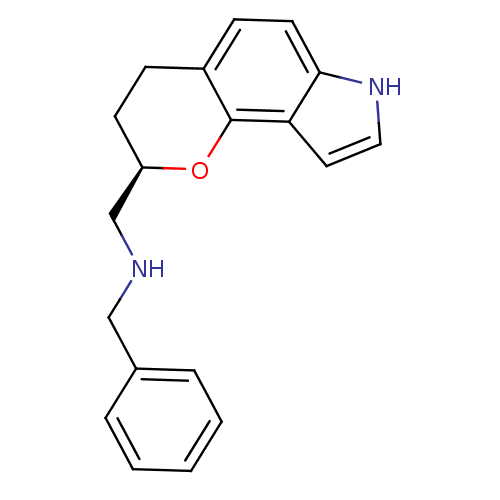 (Benzyl-[(R)-1-(2,3,4,7-tetrahydro-pyrano[2,3-e]ind...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1A receptor by the displacement of [3H]-8-OH-DPAT radioligand in rat hippocampal homogenates | J Med Chem 42: 2007-20 (1999) Article DOI: 10.1021/jm990023s BindingDB Entry DOI: 10.7270/Q25D8R1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50077588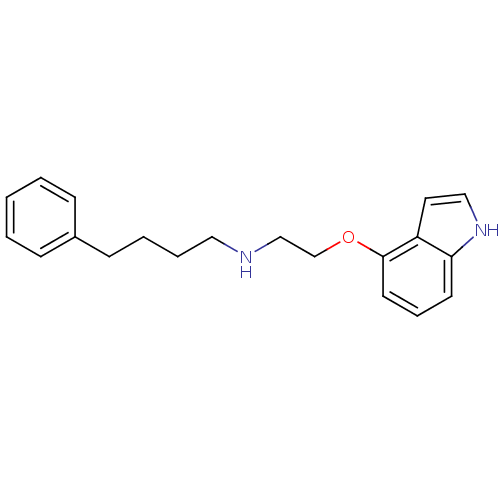 (CHEMBL59737 | [2-(1H-Indol-4-yloxy)-ethyl]-(4-phen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1A receptor by the displacement of [3H]-8-OH-DPAT radioligand in rat hippocampal homogenates | J Med Chem 42: 2007-20 (1999) Article DOI: 10.1021/jm990023s BindingDB Entry DOI: 10.7270/Q25D8R1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50061642 ((S)-3-(Benzylamino-methyl)-2,3-dihydro-benzo[1,4]d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro high binding affinity towards Dopamine receptor D2 by the displacement of [3H]-quinpirole radioligand in rat striatal membranes | J Med Chem 42: 2007-20 (1999) Article DOI: 10.1021/jm990023s BindingDB Entry DOI: 10.7270/Q25D8R1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50321509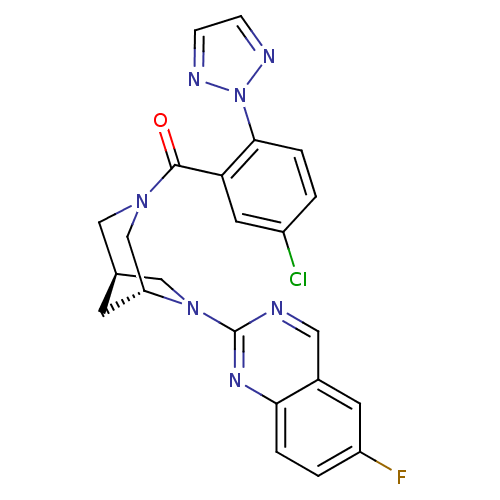 ((5-chloro-2-(2H-1,2,3-triazol-2-yl)phenyl)((1R,5R)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX1R | Bioorg Med Chem Lett 20: 4201-5 (2010) Article DOI: 10.1016/j.bmcl.2010.05.047 BindingDB Entry DOI: 10.7270/Q25Q4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50321510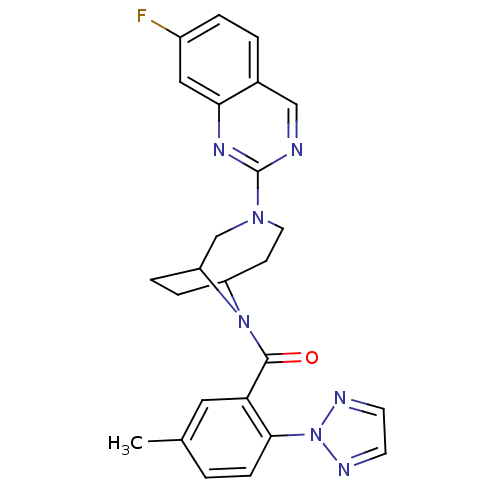 ((3-(7-fluoroquinazolin-2-yl)-3,9-di azabicyclo[4.2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX2R | Bioorg Med Chem Lett 20: 4201-5 (2010) Article DOI: 10.1016/j.bmcl.2010.05.047 BindingDB Entry DOI: 10.7270/Q25Q4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50314681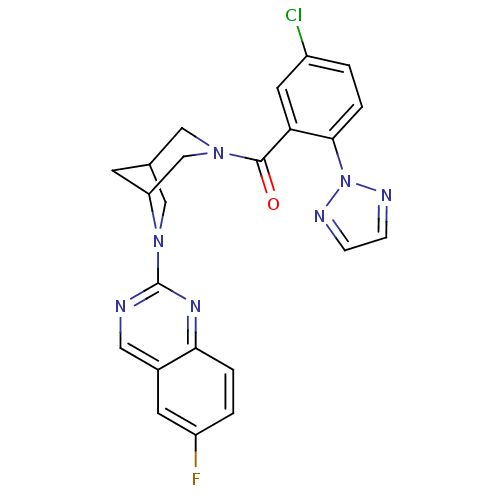 ((5-chloro-2-(2H-1,2,3-triazol-2-yl)phenyl)(6-(6-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX1 receptor by radioligand displacement assay | Bioorg Med Chem Lett 20: 2311-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.138 BindingDB Entry DOI: 10.7270/Q2MK6D26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077572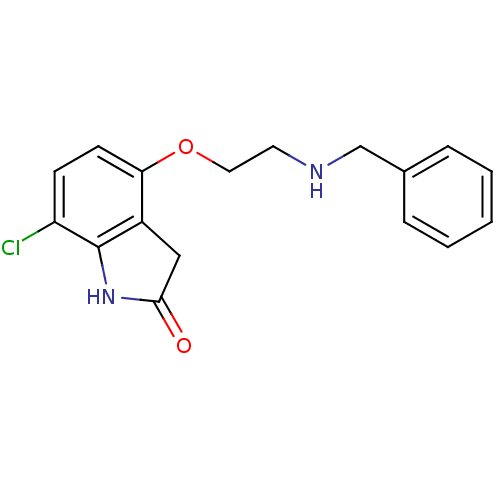 (4-(2-Benzylamino-ethoxy)-7-chloro-1,3-dihydro-indo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro high binding affinity towards Dopamine receptor D2 by the displacement of [3H]-quinpirole radioligand in rat striatal membranes | J Med Chem 42: 2007-20 (1999) Article DOI: 10.1021/jm990023s BindingDB Entry DOI: 10.7270/Q25D8R1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50077592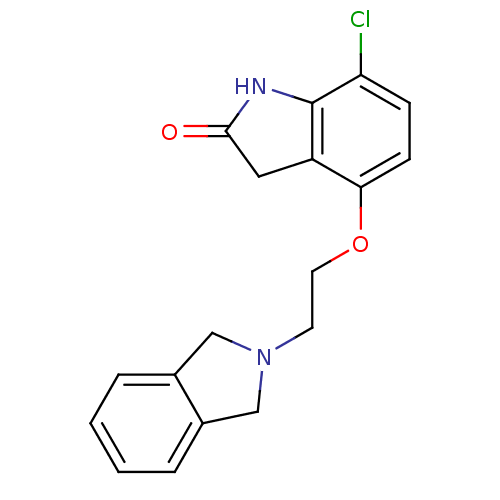 (7-Chloro-4-[2-(1,3-dihydro-isoindol-2-yl)-ethoxy]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro high binding affinity towards Dopamine receptor D2 by the displacement of [3H]-quinpirole radioligand in rat striatal membranes | J Med Chem 42: 2007-20 (1999) Article DOI: 10.1021/jm990023s BindingDB Entry DOI: 10.7270/Q25D8R1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50318696 (2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol-2-yl)benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105327 (JNJ-26481585 | Quisinostat) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50077563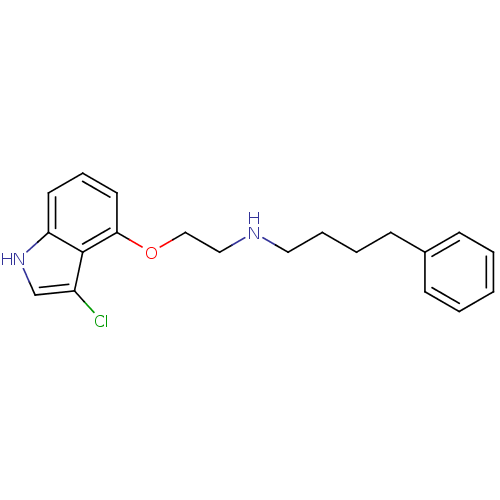 (CHEMBL64420 | [2-(3-Chloro-1H-indol-4-yloxy)-ethyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1A r receptor by the displacement of [3H]-8-OH-DPAT radioligand in rat hippocampal homogenates | J Med Chem 42: 2007-20 (1999) Article DOI: 10.1021/jm990023s BindingDB Entry DOI: 10.7270/Q25D8R1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity against rat Dopamine receptor D2. | J Med Chem 41: 5084-93 (1999) Article DOI: 10.1021/jm980452a BindingDB Entry DOI: 10.7270/Q2CZ369B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50463751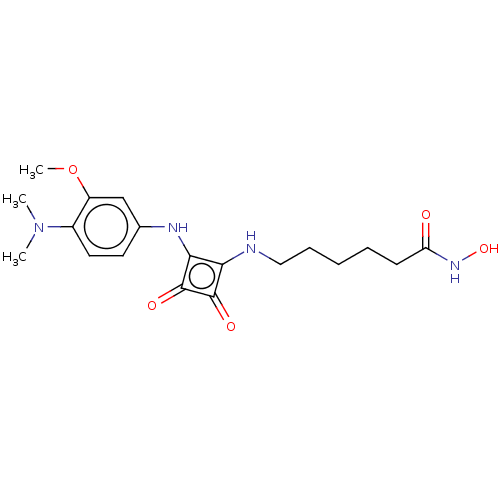 (CHEMBL4244350) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Fluor de Lys as substrate after 2 hrs by fluorescence method | Bioorg Med Chem Lett 28: 2985-2992 (2018) Article DOI: 10.1016/j.bmcl.2018.06.029 BindingDB Entry DOI: 10.7270/Q2M0484T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1157 total ) | Next | Last >> |