

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0269 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D | Assay Description Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D | Assay Description Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic tyrosine-protein kinase BMX (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.545 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D | Assay Description Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Tec (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.679 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D | Assay Description Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D | Assay Description Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 32.4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D | Assay Description Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 62.3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D | Assay Description Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088503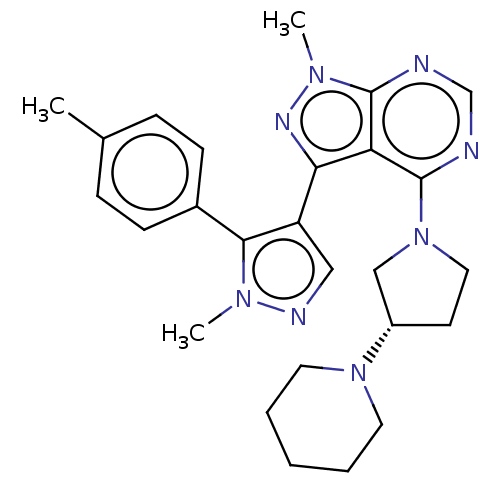 (CHEMBL3527048) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A4 harboring human P450 oxidoreductase and b5 assessed as decrease in enzyme... | Drug Metab Dispos 40: 1686-97 (2012) Article DOI: 10.1124/dmd.112.045302 BindingDB Entry DOI: 10.7270/Q2GT5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088503 (CHEMBL3527048) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of testosterone 6beta-hydroxylase activity of human recombinant CYP3A4 in presence of human P450 oxidoreductase and b5 assessed as decreas... | Drug Metab Dispos 40: 1686-97 (2012) Article DOI: 10.1124/dmd.112.045302 BindingDB Entry DOI: 10.7270/Q2GT5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50088503 (CHEMBL3527048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of testosterone 6beta-hydroxylase activity of human recombinant CYP3A5 in presence of human P450 oxidoreductase and b5 assessed as decreas... | Drug Metab Dispos 40: 1686-97 (2012) Article DOI: 10.1124/dmd.112.045302 BindingDB Entry DOI: 10.7270/Q2GT5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50088503 (CHEMBL3527048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A5 harboring human P450 oxidoreductase and b5 assessed as decrease in enzyme... | Drug Metab Dispos 40: 1686-97 (2012) Article DOI: 10.1124/dmd.112.045302 BindingDB Entry DOI: 10.7270/Q2GT5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.346 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D | Assay Description Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D | Assay Description Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM159756 (PF-02384554 | US10966980, Example 8 | US9035074, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D | Assay Description Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D | Assay Description Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM159756 (PF-02384554 | US10966980, Example 8 | US9035074, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D | Assay Description Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D | Assay Description Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113040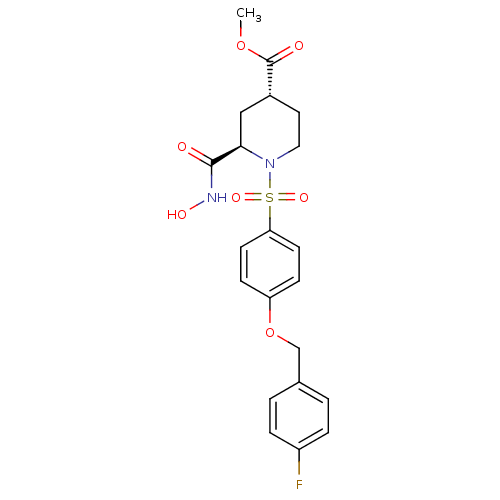 ((2R,4R)-1-[4-(4-Fluoro-benzyloxy)-benzenesulfonyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113041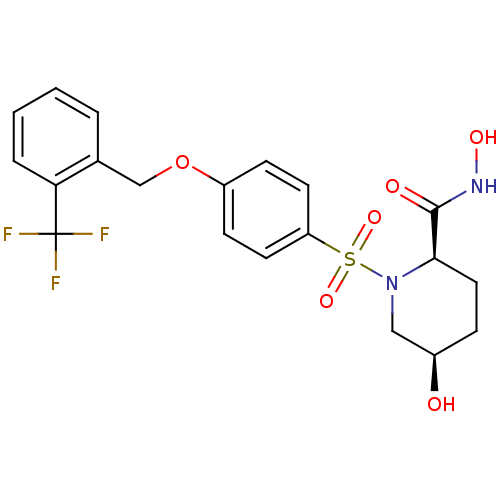 ((2R,5R)-5-Hydroxy-1-[4-(2-trifluoromethyl-benzylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195575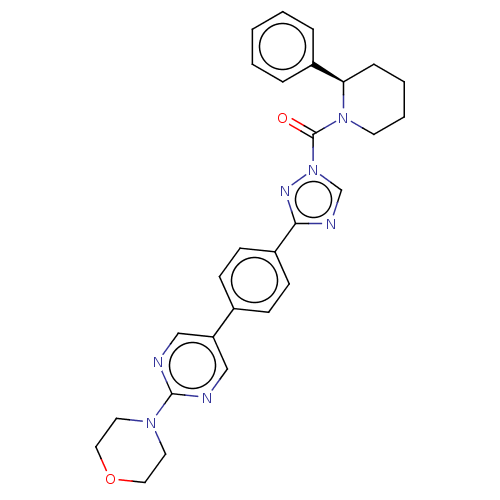 ((R)-(3-(4-(2-morpholinopyrimidin-5-yl)phenyl)-1H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195574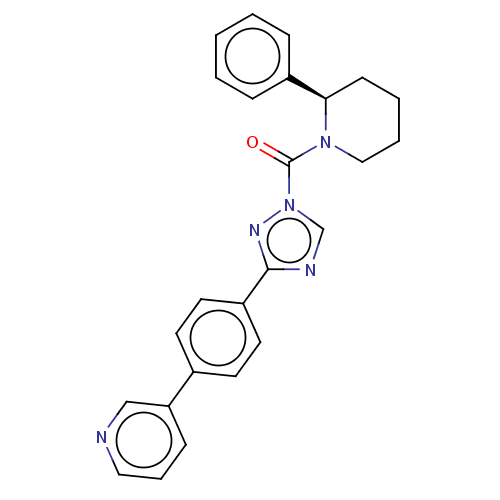 ((R)-(2-phenylpiperidin-1-yl)(3-(4-(pyridin-3-yl)ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195572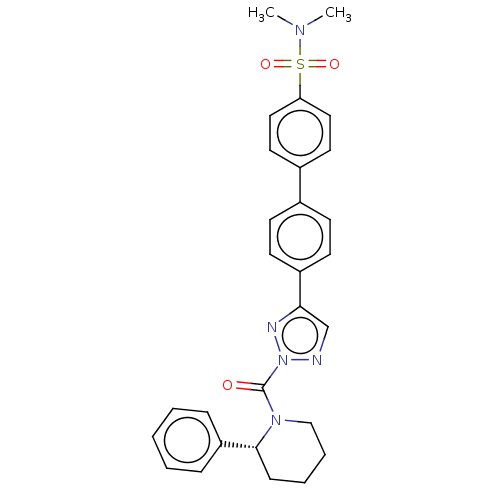 ((R)-N,N-dimethyl-4'-(2-(2-phenylpiperidine-1-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195571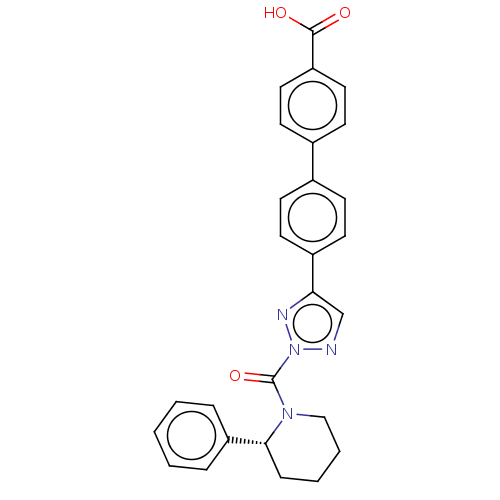 ((R)-4'-(2-(2-phenylpiperidine-1-carbonyl)-2H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195570 ((S)-4'-(2-(2-phenylpiperidine-1-carbonyl)-2H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195581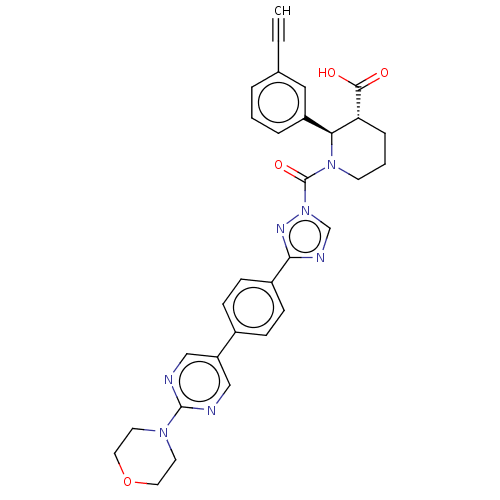 ((2R,3R)-2-(3-ethynylphenyl)-1-(3-(4-(2-morpholinop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113028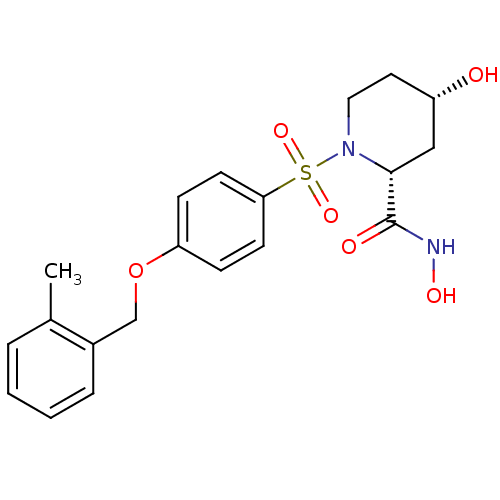 ((2R,4S)-4-Hydroxy-1-[4-(2-methyl-benzyloxy)-benzen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195580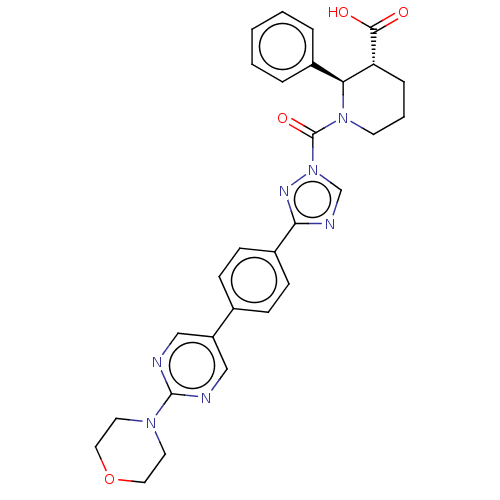 ((2R,3R)-1-(3-(4-(2-morpholinopyrimidin-5-yl)phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113035 ((2R,4S)-1-[4-(4-Chloro-benzyloxy)-benzenesulfonyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195573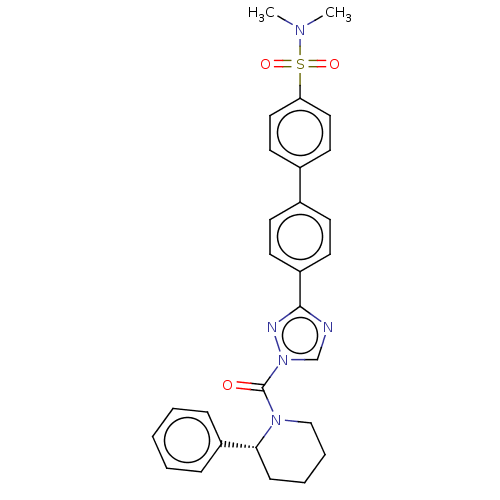 ((R)-4'-(1-(2-phenylpiperidine-1-carbonyl)-1H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113046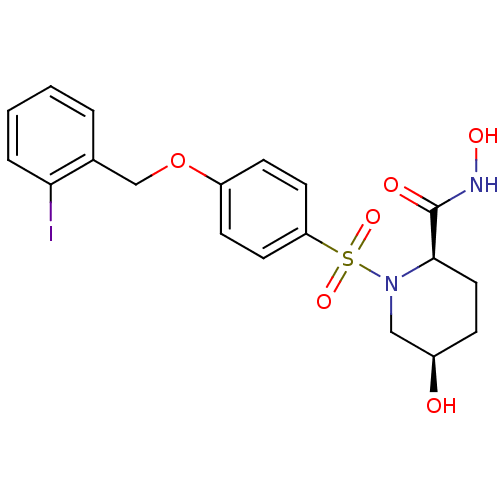 ((2R,5R)-5-Hydroxy-1-[4-(2-iodo-benzyloxy)-benzenes...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113048 ((2R,5R)-1-(4-Benzyloxy-benzenesulfonyl)-5-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195575 ((R)-(3-(4-(2-morpholinopyrimidin-5-yl)phenyl)-1H-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113044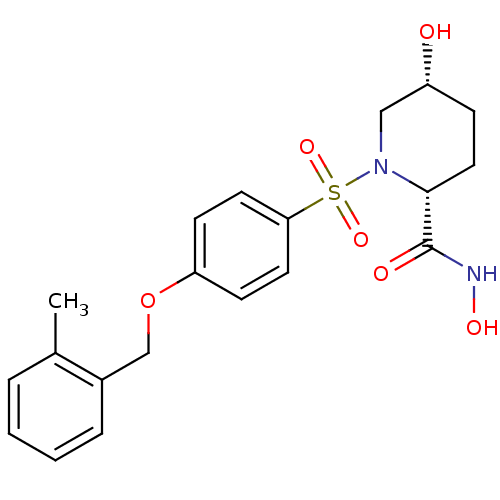 ((2R,5R)-5-Hydroxy-1-[4-(2-methyl-benzyloxy)-benzen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50113029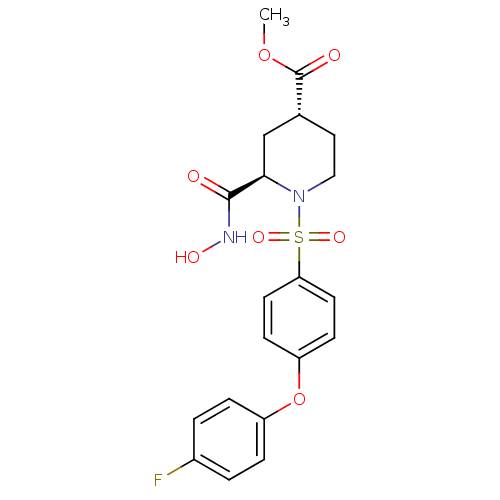 ((2R,4R)-1-[4-(4-Fluoro-phenoxy)-benzenesulfonyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Matrix metalloproteinase-1 was determined | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113038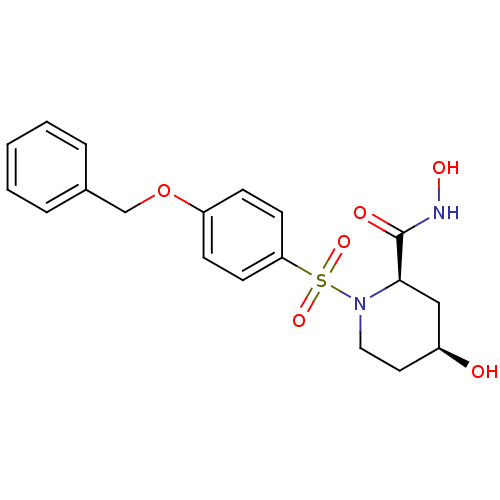 ((2R,4S)-1-(4-Benzyloxy-benzenesulfonyl)-4-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113037 ((2R,5R)-5-Hydroxy-1-[4-(4-trifluoromethyl-benzylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113034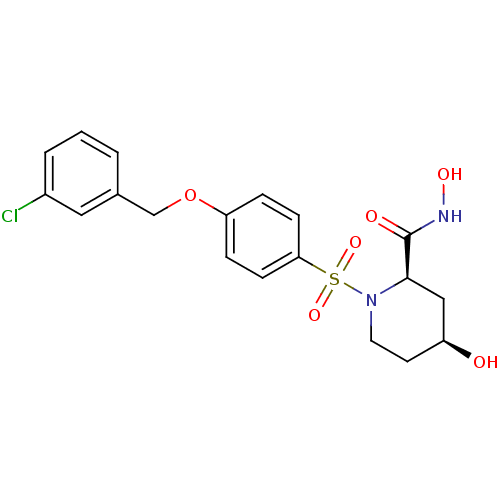 ((2R,4S)-1-[4-(3-Chloro-benzyloxy)-benzenesulfonyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113045 ((2R,5R)-1-[4-(2-Ethyl-benzyloxy)-benzenesulfonyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195578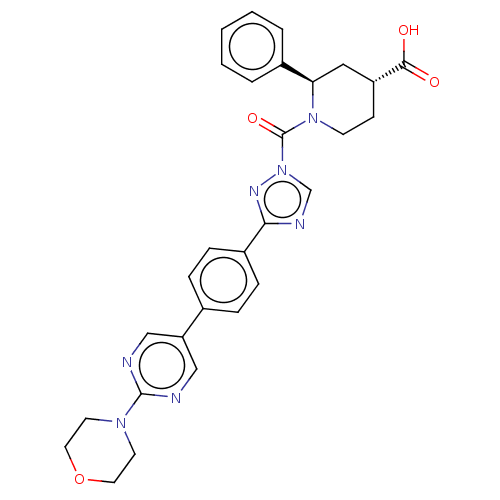 ((2R, 4R)-1-(3-(4-(2-morpholinopyrimidin-5-yl)pheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113039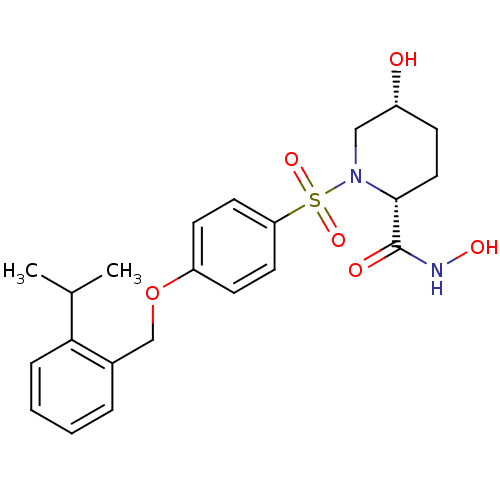 ((2R,5R)-5-Hydroxy-1-[4-(2-isopropyl-benzyloxy)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113047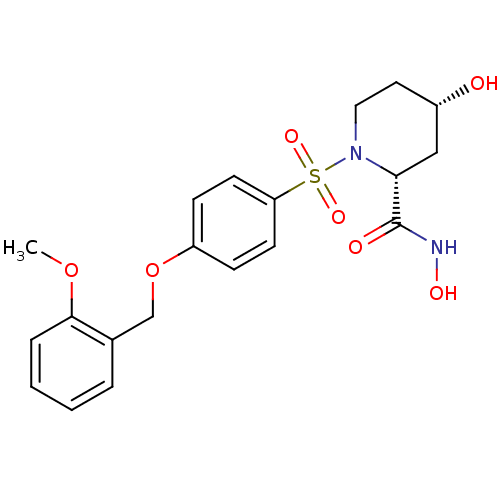 ((2R,4S)-4-Hydroxy-1-[4-(2-methoxy-benzyloxy)-benze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113024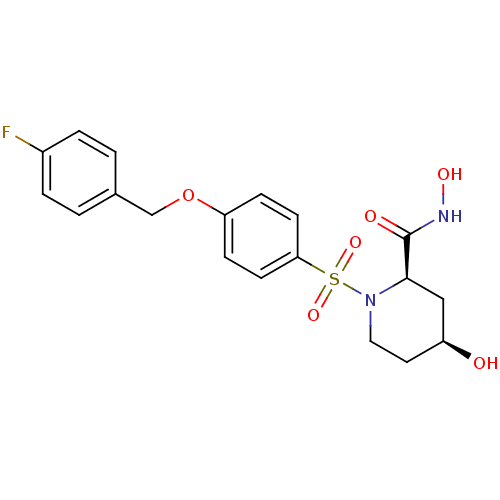 ((2R,4S)-1-[4-(4-Fluoro-benzyloxy)-benzenesulfonyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D | Assay Description Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113033 ((2R,5R)-5-Hydroxy-1-[4-(3-trifluoromethyl-benzylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113021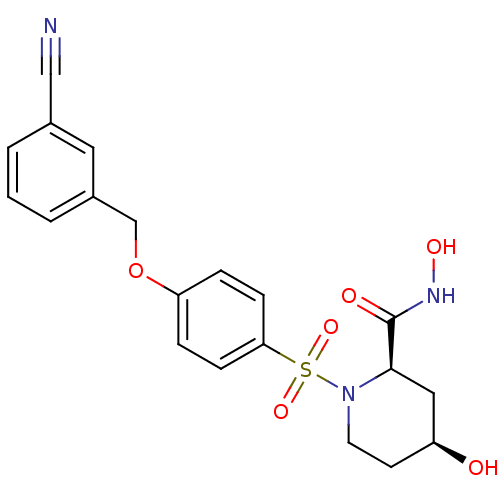 ((2R,4S)-1-[4-(3-Cyano-benzyloxy)-benzenesulfonyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113025 ((2R,5R)-5-Hydroxy-1-[4-(naphthalen-1-ylmethoxy)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113027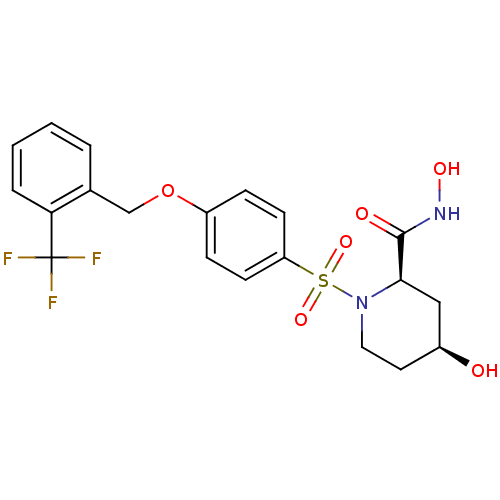 ((2R,4S)-4-Hydroxy-1-[4-(2-trifluoromethyl-benzylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113023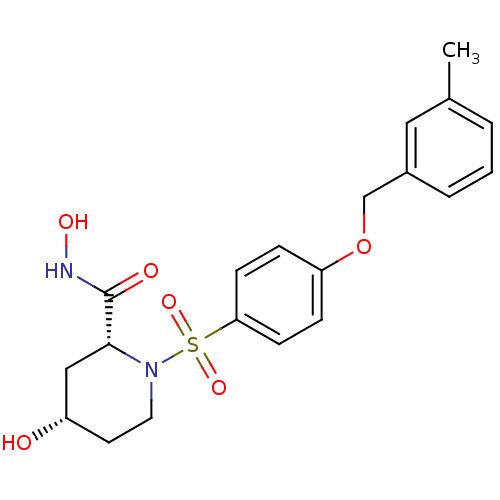 ((2R,4S)-4-Hydroxy-1-[4-(3-methyl-benzyloxy)-benzen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50113049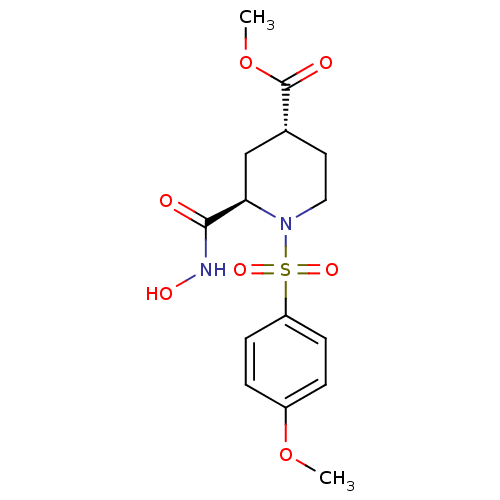 ((2R,4R)-2-Hydroxycarbamoyl-1-(4-methoxy-benzenesul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Matrix metalloproteinase-1 was determined | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50113030 ((2R,4S)-4-Hydroxy-1-[4-(4-methyl-benzyloxy)-benzen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant tumor necrosis factor alpha converting enzyme | Bioorg Med Chem Lett 12: 1387-90 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 158 total ) | Next | Last >> |