

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM50093010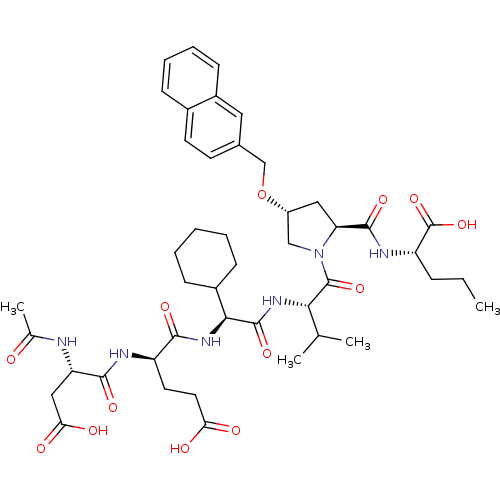 ((S)-2-{[(2S,4R)-1-((S)-2-{(S)-2-[(R)-2-((S)-2-Acet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against NS3 protease complexed with NS4A cofactor peptide (NS3-4A pep) | Bioorg Med Chem Lett 10: 2267-70 (2001) BindingDB Entry DOI: 10.7270/Q2S1831T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071982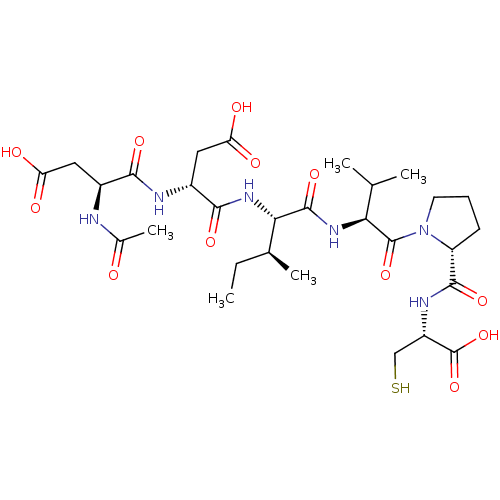 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50070797 (CHEMBL2370476 | Hexapeptide analogue) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description The apparent Ki value against NS3-4Apep protease | Bioorg Med Chem Lett 8: 1713-8 (1999) BindingDB Entry DOI: 10.7270/Q2Z89CX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50366517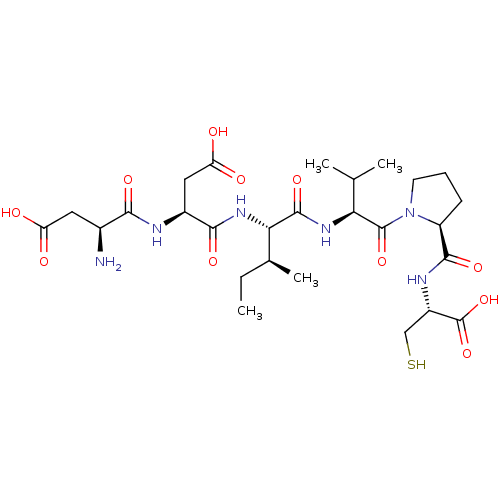 (CHEMBL1790303) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description The apparent Ki value against NS3-4Apep protease | Bioorg Med Chem Lett 8: 1713-8 (1999) BindingDB Entry DOI: 10.7270/Q2Z89CX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053967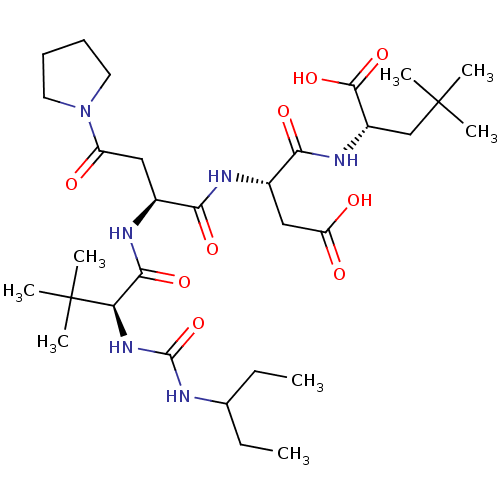 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50033458 ((S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-(2-Benzyl-3-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against HSV ribonucleotide reductase | J Med Chem 38: 3617-23 (1995) BindingDB Entry DOI: 10.7270/Q21V5FMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50033457 (1-[(S)-((S)-1-Carboxy-3-methyl-butylcarbamoyl)-((S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against HSV ribonucleotide reductase | J Med Chem 38: 3617-23 (1995) BindingDB Entry DOI: 10.7270/Q21V5FMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50369164 (CHEMBL1169533) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50369164 (CHEMBL1169533) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50050831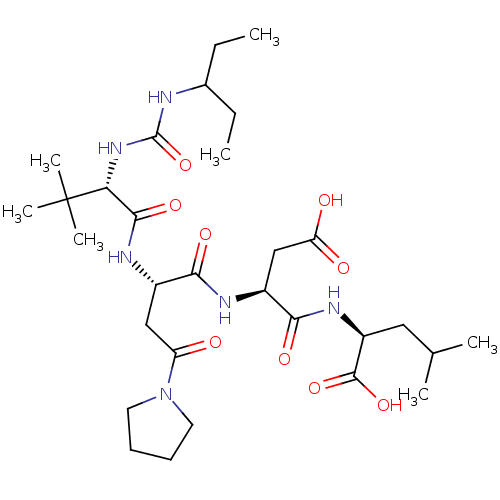 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053968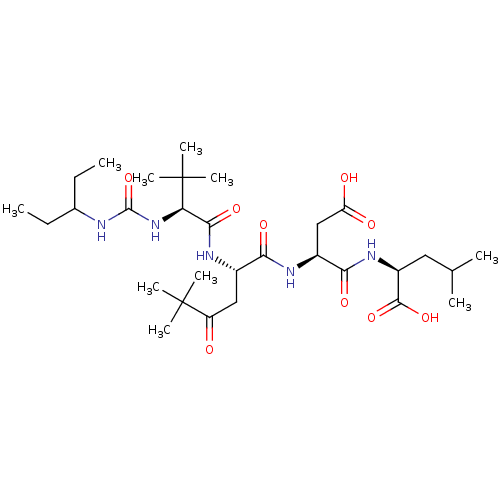 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050831 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50033463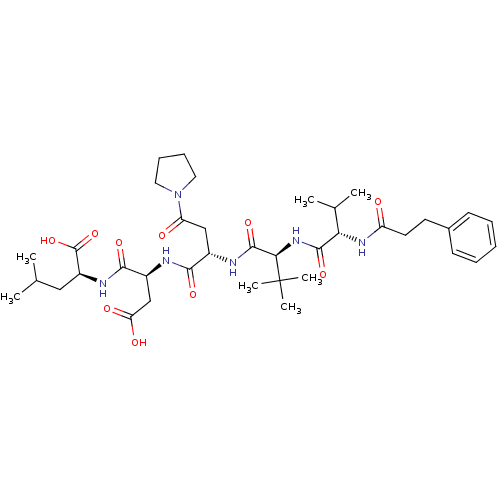 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3,3-dimethyl-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against HSV ribonucleotide reductase | J Med Chem 38: 3617-23 (1995) BindingDB Entry DOI: 10.7270/Q21V5FMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050824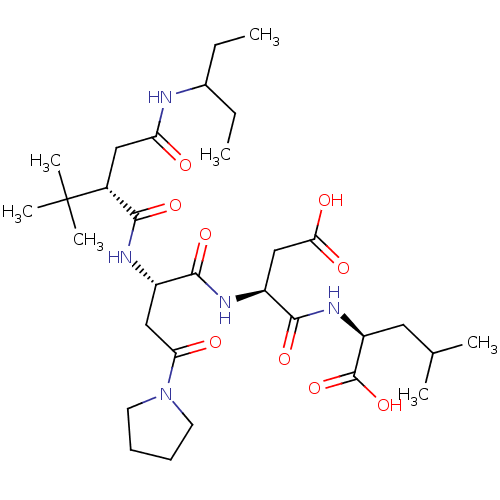 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[(1-ethyl-pro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053981 ((S)-2-((S)-3-Carboxy-2-{(2R,5S)-2-(3,3-dimethyl-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50033465 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3-methyl-2-[(S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against HSV ribonucleotide reductase | J Med Chem 38: 3617-23 (1995) BindingDB Entry DOI: 10.7270/Q21V5FMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053973 ((S)-3-((S)-2-{(S)-2-[3-(1-Ethyl-propyl)-ureido]-3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50033453 (1-((S)-((S)-1-Carboxy-3-methyl-butylcarbamoyl)-{(S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against HSV ribonucleotide reductase | J Med Chem 38: 3617-23 (1995) BindingDB Entry DOI: 10.7270/Q21V5FMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053984 ((S)-2-{(S)-3-Carboxy-2-[(2S,5S)-5-[3-(1-ethyl-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50050828 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3-methyl-2-[3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050828 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3-methyl-2-[3-(...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50033470 ((S)-2-{(S)-3-Carboxy-2-[(S)-2-((S)-3-methyl-2-{(S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against HSV ribonucleotide reductase | J Med Chem 38: 3617-23 (1995) BindingDB Entry DOI: 10.7270/Q21V5FMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142042 ((S)-2-[(1-Cyclohexyl-2-furan-3-yl-1H-benzoimidazol...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050822 ((S)-2-{(S)-3-Carboxy-2-[(1-{(S)-2-[3-(1-ethyl-prop...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50093024 (1-{[1-(2-{2-[2-(2-Acetylamino-3-carboxy-propionyla...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibitory activity against NS3 protease complexed with NS4A cofactor peptide (NS3-4A pep) | Bioorg Med Chem Lett 10: 2267-70 (2001) BindingDB Entry DOI: 10.7270/Q2S1831T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142047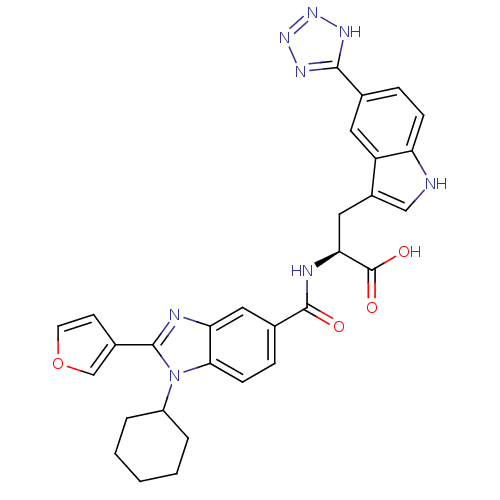 ((S)-2-[(1-Cyclohexyl-2-furan-3-yl-1H-benzoimidazol...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50050826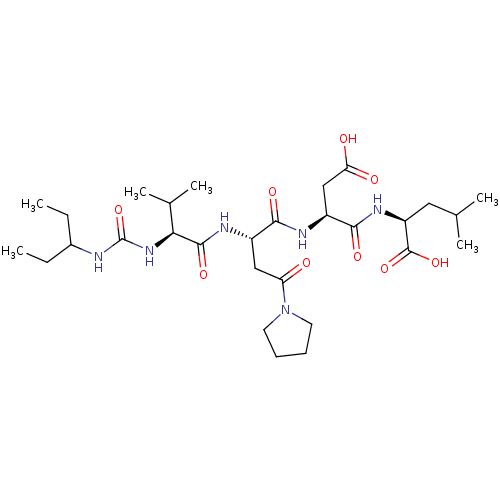 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050826 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053962 ((S)-N-((R)-1-Ethyl-2,2-dimethyl-propyl)-3-((S)-2-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142043 ((2S)-3-(5-(carboxymethoxy)-1H-indol-3-yl)-2-(1-cyc...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142041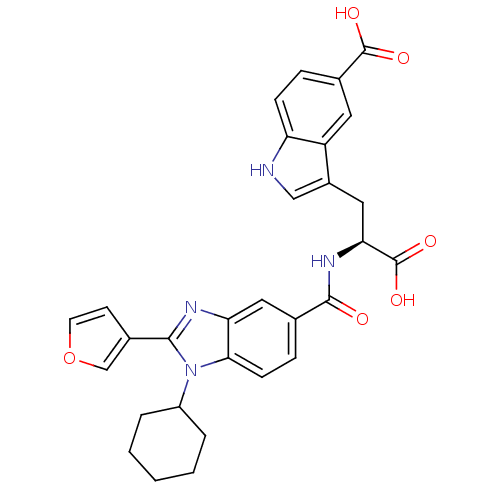 (3-{(S)-2-Carboxy-2-[(1-cyclohexyl-2-furan-3-yl-1H-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50369165 (CHEMBL1169532) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50033477 ((S)-2-{(S)-2-[(S)-2-((S)-2-{(S)-2-[(2-Benzyl-3-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against HSV ribonucleotide reductase | J Med Chem 38: 3617-23 (1995) BindingDB Entry DOI: 10.7270/Q21V5FMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50033456 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[(S)-2-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against HSV ribonucleotide reductase | J Med Chem 38: 3617-23 (1995) BindingDB Entry DOI: 10.7270/Q21V5FMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50033462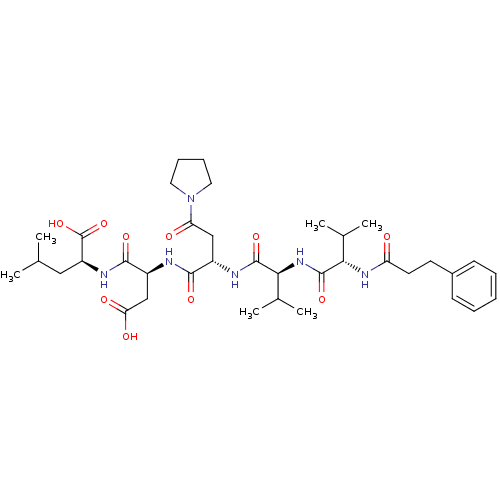 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3-methyl-2-[(S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50033462 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3-methyl-2-[(S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against HSV ribonucleotide reductase | J Med Chem 38: 3617-23 (1995) BindingDB Entry DOI: 10.7270/Q21V5FMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50033462 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3-methyl-2-[(S)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50093010 ((S)-2-{[(2S,4R)-1-((S)-2-{(S)-2-[(R)-2-((S)-2-Acet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibitory activity against NS3 protease complexed with NS4A cofactor peptide (NS3-4A pep) | Bioorg Med Chem Lett 10: 2267-70 (2001) BindingDB Entry DOI: 10.7270/Q2S1831T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053963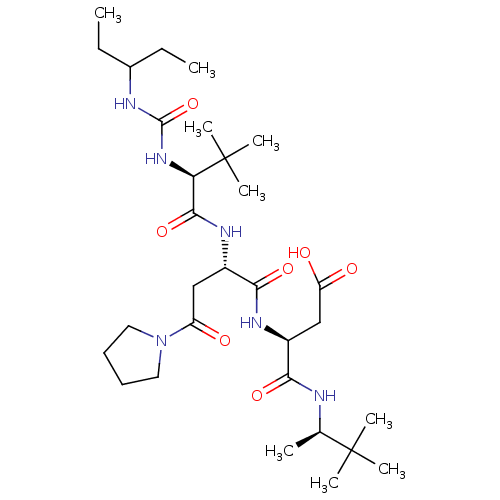 ((S)-3-((S)-2-{(S)-2-[3-(1-Ethyl-propyl)-ureido]-3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50071966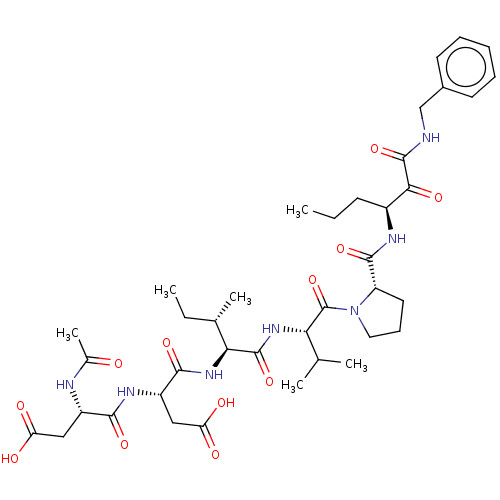 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human liver Cathepsin B | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50071966 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human leucocyte elastase (HLE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050821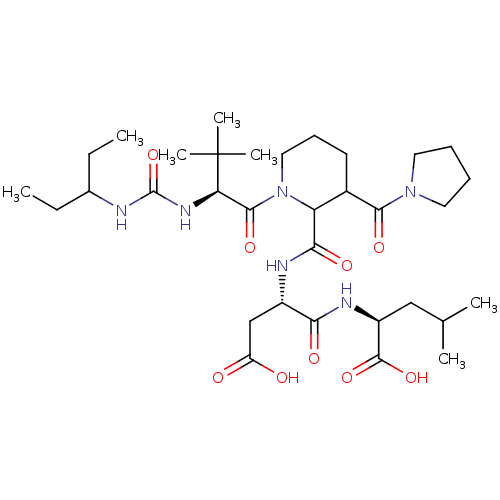 ((S)-2-((S)-3-Carboxy-2-{[1-{(S)-2-[3-(1-ethyl-prop...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050821 ((S)-2-((S)-3-Carboxy-2-{[1-{(S)-2-[3-(1-ethyl-prop...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50033461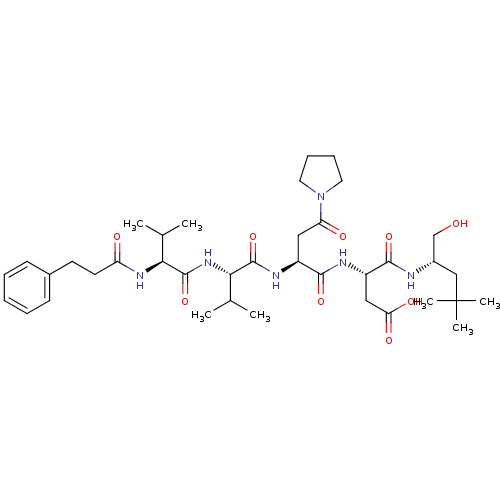 ((S)-N-((S)-1-Hydroxymethyl-3,3-dimethyl-butyl)-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against HSV ribonucleotide reductase | J Med Chem 38: 3617-23 (1995) BindingDB Entry DOI: 10.7270/Q21V5FMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50050833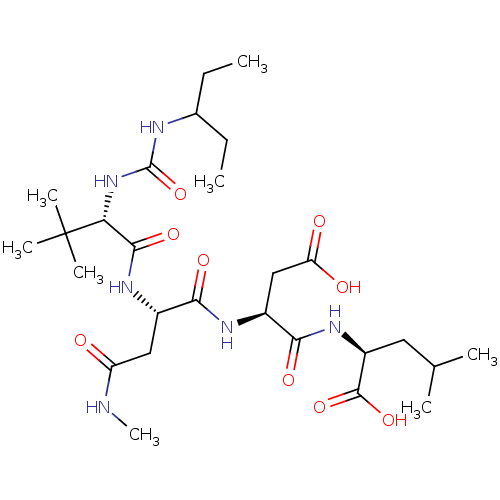 ((S)-2-[(S)-3-Carboxy-2-(2-{(S)-2-[3-(1-ethyl-propy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-Méga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Herpes simplex virus (HSV) ribonucleotide reductase (RR) | J Med Chem 39: 2178-87 (1996) Article DOI: 10.1021/jm950825x BindingDB Entry DOI: 10.7270/Q2BG2N22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50033449 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[(S)-3,3-dime...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against HSV ribonucleotide reductase | J Med Chem 38: 3617-23 (1995) BindingDB Entry DOI: 10.7270/Q21V5FMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142056 ((S)-3-(5-Carbamoyl-1H-indol-3-yl)-2-[(1-cyclohexyl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053987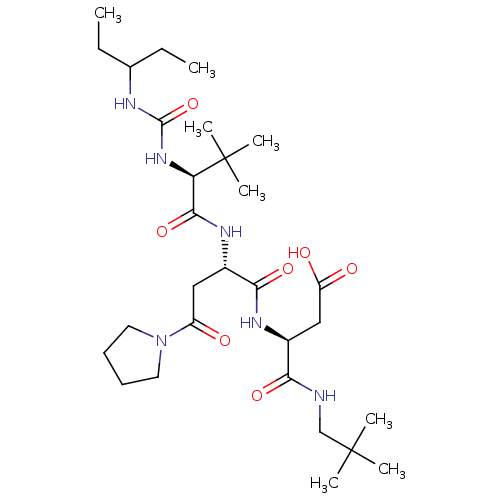 ((S)-N-(2,2-Dimethyl-propyl)-3-((S)-2-{(S)-2-[3-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50033455 ((S)-2-((S)-3-Carboxy-2-{(S)-2-[(S)-2-(2-ethyl-buty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against HSV ribonucleotide reductase | J Med Chem 38: 3617-23 (1995) BindingDB Entry DOI: 10.7270/Q21V5FMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142052 ((2S)-2-(1-cyclohexyl-2-(furan-3-yl)-1H-benzo[d]imi...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 460 total ) | Next | Last >> |