

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10439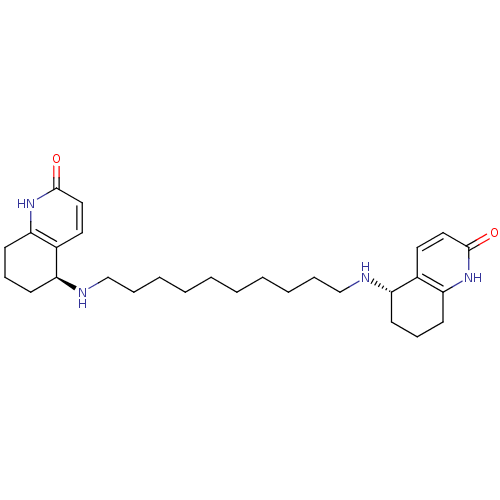 ((5S)-5-[(10-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.800 | -51.4 | 2.40 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10440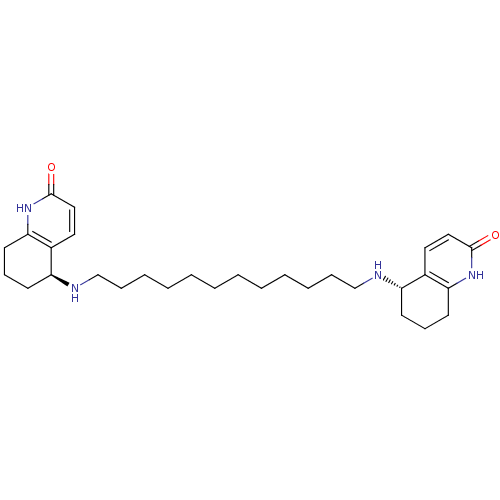 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.5 | -47.2 | 16 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10440 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 19.6 | -43.6 | 52 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 47.1 | -41.4 | 114 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 175 | -38.2 | 414 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10487 (1-chloro-6H,7H,8H,9H,10H-cyclohepta[b]quinolin-11-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10501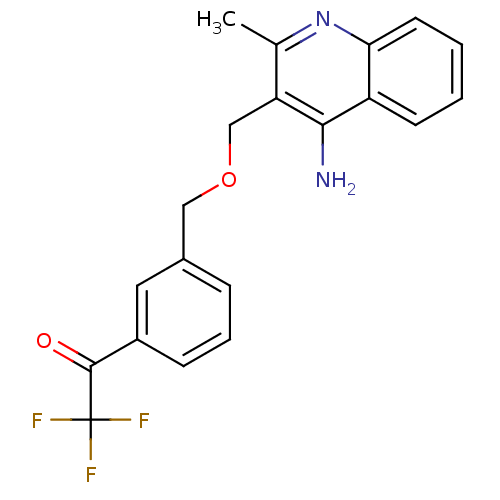 (1-(3-{[(4-amino-2-methylquinolin-3-yl)methoxy]meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10503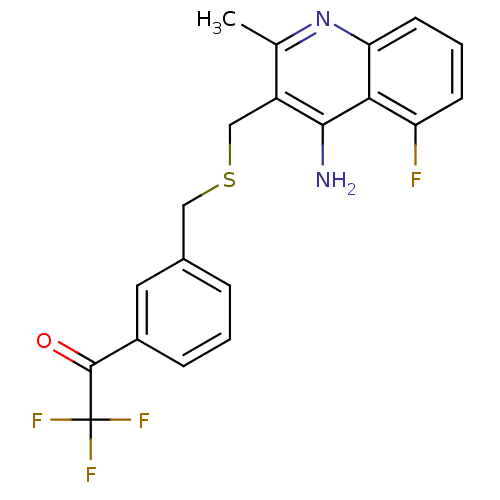 (1-[3-({[(4-amino-5-fluoro-2-methylquinolin-3-yl)me...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9047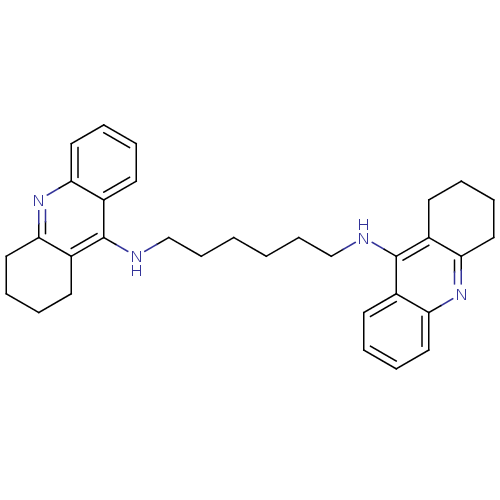 (Bis-THA inhibitor 5 | CHEMBL73800 | Hexylene-Linke...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10499 (1-(3-{[(4-amino-5-fluoro-2-methylquinolin-3-yl)met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10406 ((1S,12S,14R)-4-[8-(1,3-dioxo-2,3-dihydro-1H-isoind...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 126: 15405-11 (2004) Article DOI: 10.1021/ja0466154 BindingDB Entry DOI: 10.7270/Q2FX77N1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10500 (1-(3-{[(4-amino-5-chloro-2-methylquinolin-3-yl)met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8964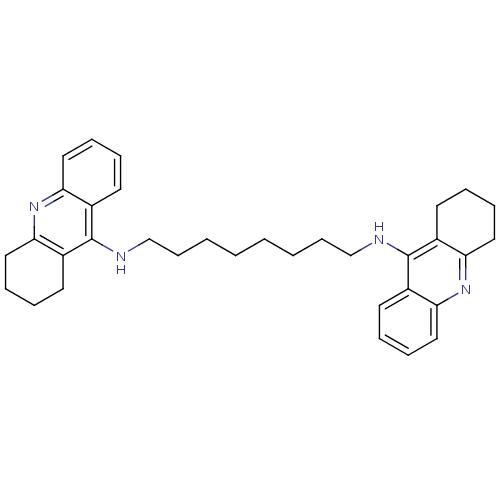 (CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10406 ((1S,12S,14R)-4-[8-(1,3-dioxo-2,3-dihydro-1H-isoind...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 126: 15405-11 (2004) Article DOI: 10.1021/ja0466154 BindingDB Entry DOI: 10.7270/Q2FX77N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10489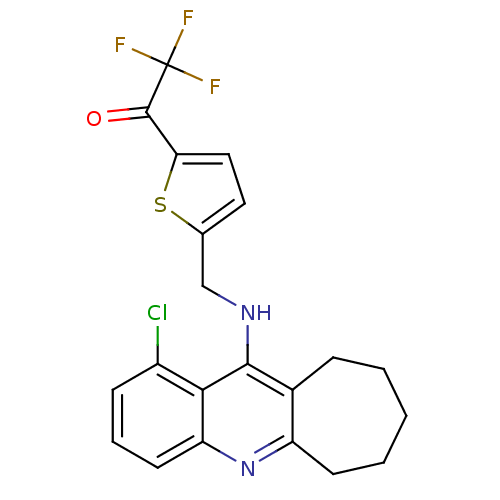 (1-(5-{[(1-chloro-7,8,9,10-tetrahydro-6H-cyclohepta...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10491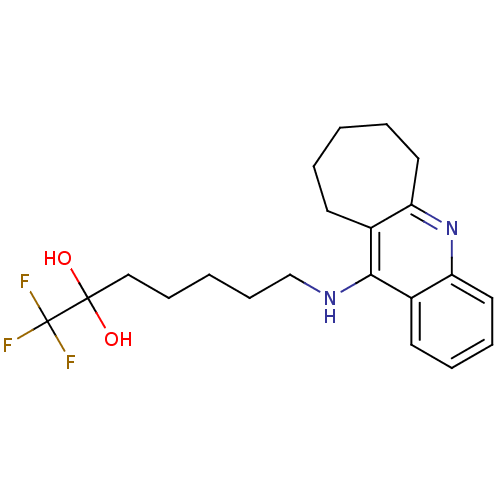 (1,1,1-trifluoro-7-(7,8,9,10-tetrahydro-6H-cyclohep...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8962 (Bis-THA inhibitor 4 | CHEMBL179732 | N-[5-(1,2,3,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10469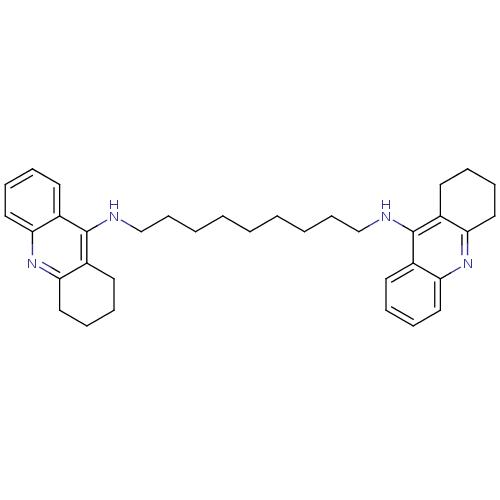 (Bis-THA inhibitor 1c | Bis-THA inhibitor 8 | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10490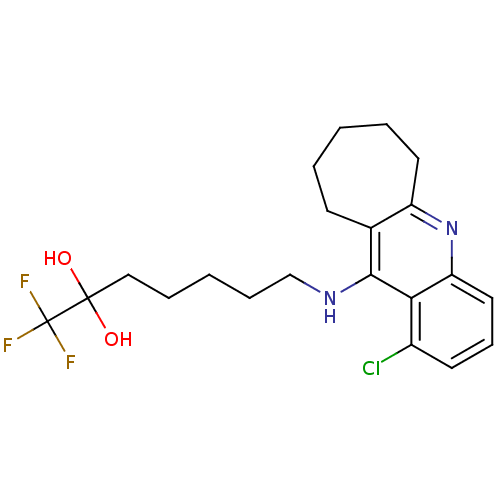 (7-({1-chloro-6H,7H,8H,9H,10H-cyclohepta[b]quinolin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10405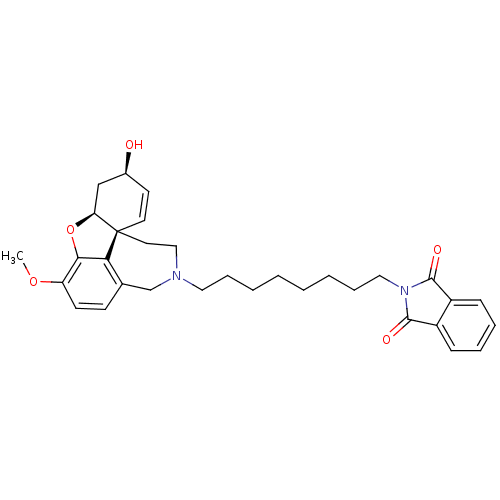 (2-{8-[(1S,12S,14R)-14-hydroxy-9-methoxy-11-oxa-4-a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 126: 15405-11 (2004) Article DOI: 10.1021/ja0466154 BindingDB Entry DOI: 10.7270/Q2FX77N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10470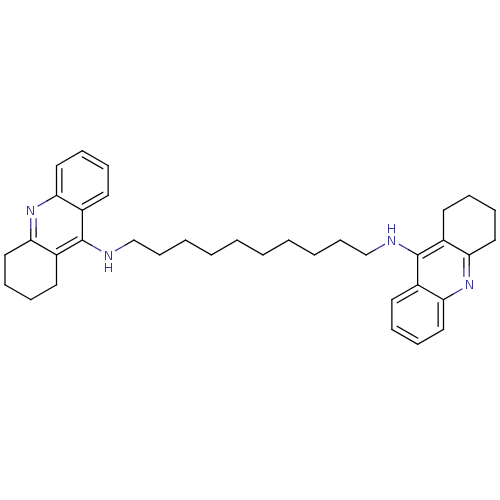 (Bis-THA inhibitor 1d | Bis-THA inhibitor 9 | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10405 (2-{8-[(1S,12S,14R)-14-hydroxy-9-methoxy-11-oxa-4-a...) | UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 126: 15405-11 (2004) Article DOI: 10.1021/ja0466154 BindingDB Entry DOI: 10.7270/Q2FX77N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat serum BuChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM10478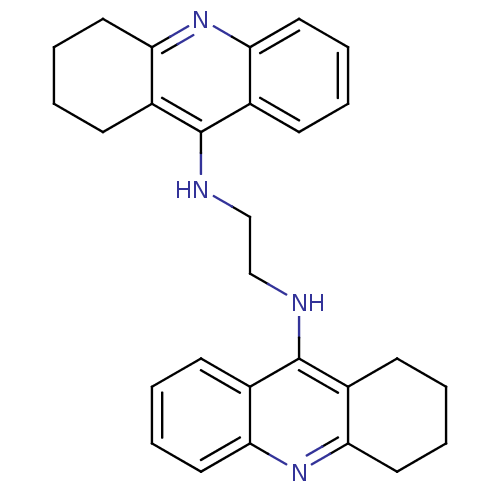 (A2A.2HCl | Bis-THA inhibitor 1 | CHEMBL213377 | N-...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat serum BuChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM8964 (CHEMBL75274 | Homodimeric Tacrine Analog 3c | N-[8...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat serum BuChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM9047 (Bis-THA inhibitor 5 | CHEMBL73800 | Hexylene-Linke...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat serum BuChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat serum BuChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10439 ((5S)-5-[(10-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 151 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM10479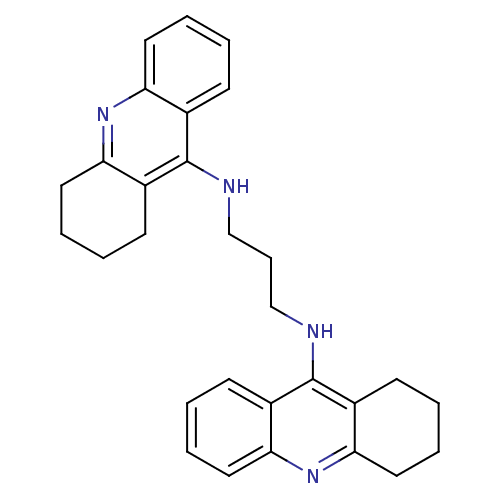 (A3A.2HCl | Bis-THA inhibitor 2 | CHEMBL378006 | N-...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat serum BuChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM10469 (Bis-THA inhibitor 1c | Bis-THA inhibitor 8 | CHEMB...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat serum BuChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10480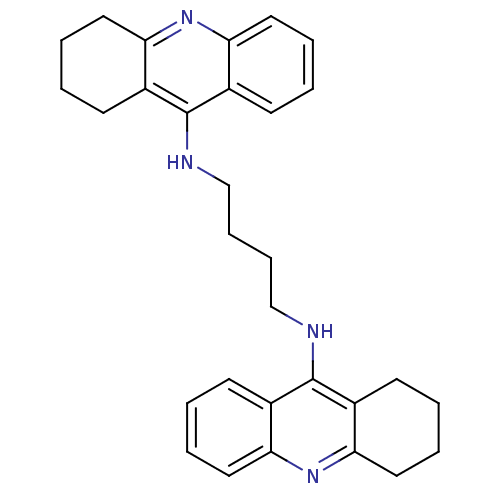 (A4A.2HCl | Bis-THA inhibitor 3 | CHEMBL211313 | N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM10470 (Bis-THA inhibitor 1d | Bis-THA inhibitor 9 | CHEMB...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat serum BuChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10407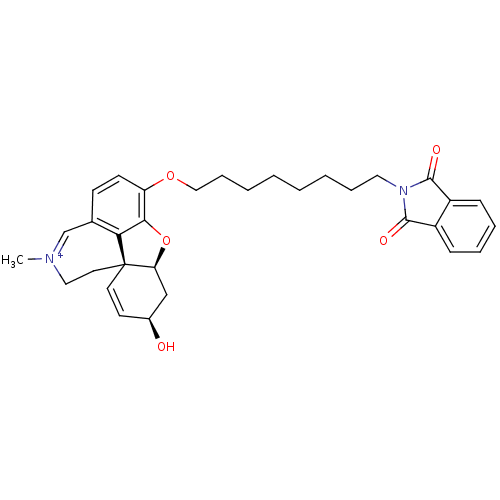 ((1S,12S,14R)-9-{[8-(1,3-dioxo-2,3-dihydro-1H-isoin...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 203 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 126: 15405-11 (2004) Article DOI: 10.1021/ja0466154 BindingDB Entry DOI: 10.7270/Q2FX77N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM10480 (A4A.2HCl | Bis-THA inhibitor 3 | CHEMBL211313 | N-...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 252 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat serum BuChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10479 (A3A.2HCl | Bis-THA inhibitor 2 | CHEMBL378006 | N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10408 ((1S,12S,14R)-14-hydroxy-9-methoxy-4-methyl-11-oxa-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 266 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 126: 15405-11 (2004) Article DOI: 10.1021/ja0466154 BindingDB Entry DOI: 10.7270/Q2FX77N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM8962 (Bis-THA inhibitor 4 | CHEMBL179732 | N-[5-(1,2,3,4...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 329 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat serum BuChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10502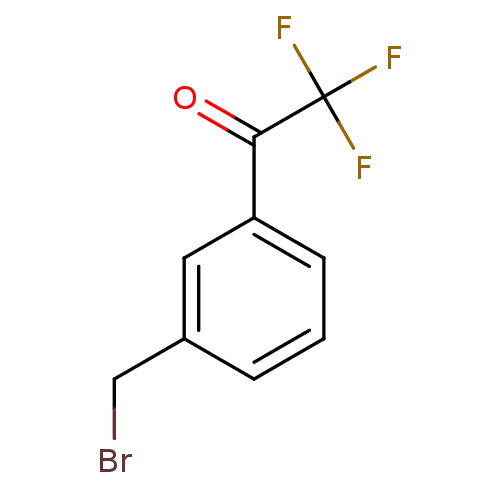 (1-[3-(bromomethyl)phenyl]-2,2,2-trifluoroethan-1-o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 418 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 126: 15405-11 (2004) Article DOI: 10.1021/ja0466154 BindingDB Entry DOI: 10.7270/Q2FX77N1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10407 ((1S,12S,14R)-9-{[8-(1,3-dioxo-2,3-dihydro-1H-isoin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 499 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 126: 15405-11 (2004) Article DOI: 10.1021/ja0466154 BindingDB Entry DOI: 10.7270/Q2FX77N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 614 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 126: 15405-11 (2004) Article DOI: 10.1021/ja0466154 BindingDB Entry DOI: 10.7270/Q2FX77N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10408 ((1S,12S,14R)-14-hydroxy-9-methoxy-4-methyl-11-oxa-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 702 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 126: 15405-11 (2004) Article DOI: 10.1021/ja0466154 BindingDB Entry DOI: 10.7270/Q2FX77N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10478 (A2A.2HCl | Bis-THA inhibitor 1 | CHEMBL213377 | N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 711 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10488 (1-chloro-N-(thien-2-ylmethyl)-7,8,9,10-tetrahydro-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10498 (3-[(benzyloxy)methyl]-5-fluoro-2-methylquinolin-4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10493 ((4-amino-5-fluoro-2-methylquinolin-3-yl)methanol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10492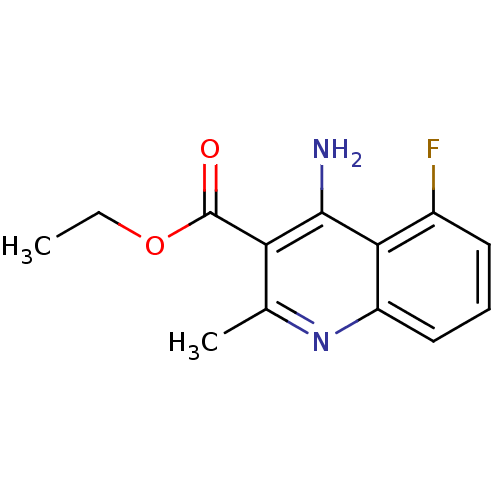 (4-Amino-3-carboxyethyl-5-fluoro-2-methylquinoline ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 56 total ) | Next | Last >> |