 Found 924 hits with Last Name = 'guilford' and Initial = 'w'
Found 924 hits with Last Name = 'guilford' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112518
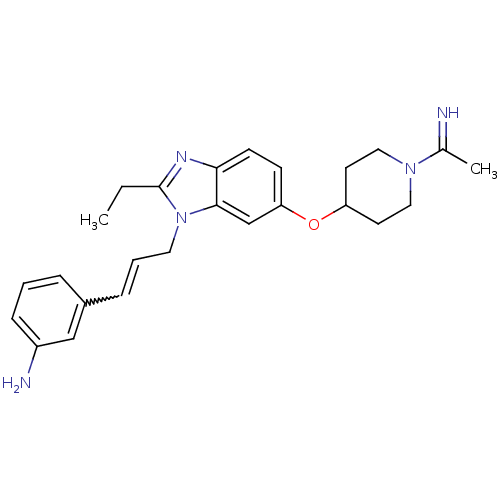
(3-(3-{2-Ethyl-6-[1-(1-imino-ethyl)-piperidin-4-ylo...)Show SMILES CCc1nc2ccc(OC3CCN(CC3)C(C)=N)cc2n1CC=Cc1cccc(N)c1 |w:23.26| Show InChI InChI=1S/C25H31N5O/c1-3-25-28-23-10-9-22(31-21-11-14-29(15-12-21)18(2)26)17-24(23)30(25)13-5-7-19-6-4-8-20(27)16-19/h4-10,16-17,21,26H,3,11-15,27H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibitory potency against Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1311-4 (2002)
BindingDB Entry DOI: 10.7270/Q2X066CP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112502
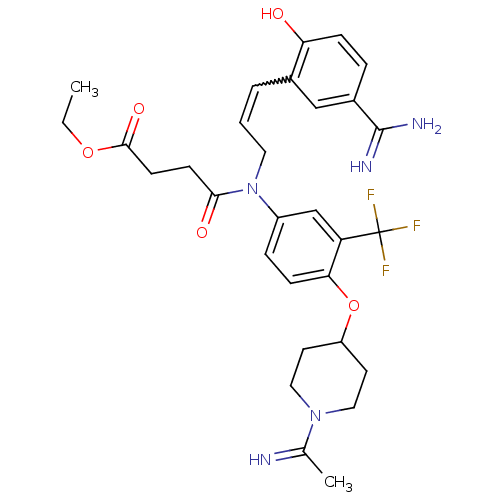
(CHEMBL26299 | N-[3-(5-Carbamimidoyl-2-hydroxy-phen...)Show SMILES CCOC(=O)CCC(=O)N(CC=Cc1cc(ccc1O)C(N)=N)c1ccc(OC2CCN(CC2)C(C)=N)c(c1)C(F)(F)F |w:12.12| Show InChI InChI=1S/C30H36F3N5O5/c1-3-42-28(41)11-10-27(40)38(14-4-5-20-17-21(29(35)36)6-8-25(20)39)22-7-9-26(24(18-22)30(31,32)33)43-23-12-15-37(16-13-23)19(2)34/h4-9,17-18,23,34,39H,3,10-16H2,1-2H3,(H3,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 12: 1307-10 (2002)
BindingDB Entry DOI: 10.7270/Q21R6PV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112506
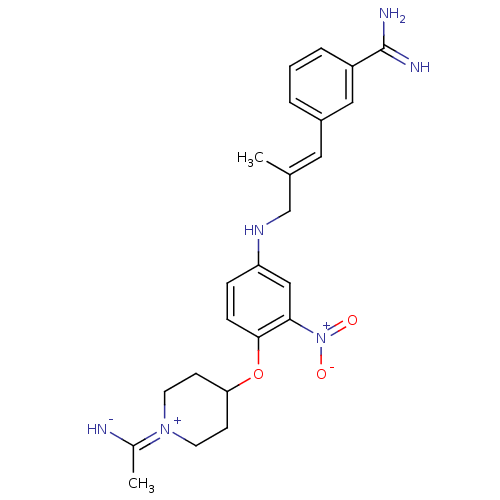
(3-(3-{4-[1-(1-Imino-ethyl)-piperidin-4-yloxy]-3-ni...)Show SMILES C\C(CNc1ccc(OC2CC[N+](CC2)=C(C)[NH-])c(c1)[N+]([O-])=O)=C/c1cccc(c1)C(N)=N |(8.92,-4.79,;7.58,-5.56,;6.23,-4.79,;6.23,-3.25,;4.91,-2.48,;3.57,-3.25,;2.24,-2.48,;2.24,-.94,;.91,-.17,;-.44,-.94,;-1.76,-.16,;-3.09,-.92,;-3.1,-2.46,;-1.76,-3.24,;-.44,-2.47,;-4.43,-3.23,;-5.78,-2.46,;-4.45,-4.77,;3.57,-.17,;4.91,-.94,;3.55,1.37,;4.9,2.14,;2.22,2.14,;7.56,-7.1,;8.89,-7.87,;8.91,-9.41,;10.25,-10.18,;11.58,-9.4,;11.57,-7.84,;10.22,-7.09,;12.9,-7.07,;12.88,-5.53,;14.23,-7.82,)| Show InChI InChI=1S/C24H30N6O3/c1-16(12-18-4-3-5-19(13-18)24(26)27)15-28-20-6-7-23(22(14-20)30(31)32)33-21-8-10-29(11-9-21)17(2)25/h3-7,12-14,21,25,28H,8-11,15H2,1-2H3,(H3,26,27)/b16-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 12: 1307-10 (2002)
BindingDB Entry DOI: 10.7270/Q21R6PV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112503

(7-({4-[1-(1-Imino-ethyl)-piperidin-4-yloxy]-3-nitr...)Show SMILES CC([NH-])=[N+]1CCC(CC1)Oc1ccc(NCc2ccc3ccc(cc3c2)C(N)=N)cc1[N+]([O-])=O |(-2.07,-4.18,;-.74,-4.97,;-.74,-6.51,;.59,-4.2,;.61,-2.66,;1.94,-1.9,;3.27,-2.68,;3.27,-4.21,;1.94,-4.98,;4.6,-1.91,;5.93,-2.68,;5.93,-4.22,;7.26,-4.99,;8.61,-4.22,;9.95,-4.99,;9.95,-6.53,;11.28,-7.29,;11.28,-8.83,;12.61,-9.6,;13.94,-8.83,;15.28,-9.59,;16.62,-8.8,;16.6,-7.26,;15.27,-6.49,;13.94,-7.28,;12.61,-6.52,;17.93,-6.47,;17.92,-4.93,;19.27,-7.24,;8.61,-2.67,;7.26,-1.9,;7.26,-.36,;8.59,.41,;5.93,.4,)| Show InChI InChI=1S/C25H28N6O3/c1-16(26)30-10-8-22(9-11-30)34-24-7-6-21(14-23(24)31(32)33)29-15-17-2-3-18-4-5-19(25(27)28)13-20(18)12-17/h2-7,12-14,22,26,29H,8-11,15H2,1H3,(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 12: 1307-10 (2002)
BindingDB Entry DOI: 10.7270/Q21R6PV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112513
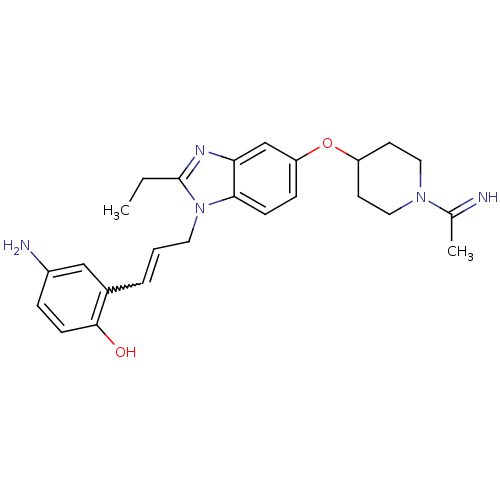
(4-Amino-2-(3-{2-ethyl-5-[1-(1-imino-ethyl)-piperid...)Show SMILES CCc1nc2cc(OC3CCN(CC3)C(C)=N)ccc2n1CC=Cc1cc(N)ccc1O |w:23.26| Show InChI InChI=1S/C25H31N5O2/c1-3-25-28-22-16-21(32-20-10-13-29(14-11-20)17(2)26)7-8-23(22)30(25)12-4-5-18-15-19(27)6-9-24(18)31/h4-9,15-16,20,26,31H,3,10-14,27H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibitory potency against Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1311-4 (2002)
BindingDB Entry DOI: 10.7270/Q2X066CP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112497

(CHEMBL26240 | N-[3-(5-Carbamimidoyl-2-hydroxy-phen...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1C(F)(F)F)N(CC=Cc1cc(ccc1O)C(N)=N)C(=O)CCC(O)=O |w:23.25| Show InChI InChI=1S/C28H32F3N5O5/c1-17(32)35-13-10-21(11-14-35)41-24-7-5-20(16-22(24)28(29,30)31)36(25(38)8-9-26(39)40)12-2-3-18-15-19(27(33)34)4-6-23(18)37/h2-7,15-16,21,32,37H,8-14H2,1H3,(H3,33,34)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 12: 1307-10 (2002)
BindingDB Entry DOI: 10.7270/Q21R6PV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112524
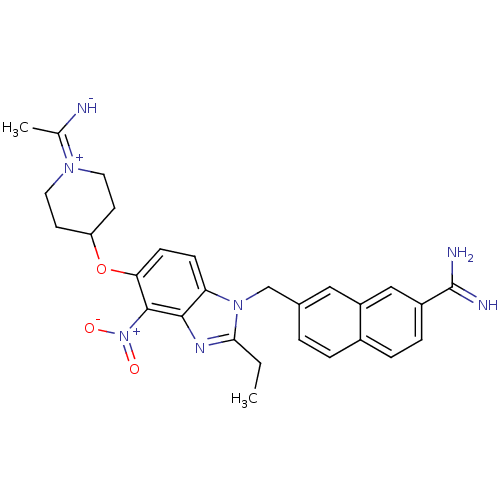
(7-{2-Ethyl-5-[1-(1-imino-ethyl)-piperidin-4-yloxy]...)Show SMILES CCc1nc2c(c(OC3CCN(CC3)C(C)=N)ccc2n1Cc1ccc2ccc(cc2c1)C(N)=N)[N+]([O-])=O Show InChI InChI=1S/C28H31N7O3/c1-3-25-32-26-23(34(25)16-18-4-5-19-6-7-20(28(30)31)15-21(19)14-18)8-9-24(27(26)35(36)37)38-22-10-12-33(13-11-22)17(2)29/h4-9,14-15,22,29H,3,10-13,16H2,1-2H3,(H3,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibitory potency against Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1311-4 (2002)
BindingDB Entry DOI: 10.7270/Q2X066CP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112491
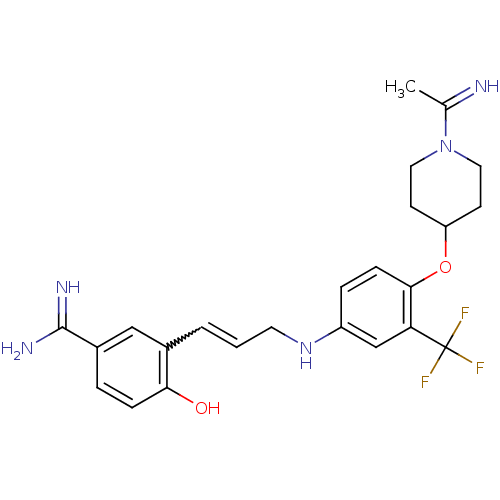
(4-Hydroxy-3-(3-{4-[1-(1-imino-ethyl)-piperidin-4-y...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(NCC=Cc2cc(ccc2O)C(N)=N)cc1C(F)(F)F |w:17.18| Show InChI InChI=1S/C24H28F3N5O2/c1-15(28)32-11-8-19(9-12-32)34-22-7-5-18(14-20(22)24(25,26)27)31-10-2-3-16-13-17(23(29)30)4-6-21(16)33/h2-7,13-14,19,28,31,33H,8-12H2,1H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 12: 1307-10 (2002)
BindingDB Entry DOI: 10.7270/Q21R6PV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112521
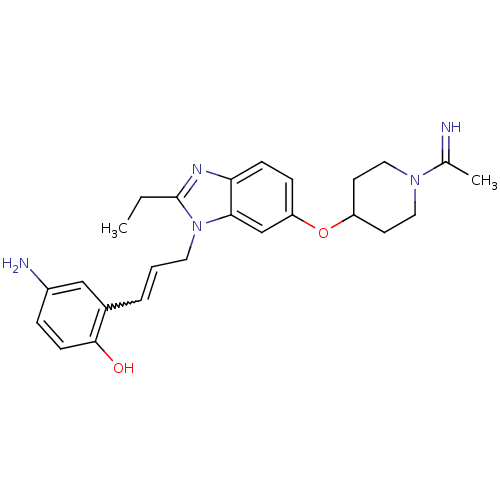
(4-Amino-2-(3-{2-ethyl-6-[1-(1-imino-ethyl)-piperid...)Show SMILES CCc1nc2ccc(OC3CCN(CC3)C(C)=N)cc2n1CC=Cc1cc(N)ccc1O |w:23.26| Show InChI InChI=1S/C25H31N5O2/c1-3-25-28-22-8-7-21(32-20-10-13-29(14-11-20)17(2)26)16-23(22)30(25)12-4-5-18-15-19(27)6-9-24(18)31/h4-9,15-16,20,26,31H,3,10-14,27H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibitory potency against Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1311-4 (2002)
BindingDB Entry DOI: 10.7270/Q2X066CP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17284
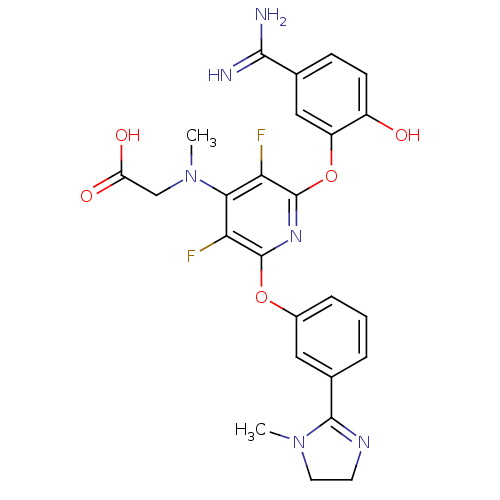
(2-{[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-diflu...)Show SMILES CN(CC(O)=O)c1c(F)c(Oc2cccc(c2)C2=NCCN2C)nc(Oc2cc(ccc2O)C(N)=N)c1F |t:18| Show InChI InChI=1S/C25H24F2N6O5/c1-32-9-8-30-23(32)14-4-3-5-15(10-14)37-24-19(26)21(33(2)12-18(35)36)20(27)25(31-24)38-17-11-13(22(28)29)6-7-16(17)34/h3-7,10-11,34H,8-9,12H2,1-2H3,(H3,28,29)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17284

(2-{[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-diflu...)Show SMILES CN(CC(O)=O)c1c(F)c(Oc2cccc(c2)C2=NCCN2C)nc(Oc2cc(ccc2O)C(N)=N)c1F |t:18| Show InChI InChI=1S/C25H24F2N6O5/c1-32-9-8-30-23(32)14-4-3-5-15(10-14)37-24-19(26)21(33(2)12-18(35)36)20(27)25(31-24)38-17-11-13(22(28)29)6-7-16(17)34/h3-7,10-11,34H,8-9,12H2,1-2H3,(H3,28,29)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17280

(1-[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-difluo...)Show SMILES CN1CCN=C1c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(N3CCCC(C3)C(O)=O)c2F)c1 |c:4| Show InChI InChI=1S/C28H28F2N6O5/c1-35-11-9-33-25(35)16-4-2-6-18(12-16)40-26-21(29)23(36-10-3-5-17(14-36)28(38)39)22(30)27(34-26)41-20-13-15(24(31)32)7-8-19(20)37/h2,4,6-8,12-13,17,37H,3,5,9-11,14H2,1H3,(H3,31,32)(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112500
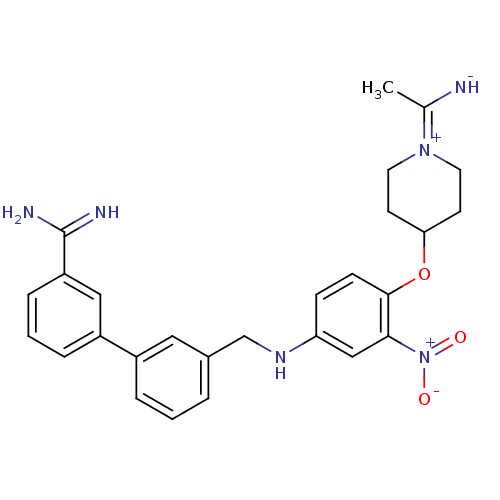
(3'-({4-[1-(1-Imino-ethyl)-piperidin-4-yloxy]-3-nit...)Show SMILES CC([NH-])=[N+]1CCC(CC1)Oc1ccc(NCc2cccc(c2)-c2cccc(c2)C(N)=N)cc1[N+]([O-])=O |(-4.64,-1.92,;-3.23,-2.6,;-3.08,-4.13,;-2,-1.75,;-2.14,-.22,;-.91,.63,;.5,-.06,;.64,-1.56,;-.61,-2.43,;1.73,.79,;3.12,.12,;3.29,-1.4,;4.68,-2.08,;5.93,-1.21,;7.32,-1.89,;7.48,-3.42,;8.87,-4.09,;10.1,-3.21,;11.49,-3.88,;11.66,-5.42,;10.42,-6.28,;9.03,-5.61,;10.59,-7.79,;9.35,-8.66,;9.51,-10.19,;10.9,-10.86,;12.14,-10,;11.98,-8.47,;13.52,-10.71,;14.79,-9.91,;13.61,-12.25,;5.76,.32,;4.36,.98,;4.2,2.52,;5.43,3.37,;2.81,3.18,)| Show InChI InChI=1S/C27H30N6O3/c1-18(28)32-12-10-24(11-13-32)36-26-9-8-23(16-25(26)33(34)35)31-17-19-4-2-5-20(14-19)21-6-3-7-22(15-21)27(29)30/h2-9,14-16,24,28,31H,10-13,17H2,1H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 12: 1307-10 (2002)
BindingDB Entry DOI: 10.7270/Q21R6PV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112516
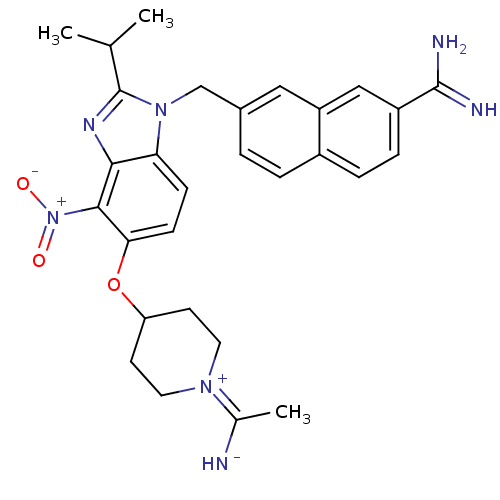
(7-{5-[1-(1-Imino-ethyl)-piperidin-4-yloxy]-2-isopr...)Show SMILES CC(C)c1nc2c(c(OC3CCN(CC3)C(C)=N)ccc2n1Cc1ccc2ccc(cc2c1)C(N)=N)[N+]([O-])=O Show InChI InChI=1S/C29H33N7O3/c1-17(2)29-33-26-24(35(29)16-19-4-5-20-6-7-21(28(31)32)15-22(20)14-19)8-9-25(27(26)36(37)38)39-23-10-12-34(13-11-23)18(3)30/h4-9,14-15,17,23,30H,10-13,16H2,1-3H3,(H3,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibitory potency against Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1311-4 (2002)
BindingDB Entry DOI: 10.7270/Q2X066CP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066635
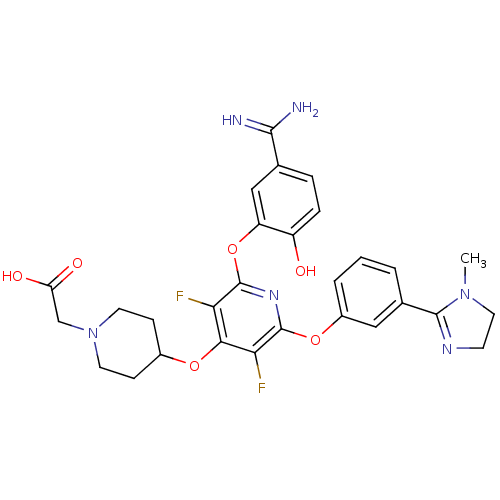
((4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-difl...)Show SMILES CN1CCN=C1c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(OC3CCN(CC(O)=O)CC3)c2F)c1 |c:4| Show InChI InChI=1S/C29H30F2N6O6/c1-36-12-9-34-27(36)17-3-2-4-19(13-17)42-28-23(30)25(41-18-7-10-37(11-8-18)15-22(39)40)24(31)29(35-28)43-21-14-16(26(32)33)5-6-20(21)38/h2-6,13-14,18,38H,7-12,15H2,1H3,(H3,32,33)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066619

(({2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-difluo...)Show SMILES CCOC(=O)CN(C)c1c(F)c(Oc2cccc(c2)C2=NCCN2C)nc(Oc2cc(ccc2O)C(N)=N)c1F |t:20| Show InChI InChI=1S/C27H28F2N6O5/c1-4-38-20(37)14-35(3)23-21(28)26(39-17-7-5-6-16(12-17)25-32-10-11-34(25)2)33-27(22(23)29)40-19-13-15(24(30)31)8-9-18(19)36/h5-9,12-13,36H,4,10-11,14H2,1-3H3,(H3,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17282

(7-({6-[(1-ethanimidoylpiperidin-4-yl)oxy]-2-methyl...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2nc(C)n(Cc3ccc4ccc(cc4c3)C(N)=N)c2c1 Show InChI InChI=1S/C27H30N6O/c1-17(28)32-11-9-23(10-12-32)34-24-7-8-25-26(15-24)33(18(2)31-25)16-19-3-4-20-5-6-21(27(29)30)14-22(20)13-19/h3-8,13-15,23,28H,9-12,16H2,1-2H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.270 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112488
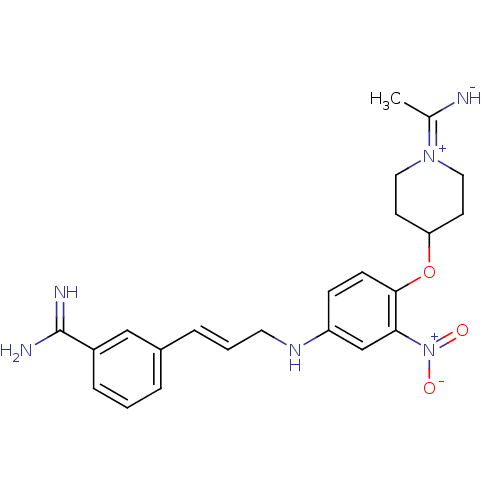
(3-(3-{4-[1-(1-Imino-ethyl)-piperidin-4-yloxy]-3-ni...)Show SMILES CC([NH-])=[N+]1CCC(CC1)Oc1ccc(NC\C=C\c2cccc(c2)C(N)=N)cc1[N+]([O-])=O |(-5.78,-2.46,;-4.43,-3.23,;-4.45,-4.77,;-3.1,-2.46,;-3.1,-.92,;-1.77,-.16,;-.44,-.94,;-.44,-2.47,;-1.77,-3.24,;.91,-.17,;2.24,-.94,;2.24,-2.48,;3.57,-3.25,;4.91,-2.48,;6.24,-3.25,;6.24,-4.79,;7.58,-5.56,;7.57,-7.1,;8.9,-7.87,;8.91,-9.41,;10.25,-10.18,;11.58,-9.4,;11.57,-7.85,;10.23,-7.09,;12.9,-7.07,;12.89,-5.53,;14.24,-7.82,;4.91,-.94,;3.57,-.17,;3.55,1.37,;4.9,2.14,;2.22,2.14,)| Show InChI InChI=1S/C23H28N6O3/c1-16(24)28-12-9-20(10-13-28)32-22-8-7-19(15-21(22)29(30)31)27-11-3-5-17-4-2-6-18(14-17)23(25)26/h2-8,14-15,20,24,27H,9-13H2,1H3,(H3,25,26)/b5-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 12: 1307-10 (2002)
BindingDB Entry DOI: 10.7270/Q21R6PV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112489
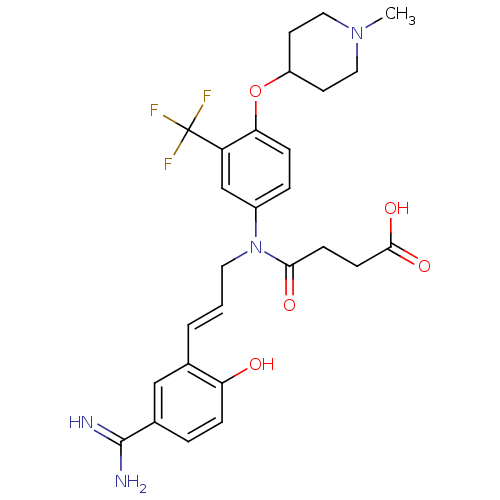
(CHEMBL277424 | N-[3-(5-Carbamimidoyl-2-hydroxy-phe...)Show SMILES CN1CCC(CC1)Oc1ccc(cc1C(F)(F)F)N(C\C=C\c1cc(ccc1O)C(N)=N)C(=O)CCC(O)=O Show InChI InChI=1S/C27H31F3N4O5/c1-33-13-10-20(11-14-33)39-23-7-5-19(16-21(23)27(28,29)30)34(24(36)8-9-25(37)38)12-2-3-17-15-18(26(31)32)4-6-22(17)35/h2-7,15-16,20,35H,8-14H2,1H3,(H3,31,32)(H,37,38)/b3-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 12: 1307-10 (2002)
BindingDB Entry DOI: 10.7270/Q21R6PV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112490
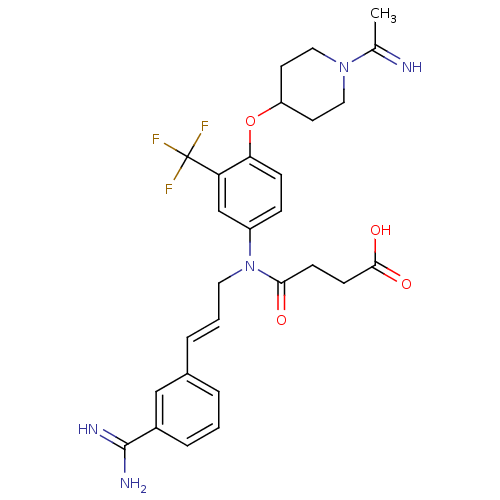
(CHEMBL424589 | N-[3-(3-Carbamimidoyl-phenyl)-allyl...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1C(F)(F)F)N(C\C=C\c1cccc(c1)C(N)=N)C(=O)CCC(O)=O Show InChI InChI=1S/C28H32F3N5O4/c1-18(32)35-14-11-22(12-15-35)40-24-8-7-21(17-23(24)28(29,30)31)36(25(37)9-10-26(38)39)13-3-5-19-4-2-6-20(16-19)27(33)34/h2-8,16-17,22,32H,9-15H2,1H3,(H3,33,34)(H,38,39)/b5-3+,32-18? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 12: 1307-10 (2002)
BindingDB Entry DOI: 10.7270/Q21R6PV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112494
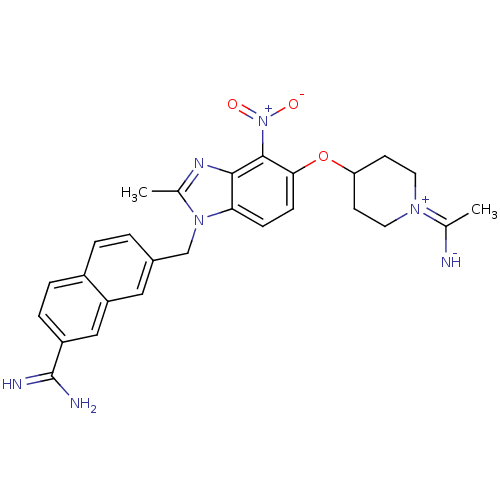
(7-{5-[1-(1-Imino-ethyl)-piperidin-4-yloxy]-2-methy...)Show SMILES CC([NH-])=[N+]1CCC(CC1)Oc1ccc2n(Cc3ccc4ccc(cc4c3)C(N)=N)c(C)nc2c1[N+]([O-])=O |(-7.32,-3.83,;-5.99,-4.62,;-5.99,-6.16,;-4.66,-3.85,;-3.33,-4.63,;-1.98,-3.86,;-1.98,-2.33,;-3.33,-1.55,;-4.64,-2.31,;-.65,-1.56,;.68,-2.33,;.68,-3.87,;2.01,-4.64,;3.36,-3.86,;4.81,-4.34,;4.83,-5.88,;6.16,-6.65,;6.16,-8.17,;7.49,-8.94,;8.82,-8.17,;10.16,-8.94,;11.49,-8.17,;11.49,-6.61,;10.13,-5.86,;8.82,-6.63,;7.47,-5.86,;12.81,-5.83,;12.79,-4.29,;14.15,-6.59,;5.72,-3.09,;7.26,-3.09,;4.81,-1.84,;3.36,-2.32,;2.01,-1.55,;2.01,-.01,;3.34,.76,;.68,.75,)| Show InChI InChI=1S/C27H29N7O3/c1-16(28)32-11-9-22(10-12-32)37-24-8-7-23-25(26(24)34(35)36)31-17(2)33(23)15-18-3-4-19-5-6-20(27(29)30)14-21(19)13-18/h3-8,13-14,22,28H,9-12,15H2,1-2H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 12: 1307-10 (2002)
BindingDB Entry DOI: 10.7270/Q21R6PV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112494

(7-{5-[1-(1-Imino-ethyl)-piperidin-4-yloxy]-2-methy...)Show SMILES CC([NH-])=[N+]1CCC(CC1)Oc1ccc2n(Cc3ccc4ccc(cc4c3)C(N)=N)c(C)nc2c1[N+]([O-])=O |(-7.32,-3.83,;-5.99,-4.62,;-5.99,-6.16,;-4.66,-3.85,;-3.33,-4.63,;-1.98,-3.86,;-1.98,-2.33,;-3.33,-1.55,;-4.64,-2.31,;-.65,-1.56,;.68,-2.33,;.68,-3.87,;2.01,-4.64,;3.36,-3.86,;4.81,-4.34,;4.83,-5.88,;6.16,-6.65,;6.16,-8.17,;7.49,-8.94,;8.82,-8.17,;10.16,-8.94,;11.49,-8.17,;11.49,-6.61,;10.13,-5.86,;8.82,-6.63,;7.47,-5.86,;12.81,-5.83,;12.79,-4.29,;14.15,-6.59,;5.72,-3.09,;7.26,-3.09,;4.81,-1.84,;3.36,-2.32,;2.01,-1.55,;2.01,-.01,;3.34,.76,;.68,.75,)| Show InChI InChI=1S/C27H29N7O3/c1-16(28)32-11-9-22(10-12-32)37-24-8-7-23-25(26(24)34(35)36)31-17(2)33(23)15-18-3-4-19-5-6-20(27(29)30)14-21(19)13-18/h3-8,13-14,22,28H,9-12,15H2,1-2H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibitory potency against Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1311-4 (2002)
BindingDB Entry DOI: 10.7270/Q2X066CP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112514
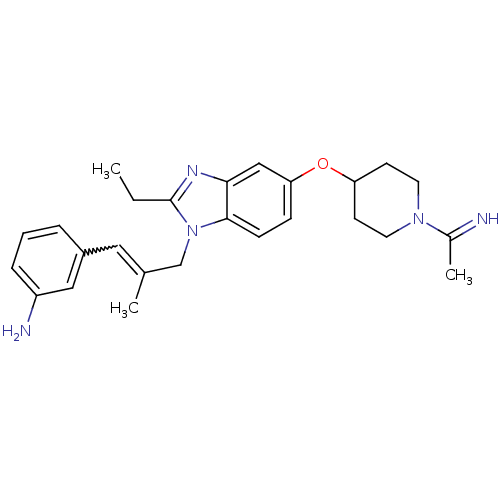
(3-(3-{2-Ethyl-5-[1-(1-imino-ethyl)-piperidin-4-ylo...)Show SMILES CCc1nc2cc(OC3CCN(CC3)C(C)=N)ccc2n1CC(C)=Cc1cccc(N)c1 |w:24.27| Show InChI InChI=1S/C26H33N5O/c1-4-26-29-24-16-23(32-22-10-12-30(13-11-22)19(3)27)8-9-25(24)31(26)17-18(2)14-20-6-5-7-21(28)15-20/h5-9,14-16,22,27H,4,10-13,17,28H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibitory potency against Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1311-4 (2002)
BindingDB Entry DOI: 10.7270/Q2X066CP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066634

((4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-difl...)Show SMILES CCOC(=O)CN1CCC(CC1)Oc1c(F)c(Oc2cccc(c2)C2=NCCN2C)nc(Oc2cc(ccc2O)C(N)=N)c1F |t:26| Show InChI InChI=1S/C31H34F2N6O6/c1-3-42-24(41)17-39-12-9-20(10-13-39)43-27-25(32)30(44-21-6-4-5-19(15-21)29-36-11-14-38(29)2)37-31(26(27)33)45-23-16-18(28(34)35)7-8-22(23)40/h4-8,15-16,20,40H,3,9-14,17H2,1-2H3,(H3,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066641
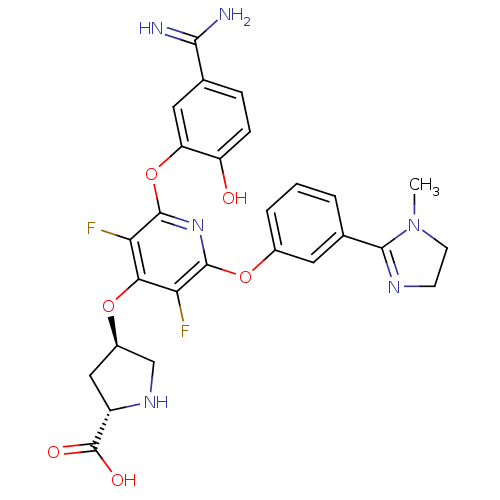
((2S,4R)-4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3...)Show SMILES CN1CCN=C1c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(O[C@H]3CN[C@@H](C3)C(O)=O)c2F)c1 |c:4| Show InChI InChI=1S/C27H26F2N6O6/c1-35-8-7-32-24(35)14-3-2-4-15(9-14)40-25-20(28)22(39-16-11-17(27(37)38)33-12-16)21(29)26(34-25)41-19-10-13(23(30)31)5-6-18(19)36/h2-6,9-10,16-17,33,36H,7-8,11-12H2,1H3,(H3,30,31)(H,37,38)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112496
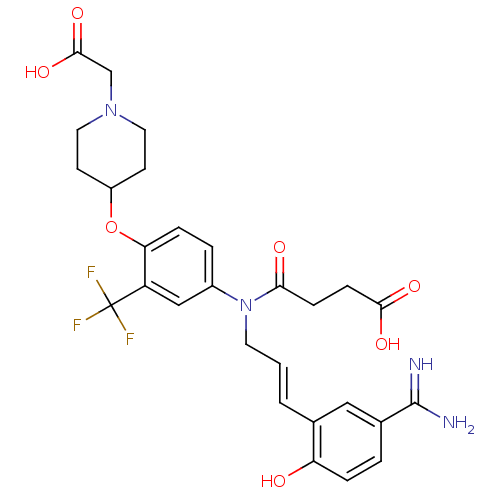
(CHEMBL26709 | N-[3-(5-Carbamimidoyl-2-hydroxy-phen...)Show SMILES NC(=N)c1ccc(O)c(\C=C\CN(C(=O)CCC(O)=O)c2ccc(OC3CCN(CC(O)=O)CC3)c(c2)C(F)(F)F)c1 Show InChI InChI=1S/C28H31F3N4O7/c29-28(30,31)21-15-19(4-6-23(21)42-20-9-12-34(13-10-20)16-26(40)41)35(24(37)7-8-25(38)39)11-1-2-17-14-18(27(32)33)3-5-22(17)36/h1-6,14-15,20,36H,7-13,16H2,(H3,32,33)(H,38,39)(H,40,41)/b2-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 12: 1307-10 (2002)
BindingDB Entry DOI: 10.7270/Q21R6PV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112528

(3-(3-{2-Ethyl-5-[1-(1-imino-ethyl)-piperidin-4-ylo...)Show SMILES CCc1nc2cc(OC3CCN(CC3)C(C)=N)ccc2n1CC=Cc1cccc(N)c1 |w:23.26| Show InChI InChI=1S/C25H31N5O/c1-3-25-28-23-17-22(31-21-11-14-29(15-12-21)18(2)26)9-10-24(23)30(25)13-5-7-19-6-4-8-20(27)16-19/h4-10,16-17,21,26H,3,11-15,27H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibitory potency against Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1311-4 (2002)
BindingDB Entry DOI: 10.7270/Q2X066CP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066623
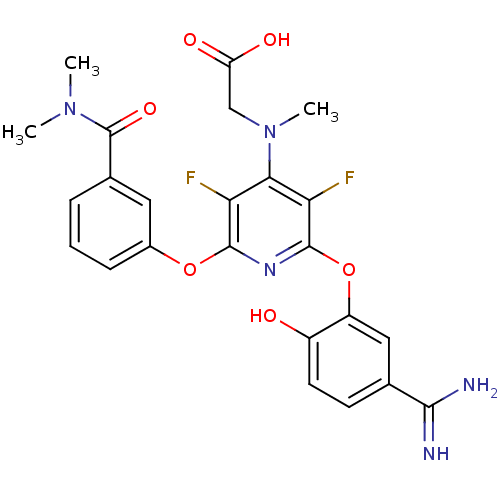
(CHEMBL72318 | {[2-(5-Carbamimidoyl-2-hydroxy-pheno...)Show SMILES CN(C)C(=O)c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(N(C)CC(O)=O)c2F)c1 Show InChI InChI=1S/C24H23F2N5O6/c1-30(2)24(35)13-5-4-6-14(9-13)36-22-18(25)20(31(3)11-17(33)34)19(26)23(29-22)37-16-10-12(21(27)28)7-8-15(16)32/h4-10,32H,11H2,1-3H3,(H3,27,28)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066639
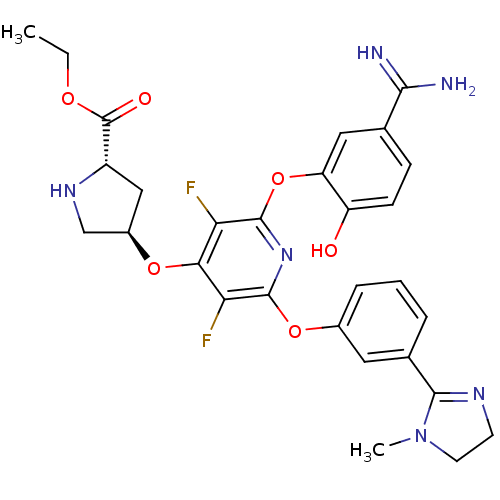
((2S,4R)-4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3...)Show SMILES CCOC(=O)[C@@H]1C[C@H](CN1)Oc1c(F)c(Oc2cccc(c2)C2=NCCN2C)nc(Oc2cc(ccc2O)C(N)=N)c1F |t:24| Show InChI InChI=1S/C29H30F2N6O6/c1-3-40-29(39)19-13-18(14-35-19)41-24-22(30)27(42-17-6-4-5-16(11-17)26-34-9-10-37(26)2)36-28(23(24)31)43-21-12-15(25(32)33)7-8-20(21)38/h4-8,11-12,18-19,35,38H,3,9-10,13-14H2,1-2H3,(H3,32,33)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112526
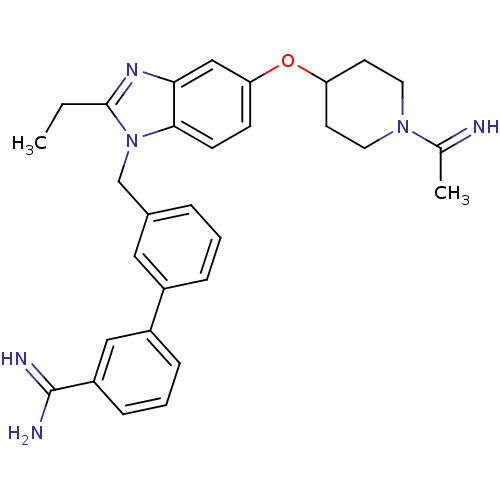
(3'-{2-Ethyl-5-[1-(1-imino-ethyl)-piperidin-4-yloxy...)Show SMILES CCc1nc2cc(OC3CCN(CC3)C(C)=N)ccc2n1Cc1cccc(c1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C30H34N6O/c1-3-29-34-27-18-26(37-25-12-14-35(15-13-25)20(2)31)10-11-28(27)36(29)19-21-6-4-7-22(16-21)23-8-5-9-24(17-23)30(32)33/h4-11,16-18,25,31H,3,12-15,19H2,1-2H3,(H3,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibitory potency against Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1311-4 (2002)
BindingDB Entry DOI: 10.7270/Q2X066CP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112492
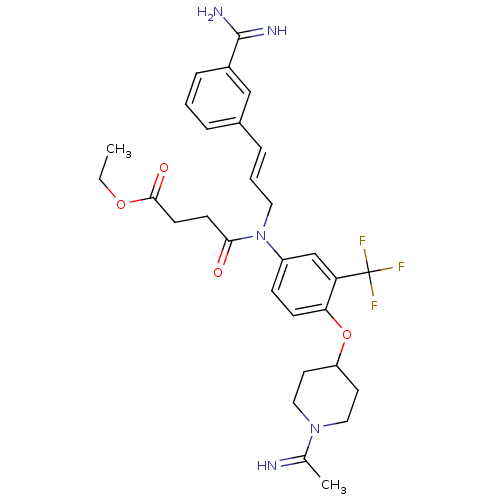
(CHEMBL26287 | N-[3-(3-Carbamimidoyl-phenyl)-allyl]...)Show SMILES CCOC(=O)CCC(=O)N(C\C=C\c1cccc(c1)C(N)=N)c1ccc(OC2CCN(CC2)C(C)=N)c(c1)C(F)(F)F Show InChI InChI=1S/C30H36F3N5O4/c1-3-41-28(40)12-11-27(39)38(15-5-7-21-6-4-8-22(18-21)29(35)36)23-9-10-26(25(19-23)30(31,32)33)42-24-13-16-37(17-14-24)20(2)34/h4-10,18-19,24,34H,3,11-17H2,1-2H3,(H3,35,36)/b7-5+,34-20? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 12: 1307-10 (2002)
BindingDB Entry DOI: 10.7270/Q21R6PV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112486
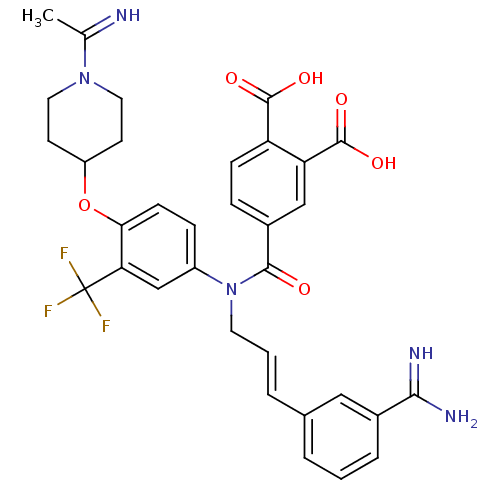
(4-([3-(3-Carbamimidoyl-phenyl)-allyl]-{4-[1-(1-imi...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(cc1C(F)(F)F)N(C\C=C\c1cccc(c1)C(N)=N)C(=O)c1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C33H32F3N5O6/c1-19(37)40-14-11-24(12-15-40)47-28-10-8-23(18-27(28)33(34,35)36)41(13-3-5-20-4-2-6-21(16-20)29(38)39)30(42)22-7-9-25(31(43)44)26(17-22)32(45)46/h2-10,16-18,24,37H,11-15H2,1H3,(H3,38,39)(H,43,44)(H,45,46)/b5-3+,37-19? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 12: 1307-10 (2002)
BindingDB Entry DOI: 10.7270/Q21R6PV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112505
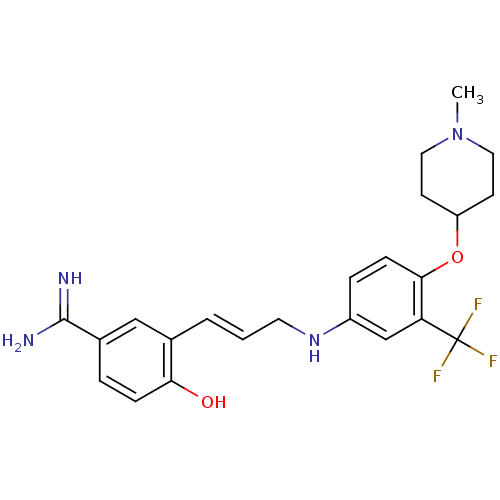
(4-Hydroxy-3-{3-[4-(1-methyl-piperidin-4-yloxy)-3-t...)Show SMILES CN1CCC(CC1)Oc1ccc(NC\C=C\c2cc(ccc2O)C(N)=N)cc1C(F)(F)F Show InChI InChI=1S/C23H27F3N4O2/c1-30-11-8-18(9-12-30)32-21-7-5-17(14-19(21)23(24,25)26)29-10-2-3-15-13-16(22(27)28)4-6-20(15)31/h2-7,13-14,18,29,31H,8-12H2,1H3,(H3,27,28)/b3-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 12: 1307-10 (2002)
BindingDB Entry DOI: 10.7270/Q21R6PV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066625

(CHEMBL120438 | {4-[2-(5-Carbamimidoyl-2-hydroxy-ph...)Show SMILES CN(C)C(=O)c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(OC3CCN(CC(O)=O)CC3)c2F)c1 Show InChI InChI=1S/C28H29F2N5O7/c1-34(2)28(39)16-4-3-5-18(12-16)41-26-22(29)24(40-17-8-10-35(11-9-17)14-21(37)38)23(30)27(33-26)42-20-13-15(25(31)32)6-7-19(20)36/h3-7,12-13,17,36H,8-11,14H2,1-2H3,(H3,31,32)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17277
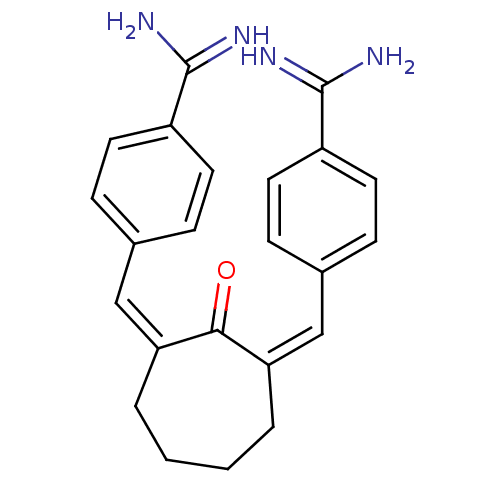
((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...)Show SMILES NC(=N)c1ccc(\C=C2\CCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13-,20-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17277

((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...)Show SMILES NC(=N)c1ccc(\C=C2\CCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13-,20-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Compound (isomer) was tested in the absence of light for inhibitory activity against Human Coagulation factor X |
J Med Chem 41: 3551-6 (1998)
Article DOI: 10.1021/jm980281+
BindingDB Entry DOI: 10.7270/Q2QJ7GFX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17277

((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...)Show SMILES NC(=N)c1ccc(\C=C2\CCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13-,20-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.660 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17277

((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...)Show SMILES NC(=N)c1ccc(\C=C2\CCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C23H24N4O/c24-22(25)17-9-5-15(6-10-17)13-19-3-1-2-4-20(21(19)28)14-16-7-11-18(12-8-16)23(26)27/h5-14H,1-4H2,(H3,24,25)(H3,26,27)/b19-13-,20-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066628
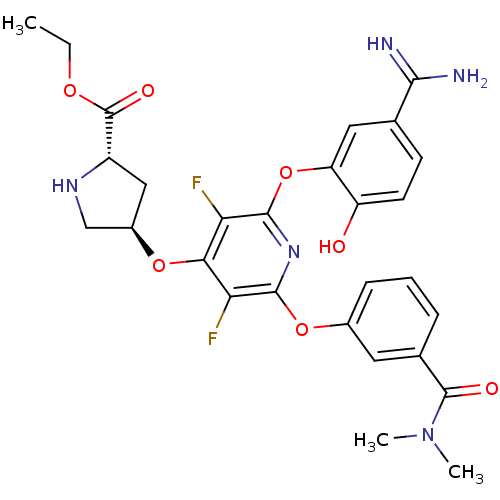
((2S,4R)-4-[2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-6...)Show SMILES CCOC(=O)[C@@H]1C[C@H](CN1)Oc1c(F)c(Oc2cccc(c2)C(=O)N(C)C)nc(Oc2cc(ccc2O)C(N)=N)c1F Show InChI InChI=1S/C28H29F2N5O7/c1-4-39-28(38)18-12-17(13-33-18)40-23-21(29)25(41-16-7-5-6-15(10-16)27(37)35(2)3)34-26(22(23)30)42-20-11-14(24(31)32)8-9-19(20)36/h5-11,17-18,33,36H,4,12-13H2,1-3H3,(H3,31,32)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112495
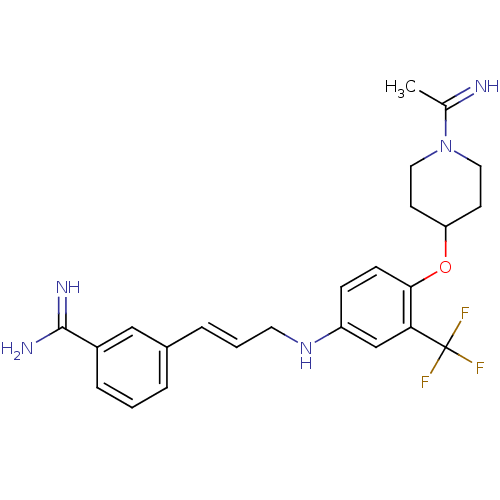
(3-(3-{4-[1-(1-Imino-ethyl)-piperidin-4-yloxy]-3-tr...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc(NC\C=C\c2cccc(c2)C(N)=N)cc1C(F)(F)F Show InChI InChI=1S/C24H28F3N5O/c1-16(28)32-12-9-20(10-13-32)33-22-8-7-19(15-21(22)24(25,26)27)31-11-3-5-17-4-2-6-18(14-17)23(29)30/h2-8,14-15,20,28,31H,9-13H2,1H3,(H3,29,30)/b5-3+,28-16? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Competitive inhibition of coagulation factor Xa |
Bioorg Med Chem Lett 12: 1307-10 (2002)
BindingDB Entry DOI: 10.7270/Q21R6PV8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066638
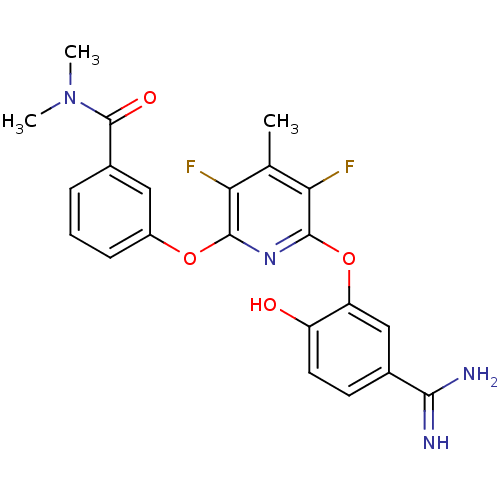
(3-[6-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-diflu...)Show SMILES CN(C)C(=O)c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(C)c2F)c1 Show InChI InChI=1S/C22H20F2N4O4/c1-11-17(23)20(31-14-6-4-5-13(9-14)22(30)28(2)3)27-21(18(11)24)32-16-10-12(19(25)26)7-8-15(16)29/h4-10,29H,1-3H3,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112512
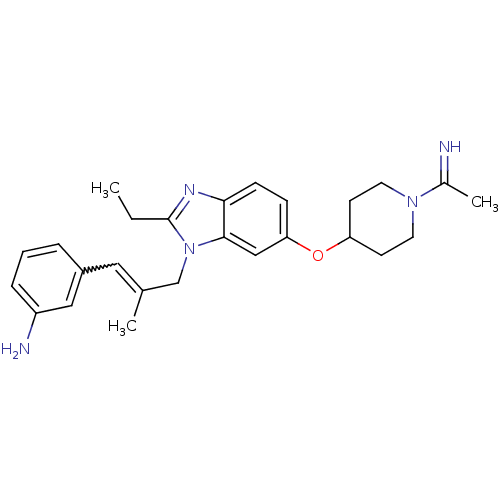
(3-(3-{2-Ethyl-6-[1-(1-imino-ethyl)-piperidin-4-ylo...)Show SMILES CCc1nc2ccc(OC3CCN(CC3)C(C)=N)cc2n1CC(C)=Cc1cccc(N)c1 |w:24.27| Show InChI InChI=1S/C26H33N5O/c1-4-26-29-24-9-8-23(32-22-10-12-30(13-11-22)19(3)27)16-25(24)31(26)17-18(2)14-20-6-5-7-21(28)15-20/h5-9,14-16,22,27H,4,10-13,17,28H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibitory potency against Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1311-4 (2002)
BindingDB Entry DOI: 10.7270/Q2X066CP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM17281

(7-({2-[(1-ethanimidoylpiperidin-4-yl)oxy]-9H-carba...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2c(c1)n(Cc1ccc3ccc(cc3c1)C(N)=N)c1ccccc21 Show InChI InChI=1S/C31H31N5O/c1-20(32)35-14-12-25(13-15-35)37-26-10-11-28-27-4-2-3-5-29(27)36(30(28)18-26)19-21-6-7-22-8-9-23(31(33)34)17-24(22)16-21/h2-11,16-18,25,32H,12-15,19H2,1H3,(H3,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.60 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex
| Assay Description
The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... |
Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999)
Article DOI: 10.1107/s0907444999007350
BindingDB Entry DOI: 10.7270/Q2H1308B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066638

(3-[6-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-diflu...)Show SMILES CN(C)C(=O)c1cccc(Oc2nc(Oc3cc(ccc3O)C(N)=N)c(F)c(C)c2F)c1 Show InChI InChI=1S/C22H20F2N4O4/c1-11-17(23)20(31-14-6-4-5-13(9-14)22(30)28(2)3)27-21(18(11)24)32-16-10-12(19(25)26)7-8-15(16)29/h4-10,29H,1-3H3,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory constant against human coagulation factor Xa (fXa) |
J Med Chem 45: 2484-93 (2002)
BindingDB Entry DOI: 10.7270/Q24J0FTH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50112529
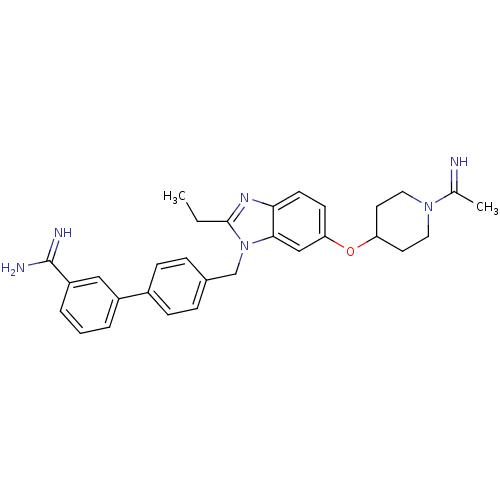
(4'-{2-Ethyl-6-[1-(1-imino-ethyl)-piperidin-4-yloxy...)Show SMILES CCc1nc2ccc(OC3CCN(CC3)C(C)=N)cc2n1Cc1ccc(cc1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C30H34N6O/c1-3-29-34-27-12-11-26(37-25-13-15-35(16-14-25)20(2)31)18-28(27)36(29)19-21-7-9-22(10-8-21)23-5-4-6-24(17-23)30(32)33/h4-12,17-18,25,31H,3,13-16,19H2,1-2H3,(H3,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Tested in vitro for the inhibitory potency against Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1311-4 (2002)
BindingDB Entry DOI: 10.7270/Q2X066CP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066637
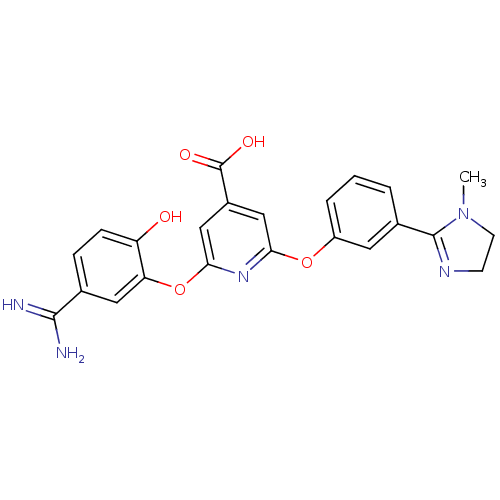
(2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-6-[3-(1-meth...)Show SMILES CN1CCN=C1c1cccc(Oc2cc(cc(Oc3cc(ccc3O)C(N)=N)n2)C(O)=O)c1 |c:4| Show InChI InChI=1S/C23H21N5O5/c1-28-8-7-26-22(28)14-3-2-4-16(9-14)32-19-11-15(23(30)31)12-20(27-19)33-18-10-13(21(24)25)5-6-17(18)29/h2-6,9-12,29H,7-8H2,1H3,(H3,24,25)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory potency was measured against human coagulation factor X |
J Med Chem 41: 3557-62 (1998)
Article DOI: 10.1021/jm980280h
BindingDB Entry DOI: 10.7270/Q2KS6QPR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50066637

(2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-6-[3-(1-meth...)Show SMILES CN1CCN=C1c1cccc(Oc2cc(cc(Oc3cc(ccc3O)C(N)=N)n2)C(O)=O)c1 |c:4| Show InChI InChI=1S/C23H21N5O5/c1-28-8-7-26-22(28)14-3-2-4-16(9-14)32-19-11-15(23(30)31)12-20(27-19)33-18-10-13(21(24)25)5-6-17(18)29/h2-6,9-12,29H,7-8H2,1H3,(H3,24,25)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibitory constant against human coagulation factor Xa (fXa) |
J Med Chem 45: 2484-93 (2002)
BindingDB Entry DOI: 10.7270/Q24J0FTH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50084175
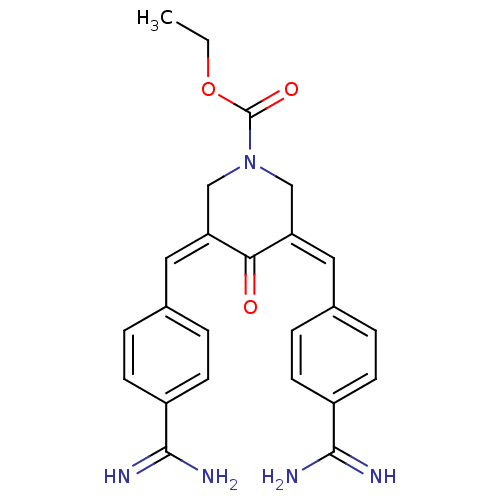
(3,5-Bis-[1-(4-carbamimidoyl-phenyl)-meth-(Z)-ylide...)Show SMILES CCOC(=O)N1C\C(=C\c2ccc(cc2)C(N)=N)C(=O)\C(C1)=C/c1ccc(cc1)C(N)=N Show InChI InChI=1S/C24H25N5O3/c1-2-32-24(31)29-13-19(11-15-3-7-17(8-4-15)22(25)26)21(30)20(14-29)12-16-5-9-18(10-6-16)23(27)28/h3-12H,2,13-14H2,1H3,(H3,25,26)(H3,27,28)/b19-11-,20-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50084186

(2,9-di[1-[4-amino(imino)methylphenyl]-(Z)-methylid...)Show SMILES NC(=N)c1ccc(\C=C2\CCCCCC\C(=C\c3ccc(cc3)C(N)=N)C2=O)cc1 Show InChI InChI=1S/C25H28N4O/c26-24(27)19-11-7-17(8-12-19)15-21-5-3-1-2-4-6-22(23(21)30)16-18-9-13-20(14-10-18)25(28)29/h7-16H,1-6H2,(H3,26,27)(H3,28,29)/b21-15-,22-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa (Xa) in a purified enzyme system. |
J Med Chem 42: 5415-25 (2000)
BindingDB Entry DOI: 10.7270/Q2TT4Q5S |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50112494

(7-{5-[1-(1-Imino-ethyl)-piperidin-4-yloxy]-2-methy...)Show SMILES CC([NH-])=[N+]1CCC(CC1)Oc1ccc2n(Cc3ccc4ccc(cc4c3)C(N)=N)c(C)nc2c1[N+]([O-])=O |(-7.32,-3.83,;-5.99,-4.62,;-5.99,-6.16,;-4.66,-3.85,;-3.33,-4.63,;-1.98,-3.86,;-1.98,-2.33,;-3.33,-1.55,;-4.64,-2.31,;-.65,-1.56,;.68,-2.33,;.68,-3.87,;2.01,-4.64,;3.36,-3.86,;4.81,-4.34,;4.83,-5.88,;6.16,-6.65,;6.16,-8.17,;7.49,-8.94,;8.82,-8.17,;10.16,-8.94,;11.49,-8.17,;11.49,-6.61,;10.13,-5.86,;8.82,-6.63,;7.47,-5.86,;12.81,-5.83,;12.79,-4.29,;14.15,-6.59,;5.72,-3.09,;7.26,-3.09,;4.81,-1.84,;3.36,-2.32,;2.01,-1.55,;2.01,-.01,;3.34,.76,;.68,.75,)| Show InChI InChI=1S/C27H29N7O3/c1-16(28)32-11-9-22(10-12-32)37-24-8-7-23-25(26(24)34(35)36)31-17(2)33(23)15-18-3-4-19-5-6-20(27(29)30)14-21(19)13-18/h3-8,13-14,22,28H,9-12,15H2,1-2H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Tested in vitro for the selectivity against trypsin (Trp) |
Bioorg Med Chem Lett 12: 1311-4 (2002)
BindingDB Entry DOI: 10.7270/Q2X066CP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data