

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50550643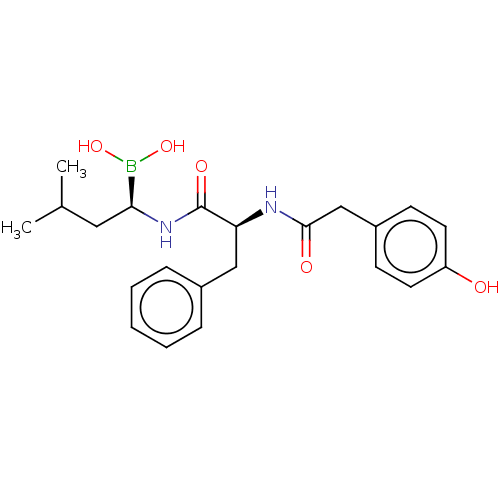 (CHEMBL4749207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human 20S constitutive proteasome beta 5 subunit assessed as equilibrium constant using fluorogenic peptide Ac-WLA-AMC as substra... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50339127 ((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant cyclophilin-associted cis-trans propyl isomerase activity | Antimicrob Agents Chemother 52: 1302-17 (2008) Article DOI: 10.1128/AAC.01324-07 BindingDB Entry DOI: 10.7270/Q21836R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50339126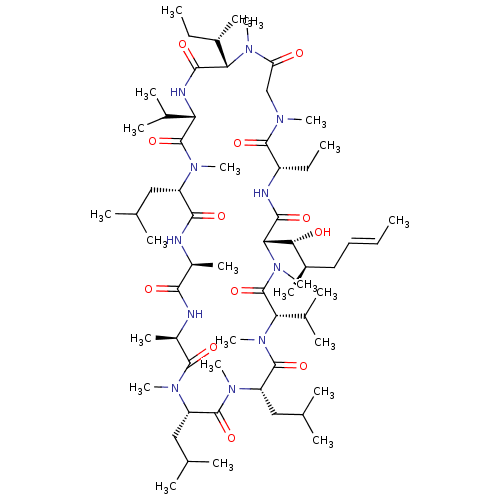 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-24-sec-buty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant cyclophilin-associted cis-trans propyl isomerase activity | Antimicrob Agents Chemother 52: 1302-17 (2008) Article DOI: 10.1128/AAC.01324-07 BindingDB Entry DOI: 10.7270/Q21836R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 9.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant cyclophilin-associted cis-trans propyl isomerase activity | Antimicrob Agents Chemother 52: 1302-17 (2008) Article DOI: 10.1128/AAC.01324-07 BindingDB Entry DOI: 10.7270/Q21836R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50550643 (CHEMBL4749207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 487 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human 20S constitutive proteasome beta 2 subunit assessed as equilibrium constant using fluorogenic peptide Ac-WLR-AMC as substra... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 2 (Rattus norvegicus) | BDBM19473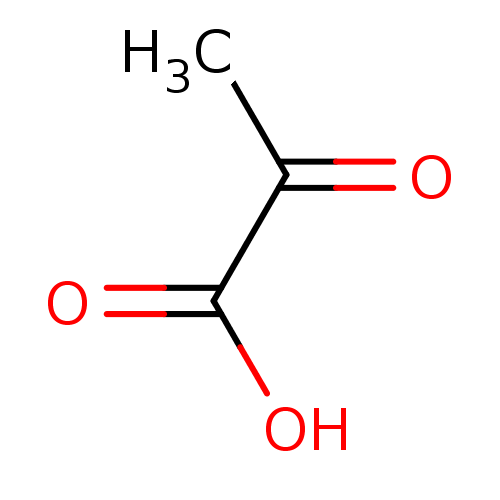 (2-Oxopropanoate | 2-oxopropanoic acid | Pyruvate) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Physiologisch-chemisches Institut der Eberhard-Karls-Universit£t T£bingen Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of lactate uptake in Xenopus laevis oocytes | Biochem J 341: 529-35 (1999) BindingDB Entry DOI: 10.7270/Q2125TQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 2 (Rattus norvegicus) | BDBM50390988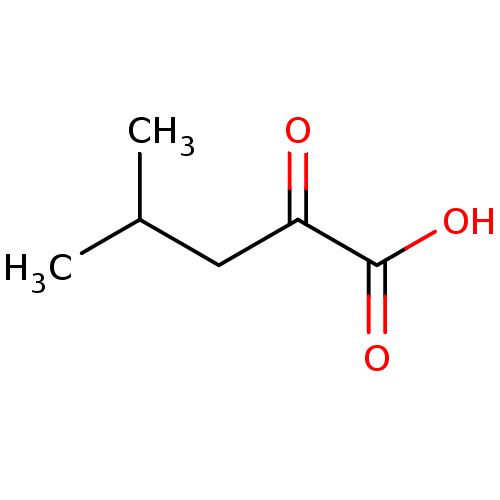 (CHEMBL445647) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem | PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Physiologisch-chemisches Institut der Eberhard-Karls-Universit£t T£bingen Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of lactate uptake in Xenopus laevis oocytes | Biochem J 341: 529-35 (1999) BindingDB Entry DOI: 10.7270/Q2125TQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 2 (Rattus norvegicus) | BDBM50390989 (CHEMBL146554) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem | PubMed | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Physiologisch-chemisches Institut der Eberhard-Karls-Universit£t T£bingen Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of lactate uptake in Xenopus laevis oocytes | Biochem J 341: 529-35 (1999) BindingDB Entry DOI: 10.7270/Q2125TQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 2 (Rattus norvegicus) | BDBM50390990 (CHEMBL2074691) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | PubMed | 8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Physiologisch-chemisches Institut der Eberhard-Karls-Universit£t T£bingen Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of lactate uptake in Xenopus laevis oocytes | Biochem J 341: 529-35 (1999) BindingDB Entry DOI: 10.7270/Q2125TQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 2 (Rattus norvegicus) | BDBM50270275 (3-OH butyrate | 3-OH-butyrate | 3-hydroxybutanoate...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Physiologisch-chemisches Institut der Eberhard-Karls-Universit£t T£bingen Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of lactate uptake in Xenopus laevis oocytes | Biochem J 341: 529-35 (1999) BindingDB Entry DOI: 10.7270/Q2125TQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21489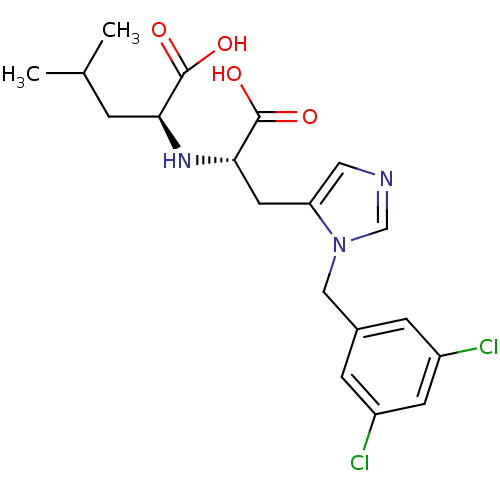 ((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,5-dichlorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals | Assay Description Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... | J Am Chem Soc 124: 11852-3 (2002) Article DOI: 10.1021/ja0277226 BindingDB Entry DOI: 10.7270/Q2KP80FN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50550643 (CHEMBL4749207) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 subunit chymotrypsin-like activity using fluorogenic peptide Ac-WLA-AMC as substrate in presence of P... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21488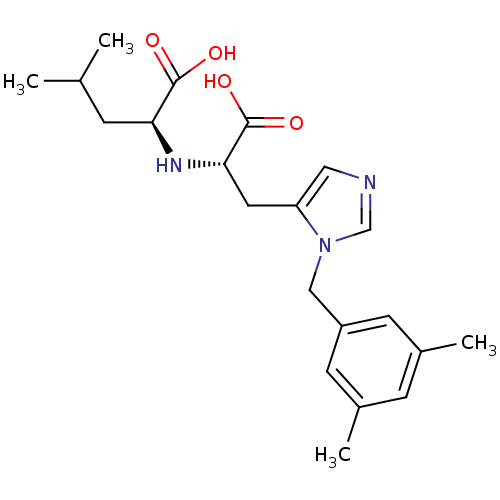 ((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,5-dimethylphenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals | Assay Description Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... | J Am Chem Soc 124: 11852-3 (2002) Article DOI: 10.1021/ja0277226 BindingDB Entry DOI: 10.7270/Q2KP80FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50550643 (CHEMBL4749207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S constitutive proteasome beta 5 subunit chymotrypsin-like activity using fluorogenic peptide Ac-WLA-AMC as substrate in presen... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 subunit chymotrypsin-like activity using fluorogenic peptide Ac-WLA-AMC as substrate in presence of P... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21486 ((2S)-2-{[(1S)-1-carboxy-2-{1-[(3-methylphenyl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals | Assay Description Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... | J Am Chem Soc 124: 11852-3 (2002) Article DOI: 10.1021/ja0277226 BindingDB Entry DOI: 10.7270/Q2KP80FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50550642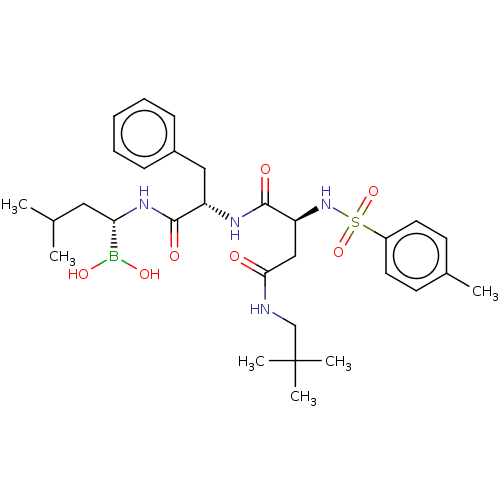 (CHEMBL4787081) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 subunit chymotrypsin-like activity using fluorogenic peptide Ac-WLA-AMC as substrate in presence of P... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-9 (Homo sapiens (Human)) | BDBM50550643 (CHEMBL4749207) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 1 subunit caspase-like activity using fluorogenic peptide Ac-nLPnLD-AMC as substrate in presence of PA2... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-9 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 1 subunit caspase-like activity using fluorogenic peptide Ac-nLPnLD-AMC as substrate in presence of PA2... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S constitutive proteasome beta 5 subunit chymotrypsin-like activity using fluorogenic peptide Ac-WLA-AMC as substrate in presen... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteasome subunit beta type-9 (Homo sapiens (Human)) | BDBM50550640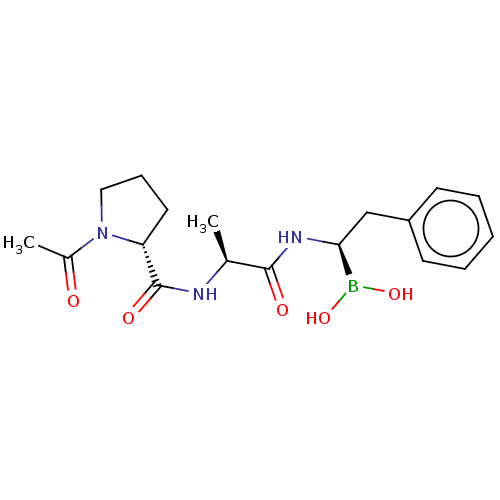 (CHEMBL4749043) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 1 subunit caspase-like activity using fluorogenic peptide Ac-nLPnLD-AMC as substrate in presence of PA2... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50550642 (CHEMBL4787081) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S constitutive proteasome beta 5 subunit chymotrypsin-like activity using fluorogenic peptide Ac-WLA-AMC as substrate in presen... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21491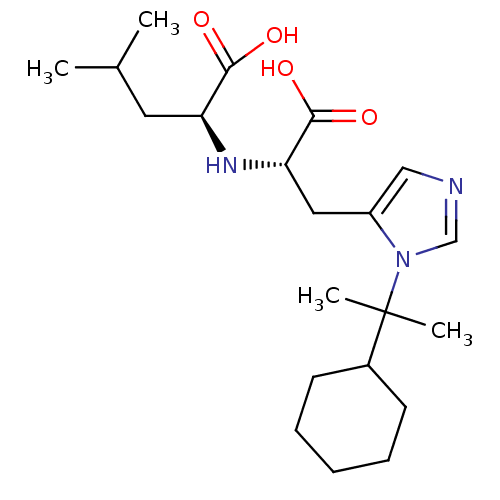 ((2S)-2-{[(1S)-1-carboxy-2-[1-(2-cyclohexylpropan-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals | Assay Description Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... | J Am Chem Soc 124: 11852-3 (2002) Article DOI: 10.1021/ja0277226 BindingDB Entry DOI: 10.7270/Q2KP80FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21487 ((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,4-dimethylphenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals | Assay Description Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... | J Am Chem Soc 124: 11852-3 (2002) Article DOI: 10.1021/ja0277226 BindingDB Entry DOI: 10.7270/Q2KP80FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-9 (Homo sapiens (Human)) | BDBM50550641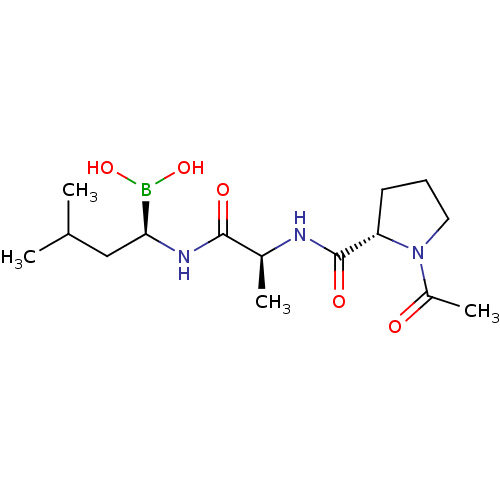 (CHEMBL4781914) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 1 subunit caspase-like activity using fluorogenic peptide Ac-nLPnLD-AMC as substrate in presence of PA2... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21482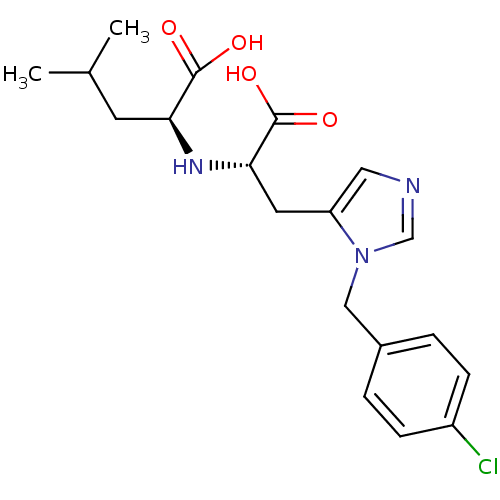 ((2S)-2-{[(1S)-1-carboxy-2-{1-[(4-chlorophenyl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals | Assay Description Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... | J Am Chem Soc 124: 11852-3 (2002) Article DOI: 10.1021/ja0277226 BindingDB Entry DOI: 10.7270/Q2KP80FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50550640 (CHEMBL4749043) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 subunit chymotrypsin-like activity using fluorogenic peptide Ac-WLA-AMC as substrate in presence of P... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-9 (Homo sapiens (Human)) | BDBM50550642 (CHEMBL4787081) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 1 subunit caspase-like activity using fluorogenic peptide Ac-nLPnLD-AMC as substrate in presence of PA2... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21484 ((2S)-2-{[(1S)-1-carboxy-2-{1-[(4-methylphenyl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals | Assay Description Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... | J Am Chem Soc 124: 11852-3 (2002) Article DOI: 10.1021/ja0277226 BindingDB Entry DOI: 10.7270/Q2KP80FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21483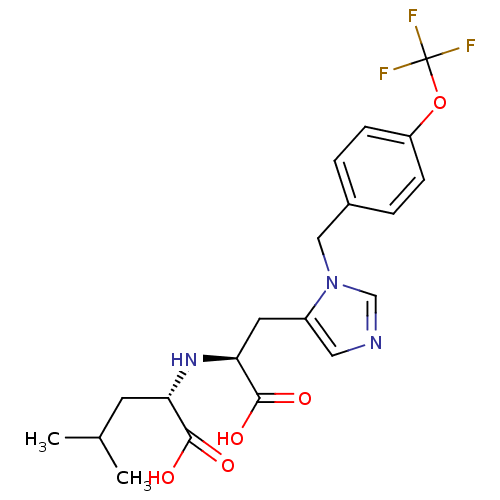 ((2S)-2-{[(1S)-1-carboxy-2-(1-{[4-(trifluoromethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals | Assay Description Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... | J Am Chem Soc 124: 11852-3 (2002) Article DOI: 10.1021/ja0277226 BindingDB Entry DOI: 10.7270/Q2KP80FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50550643 (CHEMBL4749207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S constitutive proteasome beta 1 subunit caspase-like activity using fluorogenic peptide Ac-nLPnLD-AMC as substrate in presence... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50550641 (CHEMBL4781914) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 5 subunit chymotrypsin-like activity using fluorogenic peptide Ac-WLA-AMC as substrate in presence of P... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21481 ((2S)-2-{[(1S)-1-carboxy-2-{1-[(4-nitrophenyl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals | Assay Description Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... | J Am Chem Soc 124: 11852-3 (2002) Article DOI: 10.1021/ja0277226 BindingDB Entry DOI: 10.7270/Q2KP80FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S constitutive proteasome beta 1 subunit caspase-like activity using fluorogenic peptide Ac-nLPnLD-AMC as substrate in presence... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50550641 (CHEMBL4781914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S constitutive proteasome beta 5 subunit chymotrypsin-like activity using fluorogenic peptide Ac-WLA-AMC as substrate in presen... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-10 (Homo sapiens (Human)) | BDBM50550642 (CHEMBL4787081) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 2 subunit trypsin-like activity using fluorogenic peptide Ac-WLR-AMC as substrate in presence of PA28al... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50550640 (CHEMBL4749043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S constitutive proteasome beta 5 subunit chymotrypsin-like activity using fluorogenic peptide Ac-WLA-AMC as substrate in presen... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21480 ((2S)-2-{[(1S)-1-carboxy-2-[1-(cyclohexylmethyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals | Assay Description Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... | J Am Chem Soc 124: 11852-3 (2002) Article DOI: 10.1021/ja0277226 BindingDB Entry DOI: 10.7270/Q2KP80FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21485 ((2S)-2-{[(1S)-1-carboxy-2-{1-[(2-methylphenyl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals | Assay Description Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... | J Am Chem Soc 124: 11852-3 (2002) Article DOI: 10.1021/ja0277226 BindingDB Entry DOI: 10.7270/Q2KP80FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21478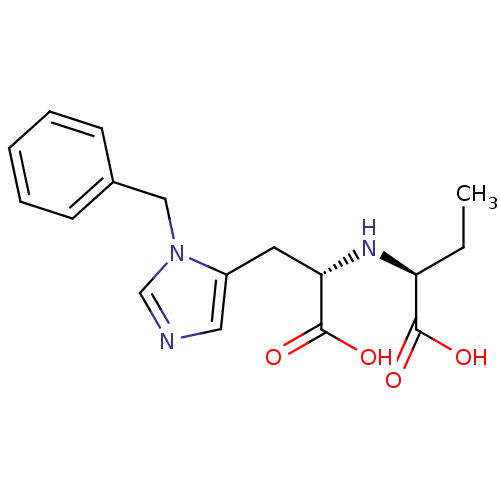 ((2S)-2-{[(1S)-2-(1-benzyl-1H-imidazol-5-yl)-1-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals | Assay Description Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... | J Am Chem Soc 124: 11852-3 (2002) Article DOI: 10.1021/ja0277226 BindingDB Entry DOI: 10.7270/Q2KP80FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-10 (Homo sapiens (Human)) | BDBM50550643 (CHEMBL4749207) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 2 subunit trypsin-like activity using fluorogenic peptide Ac-WLR-AMC as substrate in presence of PA28al... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50550641 (CHEMBL4781914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S constitutive proteasome beta 1 subunit caspase-like activity using fluorogenic peptide Ac-nLPnLD-AMC as substrate in presence... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21479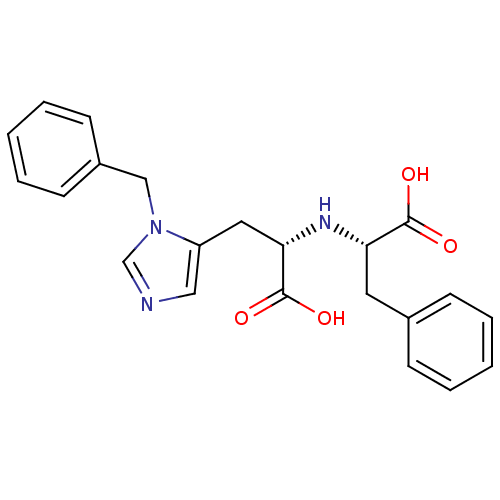 ((2S)-2-{[(1S)-2-(1-benzyl-1H-imidazol-5-yl)-1-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals | Assay Description Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... | J Am Chem Soc 124: 11852-3 (2002) Article DOI: 10.1021/ja0277226 BindingDB Entry DOI: 10.7270/Q2KP80FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50550642 (CHEMBL4787081) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S constitutive proteasome beta 2 subunit trypsin-like activity using fluorogenic peptide Ac-WLR-AMC as substrate in presence of... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-10 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S immunoproteasome beta 2 subunit trypsin-like activity using fluorogenic peptide Ac-WLR-AMC as substrate in presence of PA28al... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50550643 (CHEMBL4749207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S constitutive proteasome beta 2 subunit trypsin-like activity using fluorogenic peptide Ac-WLR-AMC as substrate in presence of... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S constitutive proteasome beta 2 subunit trypsin-like activity using fluorogenic peptide Ac-WLR-AMC as substrate in presence of... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50550642 (CHEMBL4787081) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S constitutive proteasome beta 1 subunit caspase-like activity using fluorogenic peptide Ac-nLPnLD-AMC as substrate in presence... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM21478 ((2S)-2-{[(1S)-2-(1-benzyl-1H-imidazol-5-yl)-1-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Millennium Pharmaceuticals | Assay Description Assay for the inhibition of carboxypeptidase A (CPDA) was conducted in 96-well microplates format. Activity was monitored by measuring decrease in ab... | J Am Chem Soc 124: 11852-3 (2002) Article DOI: 10.1021/ja0277226 BindingDB Entry DOI: 10.7270/Q2KP80FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50550640 (CHEMBL4749043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S constitutive proteasome beta 1 subunit caspase-like activity using fluorogenic peptide Ac-nLPnLD-AMC as substrate in presence... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01161 BindingDB Entry DOI: 10.7270/Q2K077W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 79 total ) | Next | Last >> |