 Found 266 hits with Last Name = 'hamada' and Initial = 't'
Found 266 hits with Last Name = 'hamada' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50098412

(CHEMBL3593400)Show SMILES OC(=O)\C=C\C(O)=O.CC(=O)NC[C@@H]1CNCCO[C@H]1c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H18Cl2N2O2/c1-9(19)18-8-11-7-17-4-5-20-14(11)10-2-3-12(15)13(16)6-10/h2-3,6,11,14,17H,4-5,7-8H2,1H3,(H,18,19)/t11-,14-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human NET expressed in CHOK1 cells incubated for 45 mins by [3H]-norepinephrine uptake assay |
Bioorg Med Chem 23: 5000-14 (2015)
Article DOI: 10.1016/j.bmc.2015.05.017
BindingDB Entry DOI: 10.7270/Q22F7Q6R |
More data for this
Ligand-Target Pair | |
Acyl carrier protein,/NADH dehydrogenase [ubiquinone] flavoprotein 1, mitochondrial
(Bos taurus) | BDBM50116000
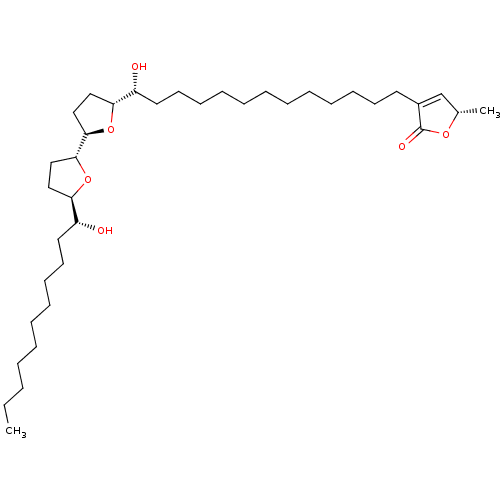
((S)-3-{(12R,13R)-13-Hydroxy-13-[(4R,2'R,5'R)-5'-((...)Show SMILES CCCCCCCCCC[C@@H](O)[C@H]1CC[C@@H](O1)[C@H]1CC[C@@H](O1)[C@H](O)CCCCCCCCCCCCC1=C[C@H](C)OC1=O |t:38| Show InChI InChI=1S/C37H66O6/c1-3-4-5-6-7-13-16-19-22-31(38)33-24-26-35(42-33)36-27-25-34(43-36)32(39)23-20-17-14-11-9-8-10-12-15-18-21-30-28-29(2)41-37(30)40/h28-29,31-36,38-39H,3-27H2,1-2H3/t29-,31+,32+,33+,34+,35+,36+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibitory concentration needed to halve the control NADH oxidase activity in bovine heart submitochondrial particles (SMP) |
Bioorg Med Chem Lett 14: 779-82 (2004)
BindingDB Entry DOI: 10.7270/Q2ZC8290 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50098413
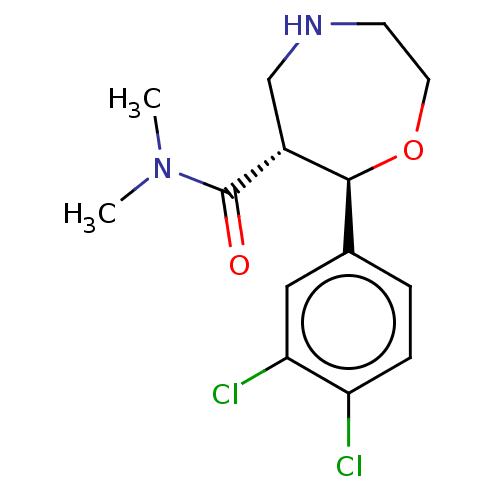
(CHEMBL3593399)Show SMILES Cl.CN(C)C(=O)[C@@H]1CNCCO[C@H]1c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H18Cl2N2O2/c1-18(2)14(19)10-8-17-5-6-20-13(10)9-3-4-11(15)12(16)7-9/h3-4,7,10,13,17H,5-6,8H2,1-2H3/t10-,13+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human NET expressed in CHOK1 cells incubated for 45 mins by [3H]-norepinephrine uptake assay |
Bioorg Med Chem 23: 5000-14 (2015)
Article DOI: 10.1016/j.bmc.2015.05.017
BindingDB Entry DOI: 10.7270/Q22F7Q6R |
More data for this
Ligand-Target Pair | |
Acyl carrier protein,/NADH dehydrogenase [ubiquinone] flavoprotein 1, mitochondrial
(Bos taurus) | BDBM50116000

((S)-3-{(12R,13R)-13-Hydroxy-13-[(4R,2'R,5'R)-5'-((...)Show SMILES CCCCCCCCCC[C@@H](O)[C@H]1CC[C@@H](O1)[C@H]1CC[C@@H](O1)[C@H](O)CCCCCCCCCCCCC1=C[C@H](C)OC1=O |t:38| Show InChI InChI=1S/C37H66O6/c1-3-4-5-6-7-13-16-19-22-31(38)33-24-26-35(42-33)36-27-25-34(43-36)32(39)23-20-17-14-11-9-8-10-12-15-18-21-30-28-29(2)41-37(30)40/h28-29,31-36,38-39H,3-27H2,1-2H3/t29-,31+,32+,33+,34+,35+,36+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of complex I activity was determined by NADH oxidase assay using bovine heart submitochondrial particles. |
Bioorg Med Chem Lett 13: 2385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2N58MWC |
More data for this
Ligand-Target Pair | |
Acyl carrier protein,/NADH dehydrogenase [ubiquinone] flavoprotein 1, mitochondrial
(Bos taurus) | BDBM50366820

(BULLATACIN)Show SMILES CCCCCCCCCC[C@@H](O)[C@@H]1CC[C@H](O1)[C@@H]1CC[C@H](O1)[C@H](O)CCCCCCCCCC(O)CC1=CC(C)OC1=O |r,t:38| Show InChI InChI=1S/C36H64O7/c1-3-4-5-6-7-10-13-16-19-30(38)32-21-23-34(42-32)35-24-22-33(43-35)31(39)20-17-14-11-8-9-12-15-18-29(37)26-28-25-27(2)41-36(28)40/h25,27,29-35,37-39H,3-24,26H2,1-2H3/t27?,29?,30-,31-,32+,33+,34+,35+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of complex I activity was determined by NADH oxidase assay using bovine heart submitochondrial particles. |
Bioorg Med Chem Lett 13: 2385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2N58MWC |
More data for this
Ligand-Target Pair | |
Acyl carrier protein,/NADH dehydrogenase [ubiquinone] flavoprotein 1, mitochondrial
(Bos taurus) | BDBM50130144
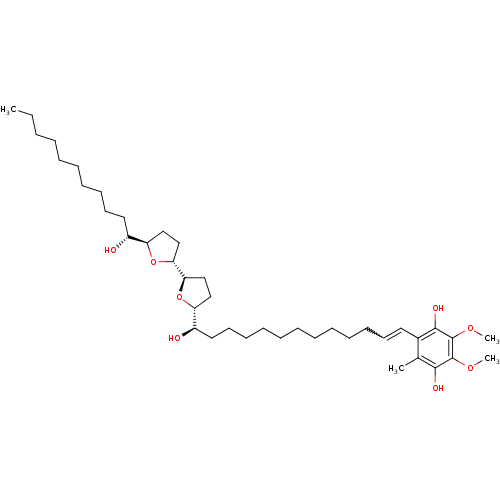
(2-{(12R,13R)-13-Hydroxy-13-[(4R,2'R,5'R)-5'-((R)-1...)Show SMILES CCCCCCCCCC[C@@H](O)[C@H]1CC[C@@H](O1)[C@H]1CC[C@@H](O1)[C@H](O)CCCCCCCCCCC=Cc1c(C)c(O)c(OC)c(OC)c1O |w:34.35| Show InChI InChI=1S/C41H70O8/c1-5-6-7-8-9-15-18-21-24-32(42)34-26-28-36(48-34)37-29-27-35(49-37)33(43)25-22-19-16-13-11-10-12-14-17-20-23-31-30(2)38(44)40(46-3)41(47-4)39(31)45/h20,23,32-37,42-45H,5-19,21-22,24-29H2,1-4H3/t32-,33-,34-,35-,36-,37-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of complex I activity was determined by NADH oxidase assay using bovine heart submitochondrial particles. |
Bioorg Med Chem Lett 13: 2385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2N58MWC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50460012

(CHEMBL4227523)Show SMILES Cc1ccc(cn1)C(=O)Nc1cc(ccn1)-c1c(nc2cccnn12)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C25H19FN6O/c1-15-12-17(7-8-20(15)26)23-24(32-22(31-23)4-3-10-29-32)18-9-11-27-21(13-18)30-25(33)19-6-5-16(2)28-14-19/h3-14H,1-2H3,(H,27,30,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of MAPK p38 in human THP1 cells assessed as reduction in LPS-induced TNFalpha production preincubated for 60 mins followed by LPS addition... |
Bioorg Med Chem 26: 647-660 (2018)
Article DOI: 10.1016/j.bmc.2017.12.031
BindingDB Entry DOI: 10.7270/Q2XP77K2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50460012

(CHEMBL4227523)Show SMILES Cc1ccc(cn1)C(=O)Nc1cc(ccn1)-c1c(nc2cccnn12)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C25H19FN6O/c1-15-12-17(7-8-20(15)26)23-24(32-22(31-23)4-3-10-29-32)18-9-11-27-21(13-18)30-25(33)19-6-5-16(2)28-14-19/h3-14H,1-2H3,(H,27,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged p38alpha expressed in baculovirus expression system using GFP-ATF2 (19 to 96 residues) as substrate prein... |
Bioorg Med Chem 26: 647-660 (2018)
Article DOI: 10.1016/j.bmc.2017.12.031
BindingDB Entry DOI: 10.7270/Q2XP77K2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50098373

(CHEMBL3593395)Show SMILES Cl.OC[C@@H]1CNCCO[C@H]1c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C12H15Cl2NO2/c13-10-2-1-8(5-11(10)14)12-9(7-16)6-15-3-4-17-12/h1-2,5,9,12,15-16H,3-4,6-7H2/t9-,12-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human NET expressed in CHOK1 cells incubated for 45 mins by [3H]-norepinephrine uptake assay |
Bioorg Med Chem 23: 5000-14 (2015)
Article DOI: 10.1016/j.bmc.2015.05.017
BindingDB Entry DOI: 10.7270/Q22F7Q6R |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50098387
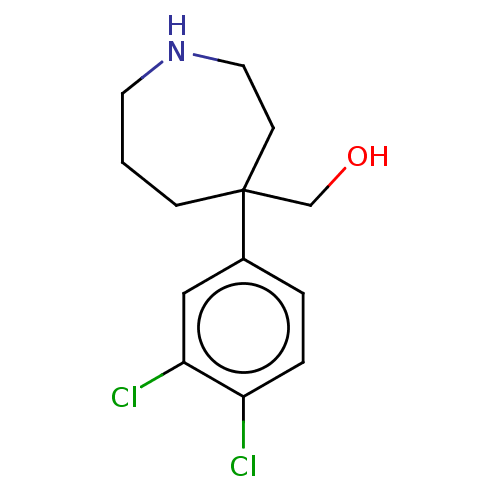
(CHEMBL3593274)Show InChI InChI=1S/C13H17Cl2NO/c14-11-3-2-10(8-12(11)15)13(9-17)4-1-6-16-7-5-13/h2-3,8,16-17H,1,4-7,9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human NET expressed in CHOK1 cells incubated for 45 mins by [3H]-norepinephrine uptake assay |
Bioorg Med Chem 23: 5000-14 (2015)
Article DOI: 10.1016/j.bmc.2015.05.017
BindingDB Entry DOI: 10.7270/Q22F7Q6R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50460026
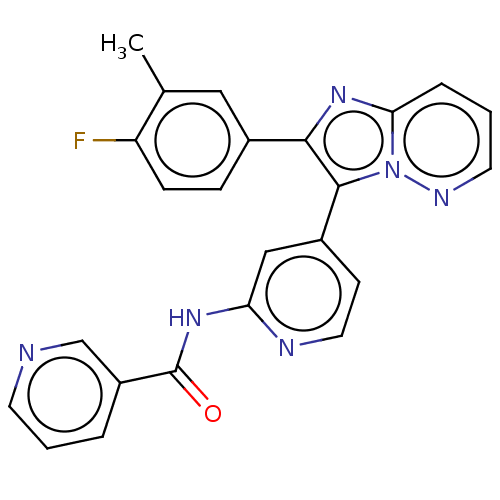
(CHEMBL4229137)Show SMILES Cc1cc(ccc1F)-c1nc2cccnn2c1-c1ccnc(NC(=O)c2cccnc2)c1 Show InChI InChI=1S/C24H17FN6O/c1-15-12-16(6-7-19(15)25)22-23(31-21(30-22)5-3-10-28-31)17-8-11-27-20(13-17)29-24(32)18-4-2-9-26-14-18/h2-14H,1H3,(H,27,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged p38alpha expressed in baculovirus expression system using GFP-ATF2 (19 to 96 residues) as substrate prein... |
Bioorg Med Chem 26: 647-660 (2018)
Article DOI: 10.1016/j.bmc.2017.12.031
BindingDB Entry DOI: 10.7270/Q2XP77K2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50098410

(CHEMBL3593402)Show SMILES Cl.CC(=O)NC[C@@H]1CNCCO[C@H]1c1ccc(Cl)c(F)c1 |r| Show InChI InChI=1S/C14H18ClFN2O2/c1-9(19)18-8-11-7-17-4-5-20-14(11)10-2-3-12(15)13(16)6-10/h2-3,6,11,14,17H,4-5,7-8H2,1H3,(H,18,19)/t11-,14-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human NET expressed in CHOK1 cells incubated for 45 mins by [3H]-norepinephrine uptake assay |
Bioorg Med Chem 23: 5000-14 (2015)
Article DOI: 10.1016/j.bmc.2015.05.017
BindingDB Entry DOI: 10.7270/Q22F7Q6R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50460017
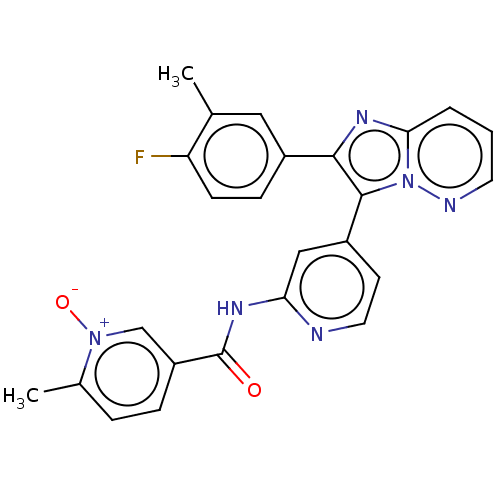
(CHEMBL4225133)Show SMILES Cc1cc(ccc1F)-c1nc2cccnn2c1-c1ccnc(NC(=O)c2ccc(C)[n+]([O-])c2)c1 Show InChI InChI=1S/C25H19FN6O2/c1-15-12-17(7-8-20(15)26)23-24(32-22(30-23)4-3-10-28-32)18-9-11-27-21(13-18)29-25(33)19-6-5-16(2)31(34)14-19/h3-14H,1-2H3,(H,27,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged p38alpha expressed in baculovirus expression system using GFP-ATF2 (19 to 96 residues) as substrate prein... |
Bioorg Med Chem 26: 647-660 (2018)
Article DOI: 10.1016/j.bmc.2017.12.031
BindingDB Entry DOI: 10.7270/Q2XP77K2 |
More data for this
Ligand-Target Pair | |
Acyl carrier protein,/NADH dehydrogenase [ubiquinone] flavoprotein 1, mitochondrial
(Bos taurus) | BDBM50139593
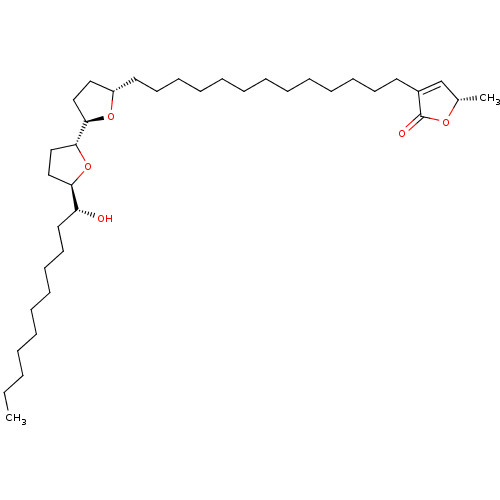
((S)-3-{13-[(2R,5S,1'R,3'R)-5'-((R)-1-Hydroxy-undec...)Show SMILES CCCCCCCCCC[C@@H](O)[C@H]1CC[C@@H](O1)[C@H]1CC[C@H](CCCCCCCCCCCCCC2=C[C@H](C)OC2=O)O1 |t:35| Show InChI InChI=1S/C37H66O5/c1-3-4-5-6-7-15-18-21-24-33(38)34-27-28-36(42-34)35-26-25-32(41-35)23-20-17-14-12-10-8-9-11-13-16-19-22-31-29-30(2)40-37(31)39/h29-30,32-36,38H,3-28H2,1-2H3/t30-,32-,33+,34+,35+,36+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibitory concentration needed to halve the control NADH oxidase activity in bovine heart submitochondrial particles (SMP) |
Bioorg Med Chem Lett 14: 779-82 (2004)
BindingDB Entry DOI: 10.7270/Q2ZC8290 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50057477
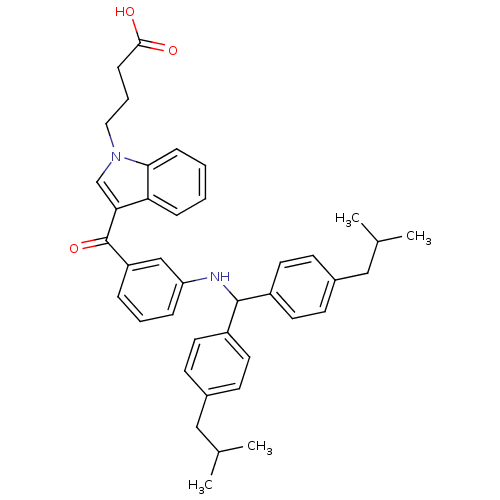
(4-(3-{3-[(4-Isobutyl-benzyl)-(4-isobutyl-phenyl)-a...)Show SMILES CC(C)Cc1ccc(cc1)C(Nc1cccc(c1)C(=O)c1cn(CCCC(O)=O)c2ccccc12)c1ccc(CC(C)C)cc1 Show InChI InChI=1S/C40H44N2O3/c1-27(2)23-29-14-18-31(19-15-29)39(32-20-16-30(17-21-32)24-28(3)4)41-34-10-7-9-33(25-34)40(45)36-26-42(22-8-13-38(43)44)37-12-6-5-11-35(36)37/h5-7,9-12,14-21,25-28,39,41H,8,13,22-24H2,1-4H3,(H,43,44) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Steroid 5-alpha-reductase type 1 in COS-1 cells was determined |
Bioorg Med Chem Lett 8: 561-6 (1999)
BindingDB Entry DOI: 10.7270/Q2571B5B |
More data for this
Ligand-Target Pair | |
Acyl carrier protein,/NADH dehydrogenase [ubiquinone] flavoprotein 1, mitochondrial
(Bos taurus) | BDBM50139595

((S)-3-[(R)-13-Hydroxy-13-((2R,5R,1'S,3'R)-5'-undec...)Show SMILES CCCCCCCCCCC[C@H]1CC[C@@H](O1)[C@H]1CC[C@@H](O1)[C@H](O)CCCCCCCCCCCCC1=C[C@H](C)OC1=O |t:37| Show InChI InChI=1S/C37H66O5/c1-3-4-5-6-7-10-14-17-20-23-32-25-26-35(41-32)36-28-27-34(42-36)33(38)24-21-18-15-12-9-8-11-13-16-19-22-31-29-30(2)40-37(31)39/h29-30,32-36,38H,3-28H2,1-2H3/t30-,32-,33+,34+,35+,36+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibitory concentration needed to halve the control NADH oxidase activity in bovine heart submitochondrial particles (SMP) |
Bioorg Med Chem Lett 14: 779-82 (2004)
BindingDB Entry DOI: 10.7270/Q2ZC8290 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50188338

(CHEMBL411711 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-(...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C36H48N14O6/c1-20(2)28(42-32(52)25(37)19-38-36(56)31-45-49-50-46-31)34(54)41-27(17-22-12-7-4-8-13-22)33(53)40-26(16-21-10-5-3-6-11-21)29(51)35(55)39-24-15-9-14-23(18-24)30-43-47-48-44-30/h3,5-6,9-11,14-15,18,20,22,25-29,51H,4,7-8,12-13,16-17,19,37H2,1-2H3,(H,38,56)(H,39,55)(H,40,53)(H,41,54)(H,42,52)(H,43,44,47,48)(H,45,46,49,50)/t25-,26-,27-,28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50098408
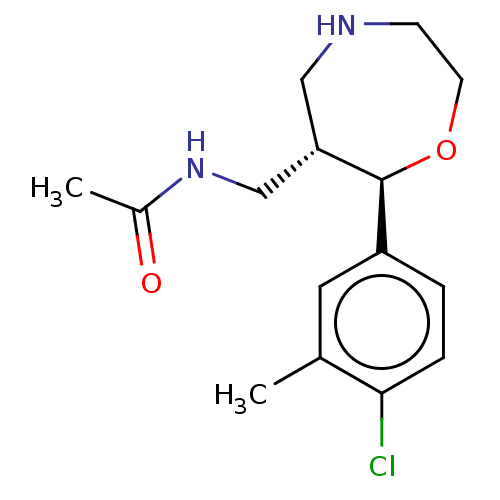
(CHEMBL3593404)Show SMILES Cl.CC(=O)NC[C@@H]1CNCCO[C@H]1c1ccc(Cl)c(C)c1 |r| Show InChI InChI=1S/C15H21ClN2O2/c1-10-7-12(3-4-14(10)16)15-13(9-18-11(2)19)8-17-5-6-20-15/h3-4,7,13,15,17H,5-6,8-9H2,1-2H3,(H,18,19)/t13-,15-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human NET expressed in CHOK1 cells incubated for 45 mins by [3H]-norepinephrine uptake assay |
Bioorg Med Chem 23: 5000-14 (2015)
Article DOI: 10.1016/j.bmc.2015.05.017
BindingDB Entry DOI: 10.7270/Q22F7Q6R |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50098407

(CHEMBL3593405)Show SMILES Cl.CC(=O)NC[C@@H]1CNCCO[C@H]1c1ccc(C)c(Cl)c1 |r| Show InChI InChI=1S/C15H21ClN2O2/c1-10-3-4-12(7-14(10)16)15-13(9-18-11(2)19)8-17-5-6-20-15/h3-4,7,13,15,17H,5-6,8-9H2,1-2H3,(H,18,19)/t13-,15-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human NET expressed in CHOK1 cells incubated for 45 mins by [3H]-norepinephrine uptake assay |
Bioorg Med Chem 23: 5000-14 (2015)
Article DOI: 10.1016/j.bmc.2015.05.017
BindingDB Entry DOI: 10.7270/Q22F7Q6R |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50141551

(5-[(S)-3-((S)-2-{(S)-2-[2-Amino-3-((S)-carboxyamin...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(O)=O)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cc(cc(c1)C(O)=O)C(O)=O Show InChI InChI=1S/C33H44N6O11/c1-16(2)10-24(38-29(43)25(17(3)4)39-27(41)22(34)15-35-33(49)50)28(42)37-23(11-18-8-6-5-7-9-18)26(40)30(44)36-21-13-19(31(45)46)12-20(14-21)32(47)48/h5-9,12-14,16-17,22-26,35,40H,10-11,15,34H2,1-4H3,(H,36,44)(H,37,42)(H,38,43)(H,39,41)(H,45,46)(H,47,48)(H,49,50)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibitory concentration of compound against Beta-secretase 1 was evaluated |
Bioorg Med Chem Lett 14: 1527-31 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.088
BindingDB Entry DOI: 10.7270/Q2V987H4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50460026

(CHEMBL4229137)Show SMILES Cc1cc(ccc1F)-c1nc2cccnn2c1-c1ccnc(NC(=O)c2cccnc2)c1 Show InChI InChI=1S/C24H17FN6O/c1-15-12-16(6-7-19(15)25)22-23(31-21(30-22)5-3-10-28-31)17-8-11-27-20(13-17)29-24(32)18-4-2-9-26-14-18/h2-14H,1H3,(H,27,29,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of MAPK p38 in human THP1 cells assessed as reduction in LPS-induced TNFalpha production preincubated for 60 mins followed by LPS addition... |
Bioorg Med Chem 26: 647-660 (2018)
Article DOI: 10.1016/j.bmc.2017.12.031
BindingDB Entry DOI: 10.7270/Q2XP77K2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50157441
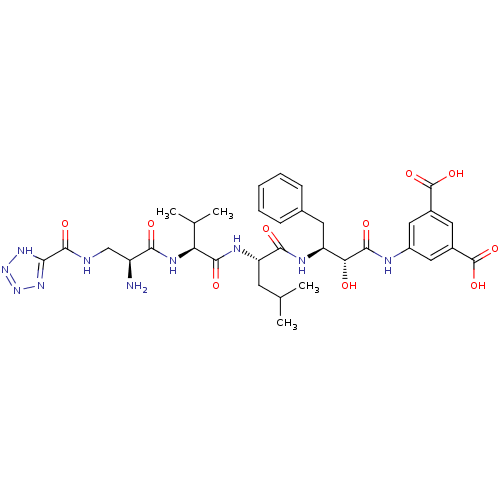
(5-((2R,3S)-3-((S)-2-((S)-2-((S)-2-amino-3-(2H-tetr...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cc(cc(c1)C(O)=O)C(O)=O Show InChI InChI=1S/C34H44N10O10/c1-16(2)10-24(39-30(48)25(17(3)4)40-28(46)22(35)15-36-32(50)27-41-43-44-42-27)29(47)38-23(11-18-8-6-5-7-9-18)26(45)31(49)37-21-13-19(33(51)52)12-20(14-21)34(53)54/h5-9,12-14,16-17,22-26,45H,10-11,15,35H2,1-4H3,(H,36,50)(H,37,49)(H,38,47)(H,39,48)(H,40,46)(H,51,52)(H,53,54)(H,41,42,43,44)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem 19: 5238-46 (2011)
Article DOI: 10.1016/j.bmc.2011.07.002
BindingDB Entry DOI: 10.7270/Q20Z73NP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50157441

(5-((2R,3S)-3-((S)-2-((S)-2-((S)-2-amino-3-(2H-tetr...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cc(cc(c1)C(O)=O)C(O)=O Show InChI InChI=1S/C34H44N10O10/c1-16(2)10-24(39-30(48)25(17(3)4)40-28(46)22(35)15-36-32(50)27-41-43-44-42-27)29(47)38-23(11-18-8-6-5-7-9-18)26(45)31(49)37-21-13-19(33(51)52)12-20(14-21)34(53)54/h5-9,12-14,16-17,22-26,45H,10-11,15,35H2,1-4H3,(H,36,50)(H,37,49)(H,38,47)(H,39,48)(H,40,46)(H,51,52)(H,53,54)(H,41,42,43,44)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-secretase 1 (BACE1) |
Bioorg Med Chem Lett 15: 211-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.090
BindingDB Entry DOI: 10.7270/Q2FN15Q2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50460024
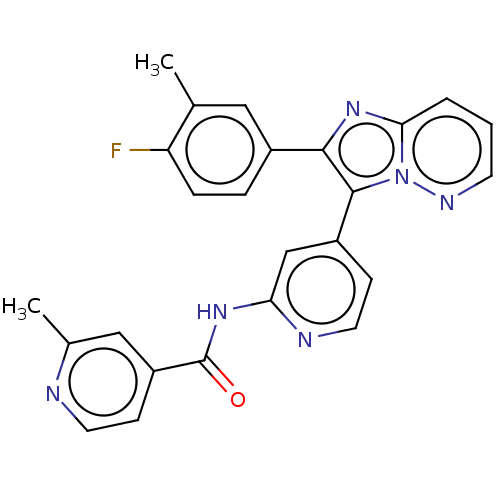
(CHEMBL4225173)Show SMILES Cc1cc(ccn1)C(=O)Nc1cc(ccn1)-c1c(nc2cccnn12)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C25H19FN6O/c1-15-12-17(5-6-20(15)26)23-24(32-22(31-23)4-3-9-29-32)18-7-11-28-21(14-18)30-25(33)19-8-10-27-16(2)13-19/h3-14H,1-2H3,(H,28,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged p38alpha expressed in baculovirus expression system using GFP-ATF2 (19 to 96 residues) as substrate prein... |
Bioorg Med Chem 26: 647-660 (2018)
Article DOI: 10.1016/j.bmc.2017.12.031
BindingDB Entry DOI: 10.7270/Q2XP77K2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50157442
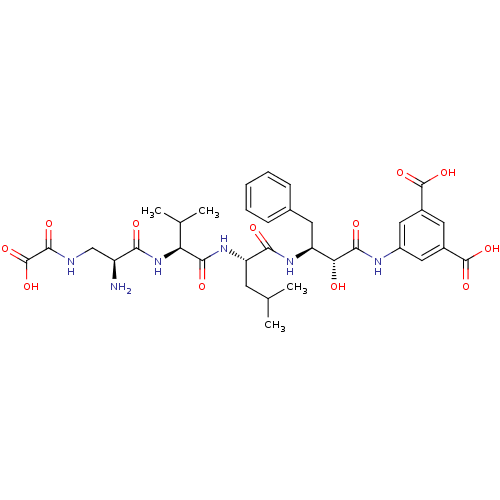
(5-{3-[2-((S)-2-{(S)-2-Amino-3-[((S)-oxalyl)-amino]...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)C(O)=O)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cc(cc(c1)C(O)=O)C(O)=O Show InChI InChI=1S/C34H44N6O12/c1-16(2)10-24(39-29(44)25(17(3)4)40-27(42)22(35)15-36-31(46)34(51)52)28(43)38-23(11-18-8-6-5-7-9-18)26(41)30(45)37-21-13-19(32(47)48)12-20(14-21)33(49)50/h5-9,12-14,16-17,22-26,41H,10-11,15,35H2,1-4H3,(H,36,46)(H,37,45)(H,38,43)(H,39,44)(H,40,42)(H,47,48)(H,49,50)(H,51,52)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-secretase 1 (BACE1) |
Bioorg Med Chem Lett 15: 211-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.090
BindingDB Entry DOI: 10.7270/Q2FN15Q2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50182871

(CHEMBL381826 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-(...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C33H44N14O6/c1-17(2)13-24(38-31(51)25(18(3)4)39-29(49)22(34)16-35-33(53)28-42-46-47-43-28)30(50)37-23(14-19-9-6-5-7-10-19)26(48)32(52)36-21-12-8-11-20(15-21)27-40-44-45-41-27/h5-12,15,17-18,22-26,48H,13-14,16,34H2,1-4H3,(H,35,53)(H,36,52)(H,37,50)(H,38,51)(H,39,49)(H,40,41,44,45)(H,42,43,46,47)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50460015
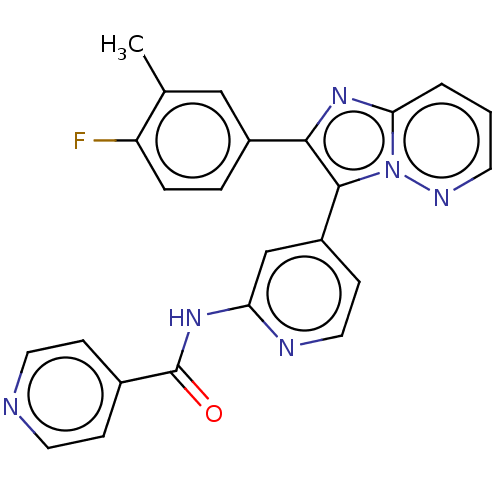
(CHEMBL4227050)Show SMILES Cc1cc(ccc1F)-c1nc2cccnn2c1-c1ccnc(NC(=O)c2ccncc2)c1 Show InChI InChI=1S/C24H17FN6O/c1-15-13-17(4-5-19(15)25)22-23(31-21(30-22)3-2-9-28-31)18-8-12-27-20(14-18)29-24(32)16-6-10-26-11-7-16/h2-14H,1H3,(H,27,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged p38alpha expressed in baculovirus expression system using GFP-ATF2 (19 to 96 residues) as substrate prein... |
Bioorg Med Chem 26: 647-660 (2018)
Article DOI: 10.1016/j.bmc.2017.12.031
BindingDB Entry DOI: 10.7270/Q2XP77K2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50460010
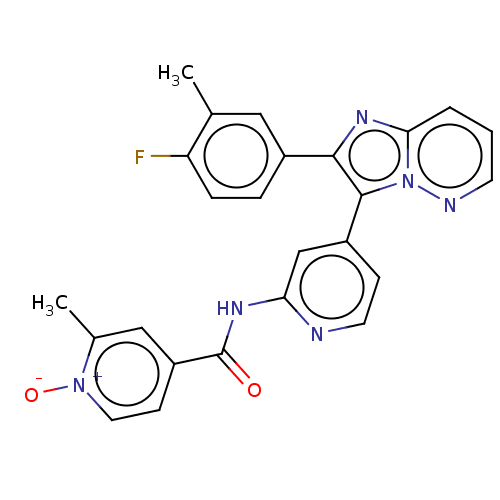
(CHEMBL4224940)Show SMILES Cc1cc(ccc1F)-c1nc2cccnn2c1-c1ccnc(NC(=O)c2cc[n+]([O-])c(C)c2)c1 Show InChI InChI=1S/C25H19FN6O2/c1-15-12-17(5-6-20(15)26)23-24(32-22(30-23)4-3-9-28-32)18-7-10-27-21(14-18)29-25(33)19-8-11-31(34)16(2)13-19/h3-14H,1-2H3,(H,27,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged p38alpha expressed in baculovirus expression system using GFP-ATF2 (19 to 96 residues) as substrate prein... |
Bioorg Med Chem 26: 647-660 (2018)
Article DOI: 10.1016/j.bmc.2017.12.031
BindingDB Entry DOI: 10.7270/Q2XP77K2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50234178
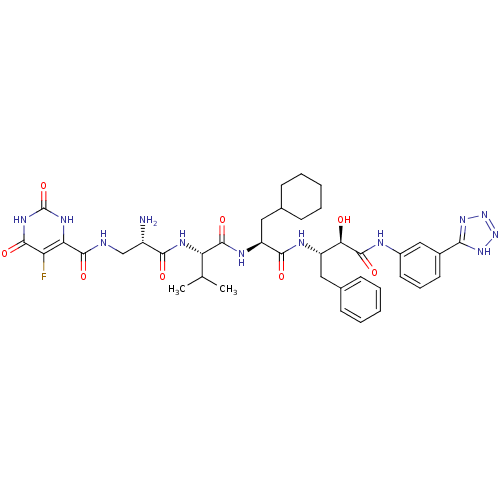
(CHEMBL255838 | N-((S)-3-((S)-1-((S)-1-((2S,3R)-4-(...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](N)CNC(=O)c1[nH]c(=O)[nH]c(=O)c1F)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C39H49FN12O8/c1-20(2)29(46-33(54)25(41)19-42-36(57)30-28(40)35(56)48-39(60)47-30)37(58)45-27(17-22-12-7-4-8-13-22)34(55)44-26(16-21-10-5-3-6-11-21)31(53)38(59)43-24-15-9-14-23(18-24)32-49-51-52-50-32/h3,5-6,9-11,14-15,18,20,22,25-27,29,31,53H,4,7-8,12-13,16-17,19,41H2,1-2H3,(H,42,57)(H,43,59)(H,44,55)(H,45,58)(H,46,54)(H2,47,48,56,60)(H,49,50,51,52)/t25-,26-,27-,29-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50098376
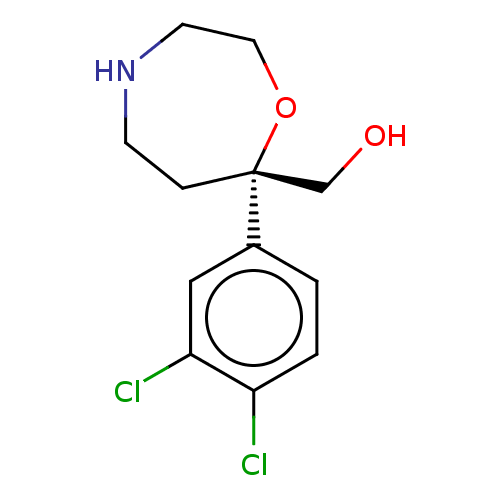
(CHEMBL3593392)Show InChI InChI=1S/C12H15Cl2NO2/c13-10-2-1-9(7-11(10)14)12(8-16)3-4-15-5-6-17-12/h1-2,7,15-16H,3-6,8H2/t12-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human NET expressed in CHOK1 cells incubated for 45 mins by [3H]-norepinephrine uptake assay |
Bioorg Med Chem 23: 5000-14 (2015)
Article DOI: 10.1016/j.bmc.2015.05.017
BindingDB Entry DOI: 10.7270/Q22F7Q6R |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50098411

(CHEMBL3593401)Show SMILES Cl.CC(=O)NCC[C@@H]1CNCCO[C@H]1c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C15H20Cl2N2O2/c1-10(20)19-5-4-12-9-18-6-7-21-15(12)11-2-3-13(16)14(17)8-11/h2-3,8,12,15,18H,4-7,9H2,1H3,(H,19,20)/t12-,15+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human NET expressed in CHOK1 cells incubated for 45 mins by [3H]-norepinephrine uptake assay |
Bioorg Med Chem 23: 5000-14 (2015)
Article DOI: 10.1016/j.bmc.2015.05.017
BindingDB Entry DOI: 10.7270/Q22F7Q6R |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50098372
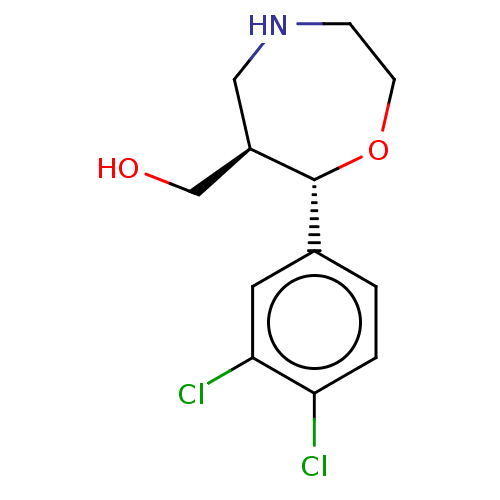
(CHEMBL3593396)Show SMILES Cl.OC[C@H]1CNCCO[C@@H]1c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C12H15Cl2NO2/c13-10-2-1-8(5-11(10)14)12-9(7-16)6-15-3-4-17-12/h1-2,5,9,12,15-16H,3-4,6-7H2/t9-,12-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human NET expressed in CHOK1 cells incubated for 45 mins by [3H]-norepinephrine uptake assay |
Bioorg Med Chem 23: 5000-14 (2015)
Article DOI: 10.1016/j.bmc.2015.05.017
BindingDB Entry DOI: 10.7270/Q22F7Q6R |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50098374

(CHEMBL3593394)Show InChI InChI=1S/C12H15Cl2NO2/c13-10-2-1-8(5-11(10)14)12-9(7-16)6-15-3-4-17-12/h1-2,5,9,12,15-16H,3-4,6-7H2/t9-,12+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human NET expressed in CHOK1 cells incubated for 45 mins by [3H]-norepinephrine uptake assay |
Bioorg Med Chem 23: 5000-14 (2015)
Article DOI: 10.1016/j.bmc.2015.05.017
BindingDB Entry DOI: 10.7270/Q22F7Q6R |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50188339

(CHEMBL380381 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-(...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1[nH]c(=O)[nH]c(=O)c1F)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C36H45FN12O8/c1-17(2)13-24(42-34(55)26(18(3)4)43-30(51)22(38)16-39-33(54)27-25(37)32(53)45-36(57)44-27)31(52)41-23(14-19-9-6-5-7-10-19)28(50)35(56)40-21-12-8-11-20(15-21)29-46-48-49-47-29/h5-12,15,17-18,22-24,26,28,50H,13-14,16,38H2,1-4H3,(H,39,54)(H,40,56)(H,41,52)(H,42,55)(H,43,51)(H2,44,45,53,57)(H,46,47,48,49)/t22-,23-,24-,26-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317669

(CHEMBL1094659 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1[nH]c(=O)[nH]c(=O)c1F)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nc(=O)o[nH]1 |r| Show InChI InChI=1S/C37H45FN10O10/c1-17(2)13-24(43-34(54)26(18(3)4)44-30(50)22(39)16-40-33(53)27-25(38)32(52)47-36(56)45-27)31(51)42-23(14-19-9-6-5-7-10-19)28(49)35(55)41-21-12-8-11-20(15-21)29-46-37(57)58-48-29/h5-12,15,17-18,22-24,26,28,49H,13-14,16,39H2,1-4H3,(H,40,53)(H,41,55)(H,42,51)(H,43,54)(H,44,50)(H,46,48,57)(H2,45,47,52,56)/t22-,23-,24-,26-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50157439

(3-((2R,3S)-3-((S)-2-((S)-2-((S)-2-amino-3-(2H-tetr...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)C(O)=O Show InChI InChI=1S/C33H44N10O8/c1-17(2)13-24(38-30(47)25(18(3)4)39-28(45)22(34)16-35-32(49)27-40-42-43-41-27)29(46)37-23(14-19-9-6-5-7-10-19)26(44)31(48)36-21-12-8-11-20(15-21)33(50)51/h5-12,15,17-18,22-26,44H,13-14,16,34H2,1-4H3,(H,35,49)(H,36,48)(H,37,46)(H,38,47)(H,39,45)(H,50,51)(H,40,41,42,43)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-secretase 1 (BACE1) |
Bioorg Med Chem Lett 15: 211-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.090
BindingDB Entry DOI: 10.7270/Q2FN15Q2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50157439

(3-((2R,3S)-3-((S)-2-((S)-2-((S)-2-amino-3-(2H-tetr...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)C(O)=O Show InChI InChI=1S/C33H44N10O8/c1-17(2)13-24(38-30(47)25(18(3)4)39-28(45)22(34)16-35-32(49)27-40-42-43-41-27)29(46)37-23(14-19-9-6-5-7-10-19)26(44)31(48)36-21-12-8-11-20(15-21)33(50)51/h5-12,15,17-18,22-26,44H,13-14,16,34H2,1-4H3,(H,35,49)(H,36,48)(H,37,46)(H,38,47)(H,39,45)(H,50,51)(H,40,41,42,43)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem 19: 5238-46 (2011)
Article DOI: 10.1016/j.bmc.2011.07.002
BindingDB Entry DOI: 10.7270/Q20Z73NP |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50098375
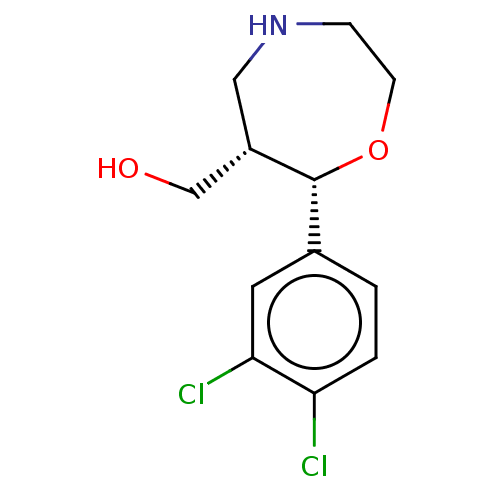
(CHEMBL3593393)Show SMILES Cl.OC[C@@H]1CNCCO[C@@H]1c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C12H15Cl2NO2/c13-10-2-1-8(5-11(10)14)12-9(7-16)6-15-3-4-17-12/h1-2,5,9,12,15-16H,3-4,6-7H2/t9-,12+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human NET expressed in CHOK1 cells incubated for 45 mins by [3H]-norepinephrine uptake assay |
Bioorg Med Chem 23: 5000-14 (2015)
Article DOI: 10.1016/j.bmc.2015.05.017
BindingDB Entry DOI: 10.7270/Q22F7Q6R |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50277598
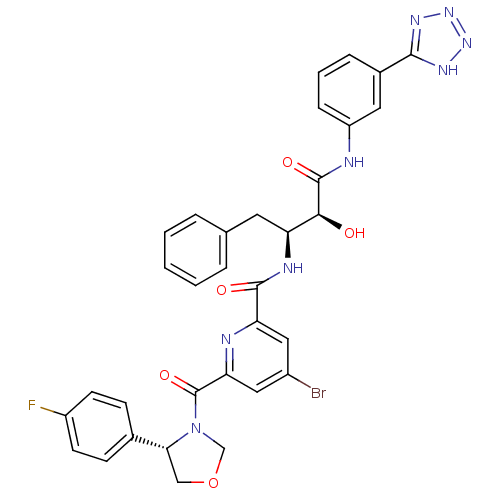
(CHEMBL453211 | N-((2S,3S)-4-(3-(2H-tetrazol-5-yl)p...)Show SMILES O[C@@H]([C@H](Cc1ccccc1)NC(=O)c1cc(Br)cc(n1)C(=O)N1COC[C@@H]1c1ccc(F)cc1)C(=O)Nc1cccc(c1)-c1nnn[nH]1 |r| Show InChI InChI=1S/C33H28BrFN8O5/c34-22-15-26(37-27(16-22)33(47)43-18-48-17-28(43)20-9-11-23(35)12-10-20)31(45)38-25(13-19-5-2-1-3-6-19)29(44)32(46)36-24-8-4-7-21(14-24)30-39-41-42-40-30/h1-12,14-16,25,28-29,44H,13,17-18H2,(H,36,46)(H,38,45)(H,39,40,41,42)/t25-,28+,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Arg235 region of BACE1 by FRET assay |
Bioorg Med Chem Lett 19: 2435-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.049
BindingDB Entry DOI: 10.7270/Q27D2V1G |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50460009
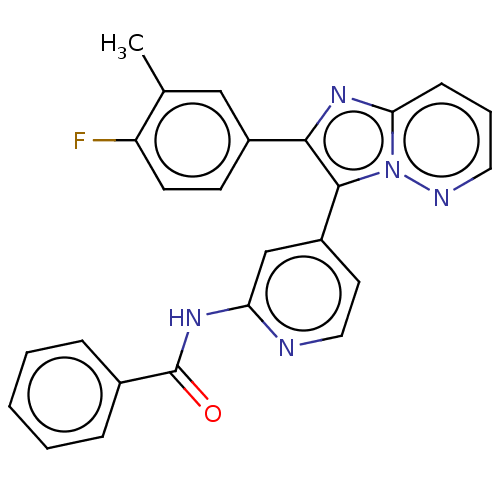
(CHEMBL4228109)Show SMILES Cc1cc(ccc1F)-c1nc2cccnn2c1-c1ccnc(NC(=O)c2ccccc2)c1 Show InChI InChI=1S/C25H18FN5O/c1-16-14-18(9-10-20(16)26)23-24(31-22(30-23)8-5-12-28-31)19-11-13-27-21(15-19)29-25(32)17-6-3-2-4-7-17/h2-15H,1H3,(H,27,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged p38alpha expressed in baculovirus expression system using GFP-ATF2 (19 to 96 residues) as substrate prein... |
Bioorg Med Chem 26: 647-660 (2018)
Article DOI: 10.1016/j.bmc.2017.12.031
BindingDB Entry DOI: 10.7270/Q2XP77K2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50277599
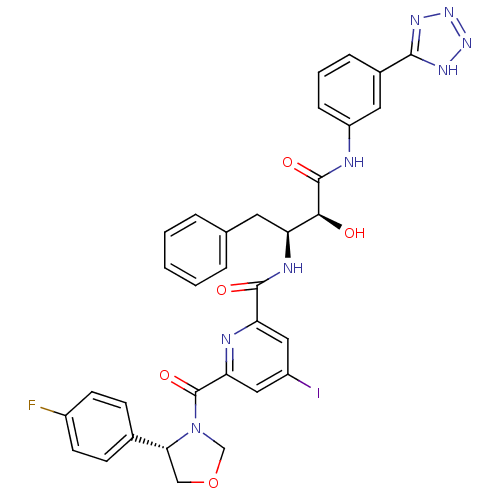
(CHEMBL507541 | N-((2S,3S)-4-(3-(2H-tetrazol-5-yl)p...)Show SMILES O[C@@H]([C@H](Cc1ccccc1)NC(=O)c1cc(I)cc(n1)C(=O)N1COC[C@@H]1c1ccc(F)cc1)C(=O)Nc1cccc(c1)-c1nnn[nH]1 |r| Show InChI InChI=1S/C33H28FIN8O5/c34-22-11-9-20(10-12-22)28-17-48-18-43(28)33(47)27-16-23(35)15-26(37-27)31(45)38-25(13-19-5-2-1-3-6-19)29(44)32(46)36-24-8-4-7-21(14-24)30-39-41-42-40-30/h1-12,14-16,25,28-29,44H,13,17-18H2,(H,36,46)(H,38,45)(H,39,40,41,42)/t25-,28+,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Arg235 region of BACE1 by FRET assay |
Bioorg Med Chem Lett 19: 2435-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.049
BindingDB Entry DOI: 10.7270/Q27D2V1G |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317671
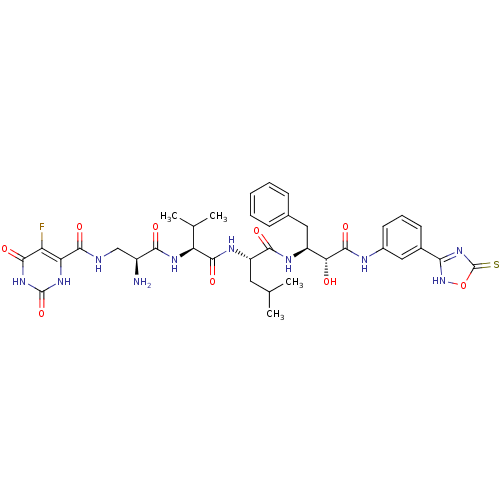
(CHEMBL1094663 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1[nH]c(=O)[nH]c(=O)c1F)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nc(=S)o[nH]1 |r| Show InChI InChI=1S/C37H45FN10O9S/c1-17(2)13-24(43-34(54)26(18(3)4)44-30(50)22(39)16-40-33(53)27-25(38)32(52)47-36(56)45-27)31(51)42-23(14-19-9-6-5-7-10-19)28(49)35(55)41-21-12-8-11-20(15-21)29-46-37(58)57-48-29/h5-12,15,17-18,22-24,26,28,49H,13-14,16,39H2,1-4H3,(H,40,53)(H,41,55)(H,42,51)(H,43,54)(H,44,50)(H,46,48,58)(H2,45,47,52,56)/t22-,23-,24-,26-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317670
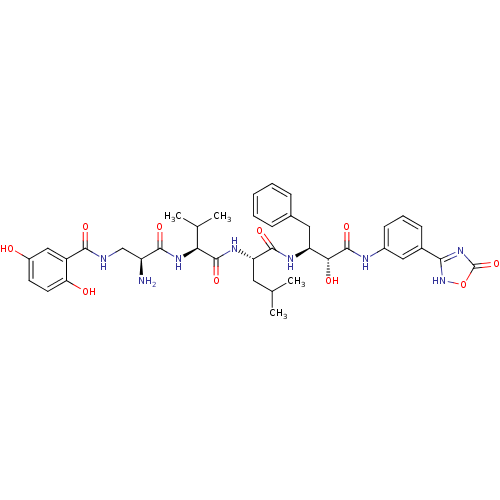
(CHEMBL1094660 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1cc(O)ccc1O)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nc(=O)o[nH]1 |r| Show InChI InChI=1S/C39H48N8O10/c1-20(2)15-29(44-37(54)31(21(3)4)45-35(52)27(40)19-41-34(51)26-18-25(48)13-14-30(26)49)36(53)43-28(16-22-9-6-5-7-10-22)32(50)38(55)42-24-12-8-11-23(17-24)33-46-39(56)57-47-33/h5-14,17-18,20-21,27-29,31-32,48-50H,15-16,19,40H2,1-4H3,(H,41,51)(H,42,55)(H,43,53)(H,44,54)(H,45,52)(H,46,47,56)/t27-,28-,29-,31-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50460019
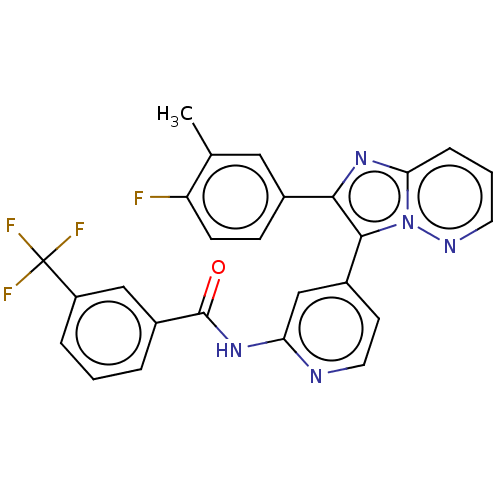
(CHEMBL4226320)Show SMILES Cc1cc(ccc1F)-c1nc2cccnn2c1-c1ccnc(NC(=O)c2cccc(c2)C(F)(F)F)c1 Show InChI InChI=1S/C26H17F4N5O/c1-15-12-16(7-8-20(15)27)23-24(35-22(34-23)6-3-10-32-35)17-9-11-31-21(14-17)33-25(36)18-4-2-5-19(13-18)26(28,29)30/h2-14H,1H3,(H,31,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged p38alpha expressed in baculovirus expression system using GFP-ATF2 (19 to 96 residues) as substrate prein... |
Bioorg Med Chem 26: 647-660 (2018)
Article DOI: 10.1016/j.bmc.2017.12.031
BindingDB Entry DOI: 10.7270/Q2XP77K2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50460016
(CHEMBL4226563)Show SMILES Cc1cccc(c1)C(=O)Nc1cc(ccn1)-c1c(nc2cccnn12)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C26H20FN5O/c1-16-5-3-6-20(13-16)26(33)30-22-15-19(10-12-28-22)25-24(18-8-9-21(27)17(2)14-18)31-23-7-4-11-29-32(23)25/h3-15H,1-2H3,(H,28,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FLAG-tagged p38alpha expressed in baculovirus expression system using GFP-ATF2 (19 to 96 residues) as substrate prein... |
Bioorg Med Chem 26: 647-660 (2018)
Article DOI: 10.1016/j.bmc.2017.12.031
BindingDB Entry DOI: 10.7270/Q2XP77K2 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317672
(CHEMBL1094664 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1cc(O)ccc1O)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nc(=S)o[nH]1 |r| Show InChI InChI=1S/C39H48N8O9S/c1-20(2)15-29(44-37(54)31(21(3)4)45-35(52)27(40)19-41-34(51)26-18-25(48)13-14-30(26)49)36(53)43-28(16-22-9-6-5-7-10-22)32(50)38(55)42-24-12-8-11-23(17-24)33-46-39(57)56-47-33/h5-14,17-18,20-21,27-29,31-32,48-50H,15-16,19,40H2,1-4H3,(H,41,51)(H,42,55)(H,43,53)(H,44,54)(H,45,52)(H,46,47,57)/t27-,28-,29-,31-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50098375

(CHEMBL3593393)Show SMILES Cl.OC[C@@H]1CNCCO[C@@H]1c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C12H15Cl2NO2/c13-10-2-1-8(5-11(10)14)12-9(7-16)6-15-3-4-17-12/h1-2,5,9,12,15-16H,3-4,6-7H2/t9-,12+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human SERT expressed in CHOK1 cells incubated for 20 mins by [3H]-5-hydroxytryptamine uptake assay |
Bioorg Med Chem 23: 5000-14 (2015)
Article DOI: 10.1016/j.bmc.2015.05.017
BindingDB Entry DOI: 10.7270/Q22F7Q6R |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057477

(4-(3-{3-[(4-Isobutyl-benzyl)-(4-isobutyl-phenyl)-a...)Show SMILES CC(C)Cc1ccc(cc1)C(Nc1cccc(c1)C(=O)c1cn(CCCC(O)=O)c2ccccc12)c1ccc(CC(C)C)cc1 Show InChI InChI=1S/C40H44N2O3/c1-27(2)23-29-14-18-31(19-15-29)39(32-20-16-30(17-21-32)24-28(3)4)41-34-10-7-9-33(25-34)40(45)36-26-42(22-8-13-38(43)44)37-12-6-5-11-35(36)37/h5-7,9-12,14-21,25-28,39,41H,8,13,22-24H2,1-4H3,(H,43,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Steroid 5-alpha-reductase type 2 in COS-1 cells was determined |
Bioorg Med Chem Lett 8: 561-6 (1999)
BindingDB Entry DOI: 10.7270/Q2571B5B |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50336049
(CHEMBL1669334 | N-benzyl-4-(6-(cyclopropylmethylam...)Show SMILES F[C@H]1CN[C@H](CN(Cc2ccccc2)C(=O)c2ccc(cc2)-c2cnc3ccc(NCC4CC4)nn23)C1 |r| Show InChI InChI=1S/C29H31FN6O/c30-24-14-25(31-16-24)19-35(18-21-4-2-1-3-5-21)29(37)23-10-8-22(9-11-23)26-17-33-28-13-12-27(34-36(26)28)32-15-20-6-7-20/h1-5,8-13,17,20,24-25,31H,6-7,14-16,18-19H2,(H,32,34)/t24-,25+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of purified His-tagged IKKbeta after 15 mins |
Bioorg Med Chem Lett 21: 904-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.078
BindingDB Entry DOI: 10.7270/Q26Q1XJF |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50351103
(CHEMBL1817745)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1[nH]c(=O)[nH]c(=O)c1F)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)NC1(CCCC1)c1ccccc1 |r| Show InChI InChI=1S/C40H53FN8O8/c1-22(2)19-28(45-37(55)30(23(3)4)46-33(51)26(42)21-43-36(54)31-29(41)35(53)48-39(57)47-31)34(52)44-27(20-24-13-7-5-8-14-24)32(50)38(56)49-40(17-11-12-18-40)25-15-9-6-10-16-25/h5-10,13-16,22-23,26-28,30,32,50H,11-12,17-21,42H2,1-4H3,(H,43,54)(H,44,52)(H,45,55)(H,46,51)(H,49,56)(H2,47,48,53,57)/t26-,27-,28-,30-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 by FRET assay |
Bioorg Med Chem 19: 5238-46 (2011)
Article DOI: 10.1016/j.bmc.2011.07.002
BindingDB Entry DOI: 10.7270/Q20Z73NP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data