

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50457437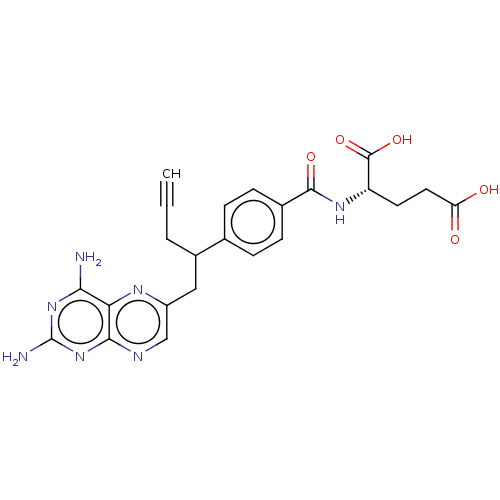 (CHEBI:71223 | Folotyn | PDX | Pralatrexate) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113218 BindingDB Entry DOI: 10.7270/Q2PN99PG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged and C-terminal FLAG-tagged full length human recombinant HDAC1 expressed in baculovirus coexpressed in fall armyw... | Bioorg Med Chem 24: 4008-4015 (2016) Article DOI: 10.1016/j.bmc.2016.06.040 BindingDB Entry DOI: 10.7270/Q23B6220 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine D3 receptor (unknown origin) expressed in HEK293 cell membranes incubated for 15 mins by liquid scintill... | Bioorg Med Chem Lett 25: 3970-4 (2015) Article DOI: 10.1016/j.bmcl.2015.07.030 BindingDB Entry DOI: 10.7270/Q2CC12G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304444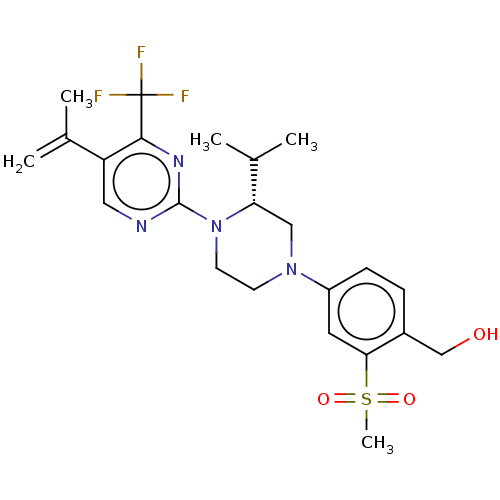 ((R)-(4-(3-isopropyl-4-(5-(prop-1-en-2-yl)-4-(trifl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177012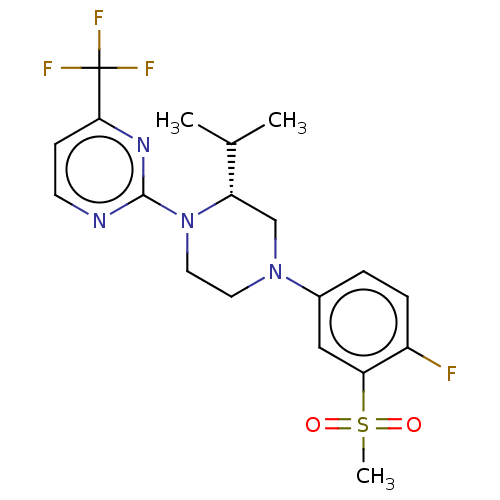 (CHEMBL3814153 | US10144715, Compound 7-32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304505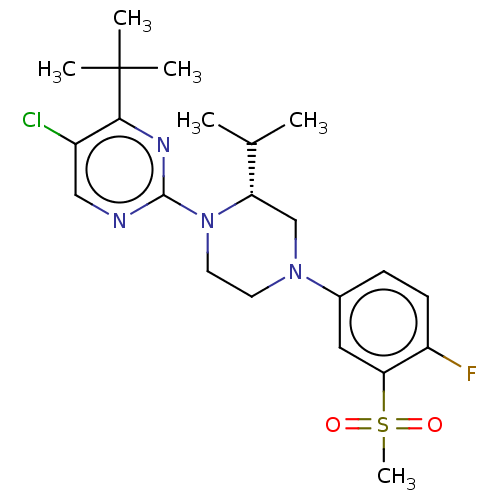 ((R)-5-chloro-2-(4-(4-fluoro-3-(methylsulfonyl)phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177012 (CHEMBL3814153 | US10144715, Compound 7-32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304452 ((R)-2-(4-(4-fluoro-3-(methylsulfonyl)phenyl)-2-iso...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177016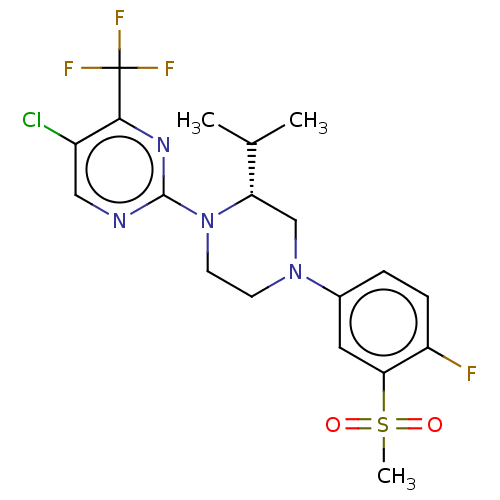 (CHEMBL3814501) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192752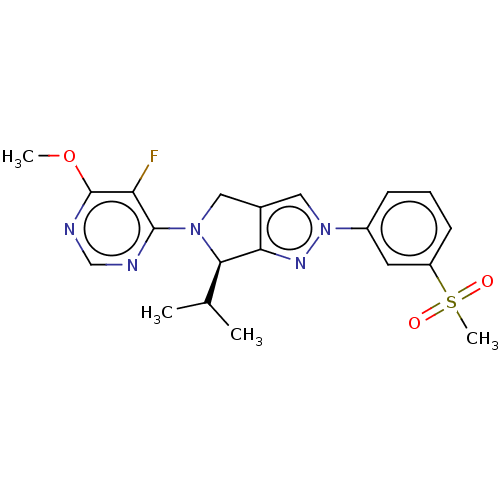 (CHEMBL3905741) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) | Bioorg Med Chem Lett 26: 5044-5050 (2016) Article DOI: 10.1016/j.bmcl.2016.08.089 BindingDB Entry DOI: 10.7270/Q28P62GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304526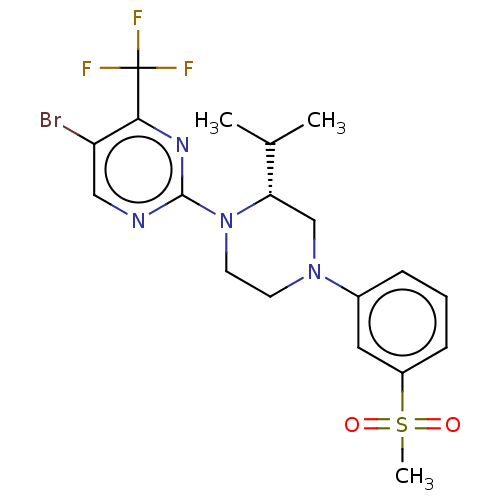 ((R)-5-cyclopropyl-2-(4-(4-fluoro-3-(methylsulfonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from dopamine D2 receptor (unknown origin) expressed in HEK293 cell membranes incubated for 15 mins by liquid scintill... | Bioorg Med Chem Lett 25: 3970-4 (2015) Article DOI: 10.1016/j.bmcl.2015.07.030 BindingDB Entry DOI: 10.7270/Q2CC12G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108220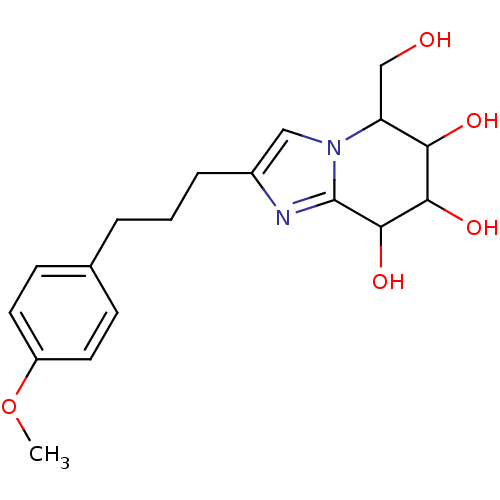 (5-(hydroxymethyl)-2-[3-(4-methoxyphenyl)propyl]- 5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177010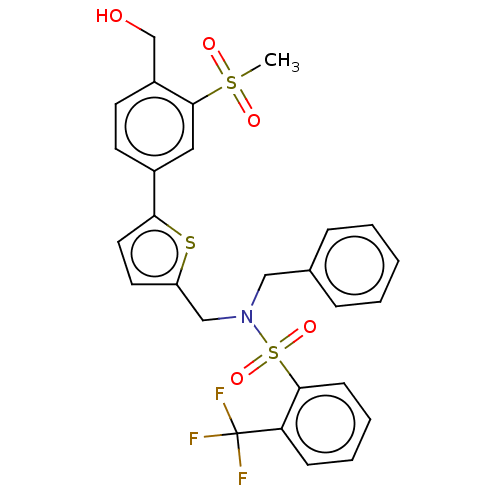 (CHEMBL3814006) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50027656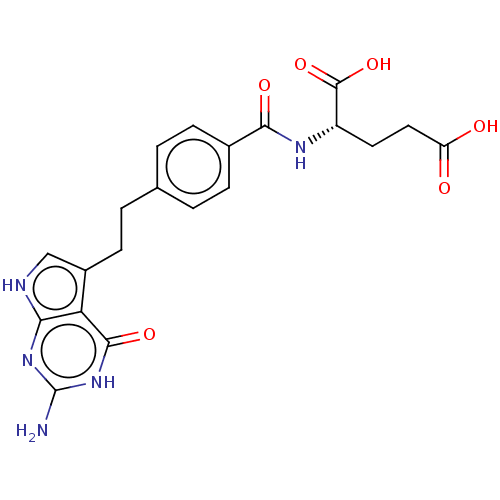 (CHEBI:63616 | LY-2315 | LY-231514 | PEMETREXED | U...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113218 BindingDB Entry DOI: 10.7270/Q2PN99PG | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108219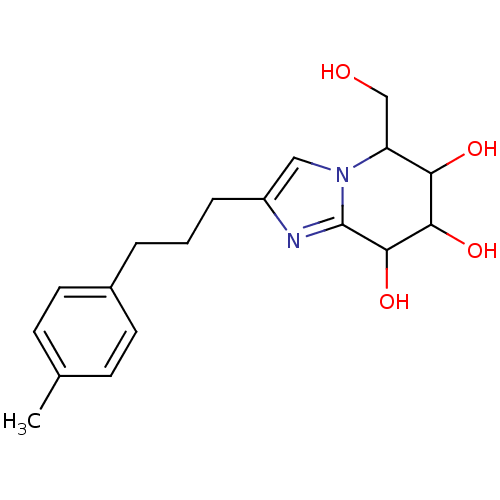 (5-(hydroxymethyl)-2-[3-(4-methylphenyl)propyl]- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.46 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Inhibition full length human recombinant HDAC2 expressed in baculovirus coexpressed in fall armyworm Sf9 cells using carboxyfluorescein (FAM)-labeled... | Bioorg Med Chem 24: 4008-4015 (2016) Article DOI: 10.1016/j.bmc.2016.06.040 BindingDB Entry DOI: 10.7270/Q23B6220 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50409510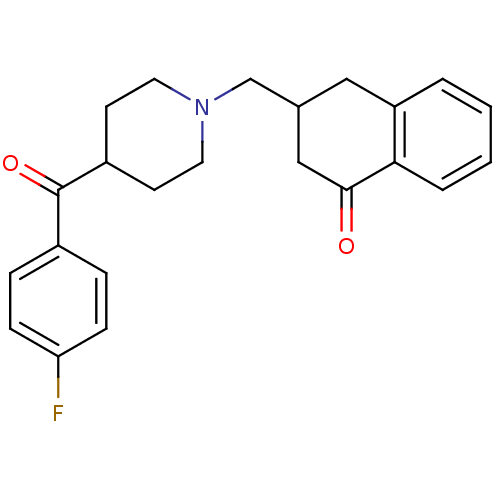 (CHEMBL308480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113964 BindingDB Entry DOI: 10.7270/Q2BP06TV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]-SCH-23390 from dopamine D1 receptor (unknown origin) expressed in HEK293 cell membranes incubated for 15 mins by liquid scintill... | Bioorg Med Chem Lett 25: 3970-4 (2015) Article DOI: 10.1016/j.bmcl.2015.07.030 BindingDB Entry DOI: 10.7270/Q2CC12G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50403163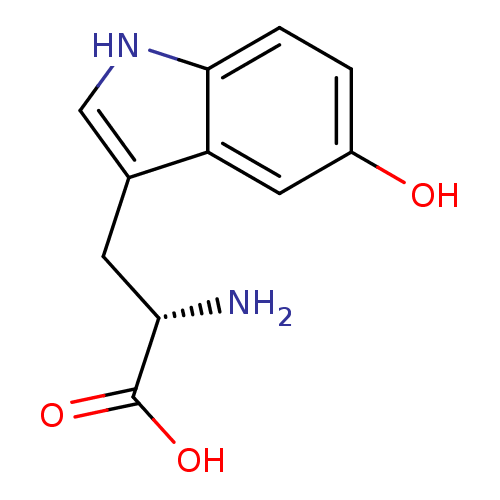 (OXITRIPTAN) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-HT1A receptor (unknown origin) expressed in HEK293 cell membranes incubated for 15 mins by liquid scintillation... | Bioorg Med Chem Lett 25: 3970-4 (2015) Article DOI: 10.1016/j.bmcl.2015.07.030 BindingDB Entry DOI: 10.7270/Q2CC12G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108222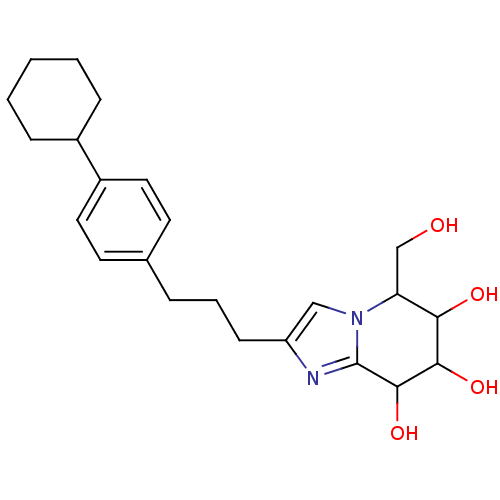 (2-[3-(4-cyclohexylphenyl)propyl]-5-(hydroxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304419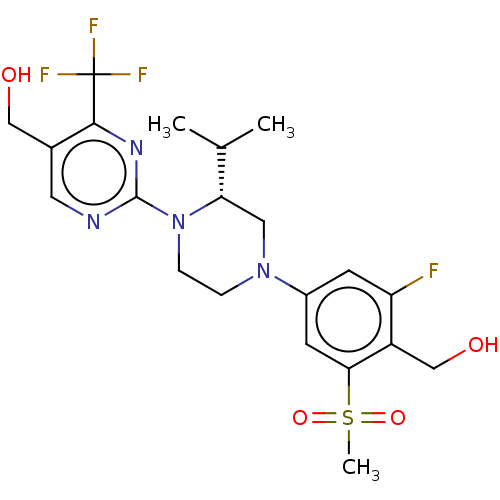 ((R)-(2-fluoro-4-(4-(5-(hydroxymethyl)-4-(trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304616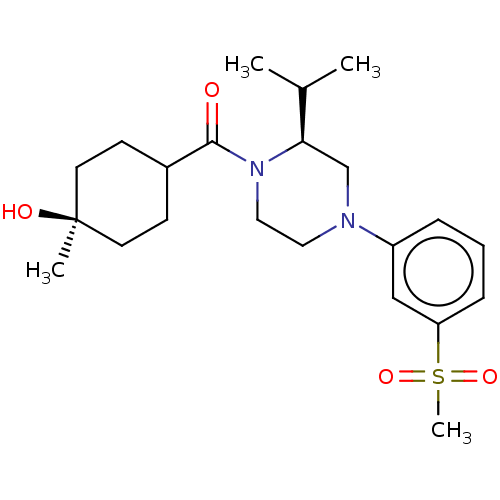 ((S)-2-isopropyl-4-(3-(methylsulfonyl)phenyl)-1-(((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304523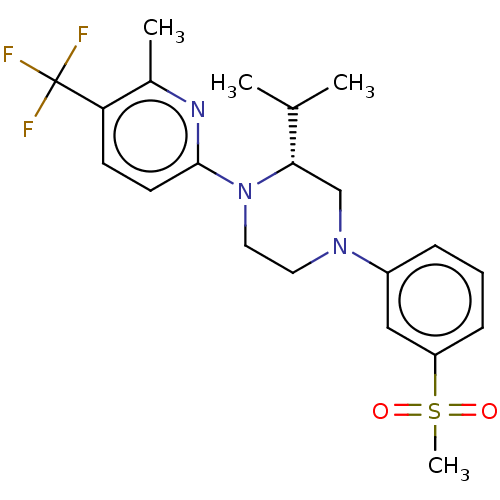 ((R)-2-isopropyl-1-(6-methyl-5-(trifluoromethyl)pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304525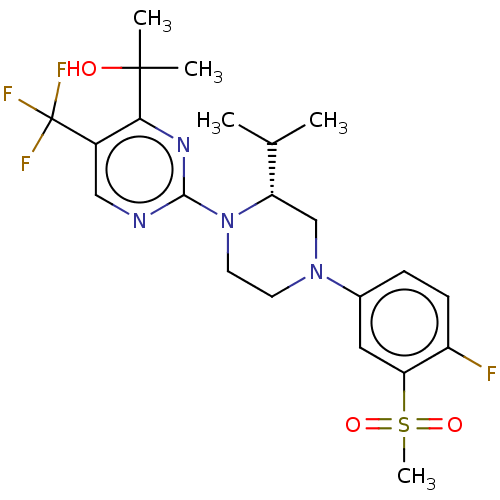 ((R)-2-(2-(4-(4-fluoro-3-(methylsulfonyl)phenyl)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304681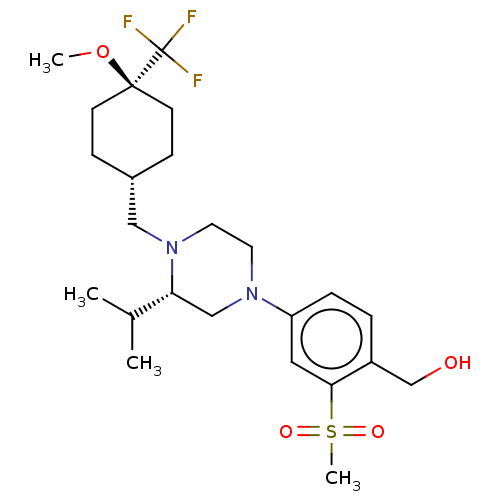 ((4-((S)-3-isopropyl-4-(((1r,4S)-4-methoxy-4-(trifl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DCN1-like protein 5 (Homo sapiens) | BDBM50525313 (CHEMBL4592844) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Binding affinity to N-terminal GST tagged recombinant DCN5 (unknown origin) (47 to 237 residues) expressed in Escherichia coli BL21(DE3) assessed as ... | J Med Chem 62: 5382-5403 (2019) Article DOI: 10.1021/acs.jmedchem.9b00003 BindingDB Entry DOI: 10.7270/Q2GT5RM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50223744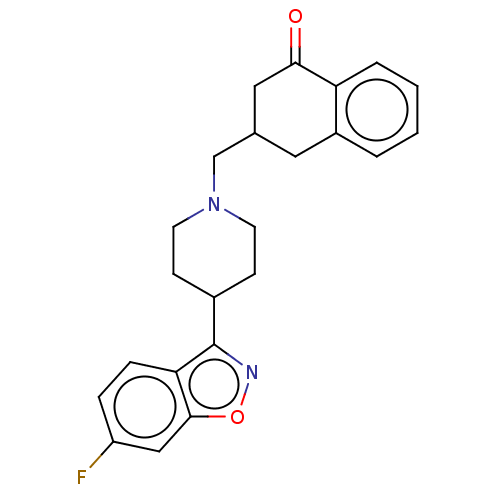 (CHEMBL155486) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113964 BindingDB Entry DOI: 10.7270/Q2BP06TV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50366495 ((+)butaclamol | CHEMBL1255588) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin hydrochloride from 5-HT2A receptor (unknown origin) expressed in HEK293 cell membranes incubated for 15 mins by liquid... | Bioorg Med Chem Lett 25: 3970-4 (2015) Article DOI: 10.1016/j.bmcl.2015.07.030 BindingDB Entry DOI: 10.7270/Q2CC12G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304682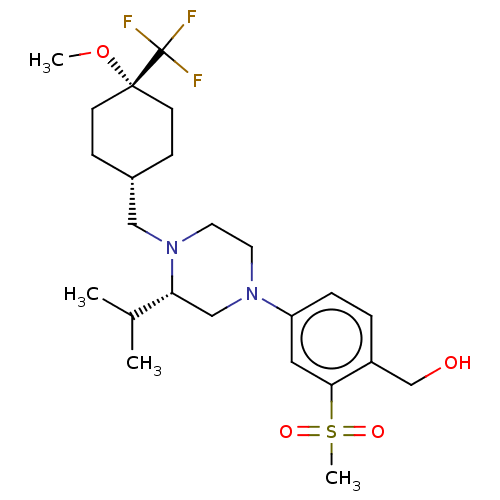 ((4-((S)-3-isopropyl-4-(((1s,4R)-4-methoxy-4-(trifl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177015 (CHEMBL3814206 | US10144715, Compound 19-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50177016 (CHEMBL3814501) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM304505 ((R)-5-chloro-2-(4-(4-fluoro-3-(methylsulfonyl)phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177015 (CHEMBL3814206 | US10144715, Compound 19-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304512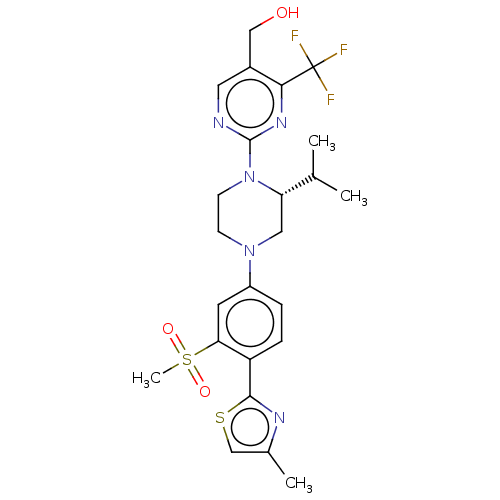 ((R)-2-(2-(4-(4-(hydroxymethyl)-3-(methylsulfonyl)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304431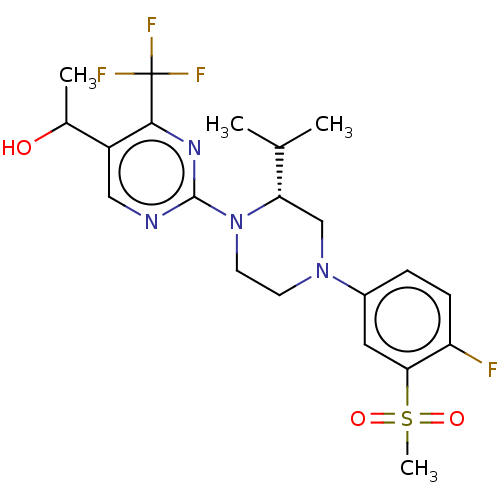 (1-(2-((R)-4-(4-fluoro-3-(methylsulfonyl)phenyl)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304441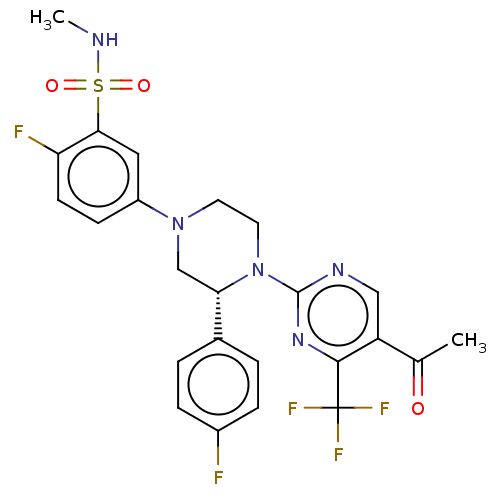 ((R)-5-(4-(5-acetyl-4-(trifluoromethyl)pyrimidin-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177015 (CHEMBL3814206 | US10144715, Compound 19-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) | Bioorg Med Chem Lett 26: 5044-5050 (2016) Article DOI: 10.1016/j.bmcl.2016.08.089 BindingDB Entry DOI: 10.7270/Q28P62GC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192753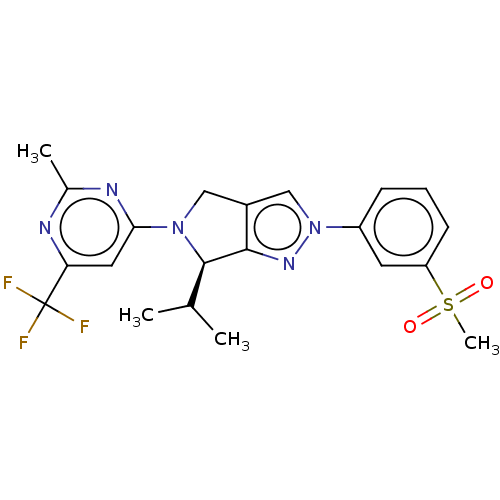 (CHEMBL3985591) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) | Bioorg Med Chem Lett 26: 5044-5050 (2016) Article DOI: 10.1016/j.bmcl.2016.08.089 BindingDB Entry DOI: 10.7270/Q28P62GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304514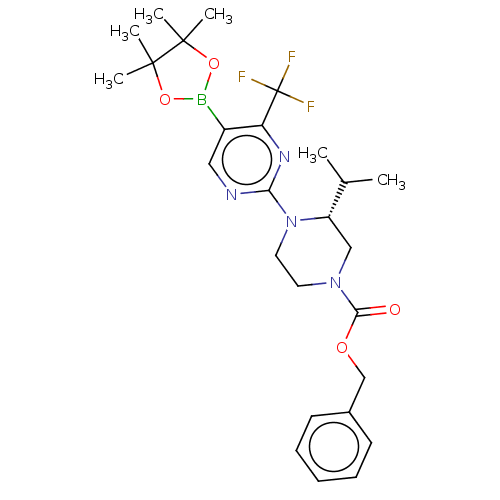 ((R)-2-(4-(4-fluoro-3-(methylsulfonyl)phenyl)-2-iso...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304522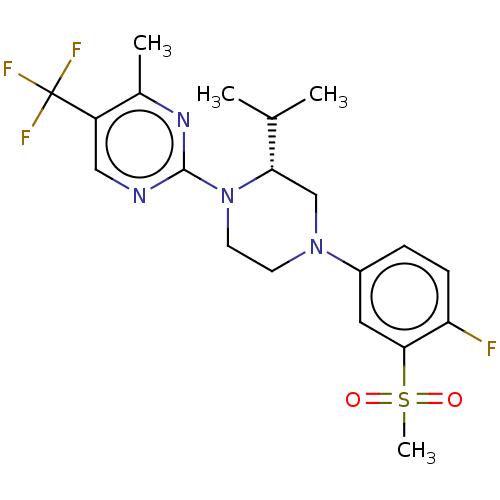 ((R)-2-(4-(4-fluoro-3-(methylsulfonyl)phenyl)-2-iso...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304524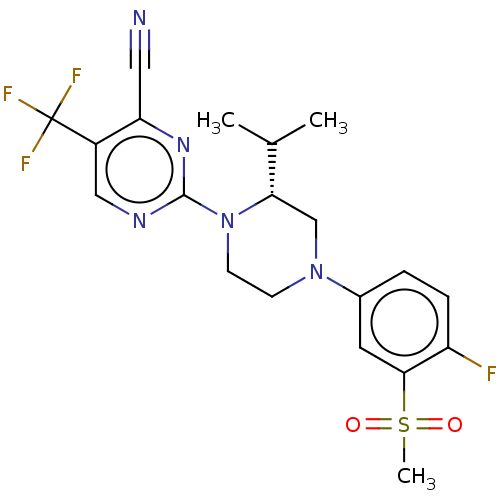 (1-(2-((R)-4-(4-fluoro-3-(methylsulfonyl)phenyl)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108216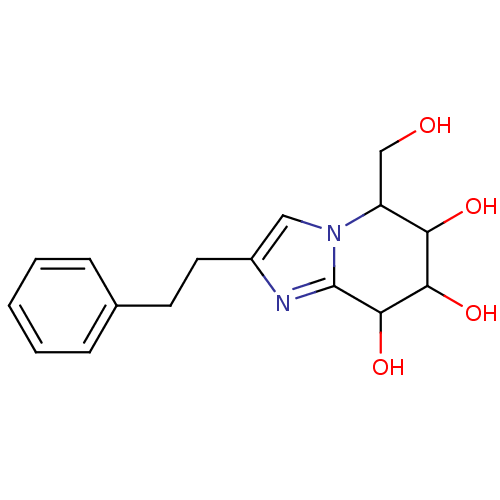 (5-(hydroxymethyl)-2-(2-phenylethyl)-5H,6H,7H,8H- i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.19 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108223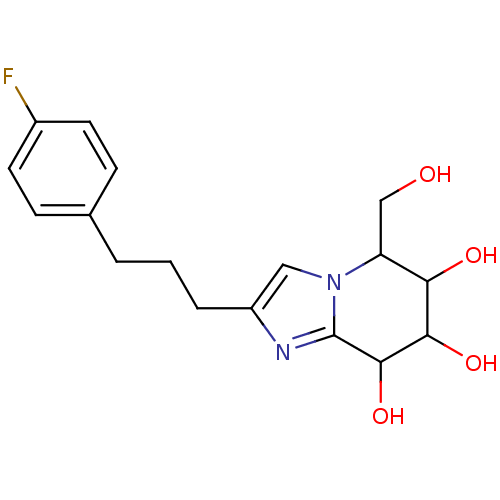 (2-[3-(4-fluorophenyl)propyl]-5-(hydroxymethyl)- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.48 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108221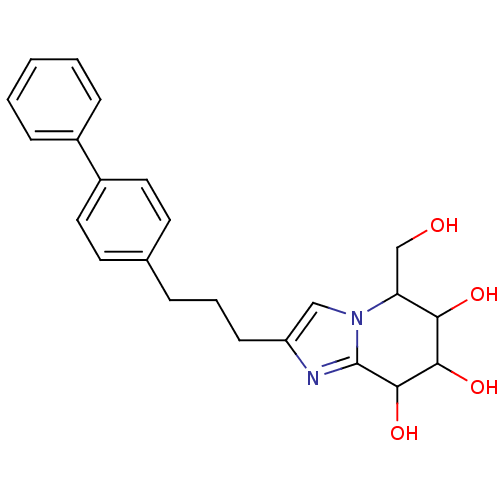 (5-(hydroxymethyl)-2-[3-(4-phenylphenyl)propyl]- 5H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.62 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Nankai University | Assay Description To determine inhibition constant (Ki), substrate (7.5µL, various concentrations in Mcllvaine buffer, pH 5.2) and enzyme (12.5µL, 0.1mg/mL) ... | Chembiochem 14: 1239-47 (2013) Article DOI: 10.1002/cbic.201300197 BindingDB Entry DOI: 10.7270/Q2NS0SHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177011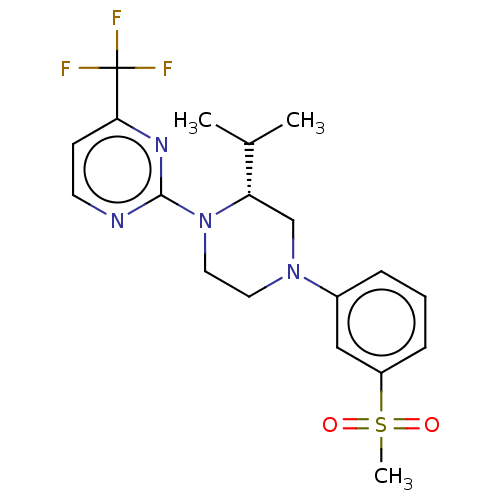 (CHEMBL3815014 | US10144715, Compound 7-13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation counting | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50192757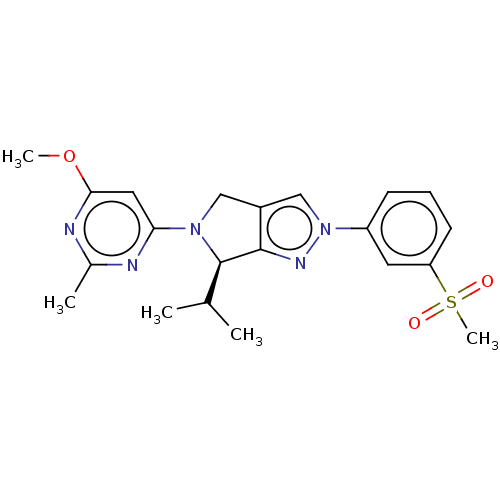 (CHEMBL3914727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) | Bioorg Med Chem Lett 26: 5044-5050 (2016) Article DOI: 10.1016/j.bmcl.2016.08.089 BindingDB Entry DOI: 10.7270/Q28P62GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM155261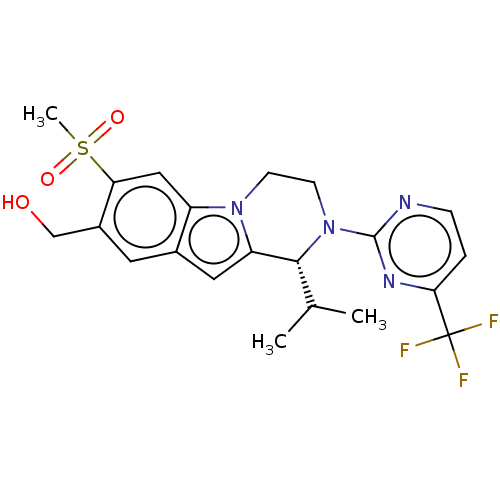 (US9006244, E4a | US9814715, Example 4, isomer 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US9006244 (2015) BindingDB Entry DOI: 10.7270/Q2X92911 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM304506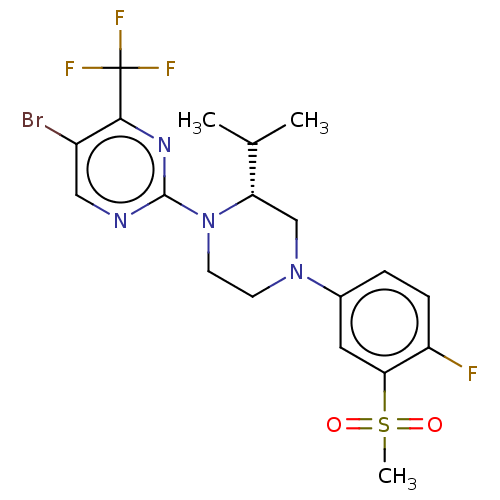 ((R)-5-bromo-2-(4-(4-fluoro-3-(methylsulfonyl)pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304509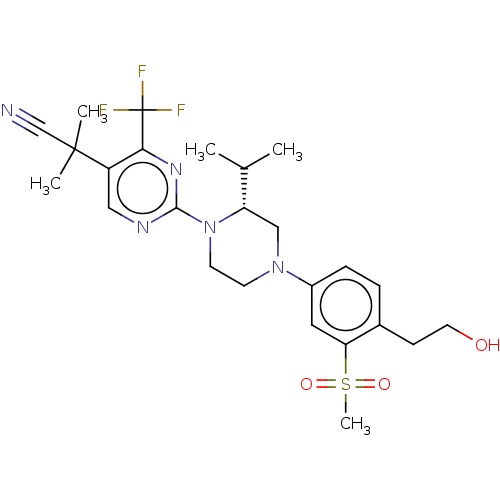 ((R)-2-(2-(4-(4-(hydroxymethyl)-3-(methylsulfonyl)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7049 total ) | Next | Last >> |