

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50163152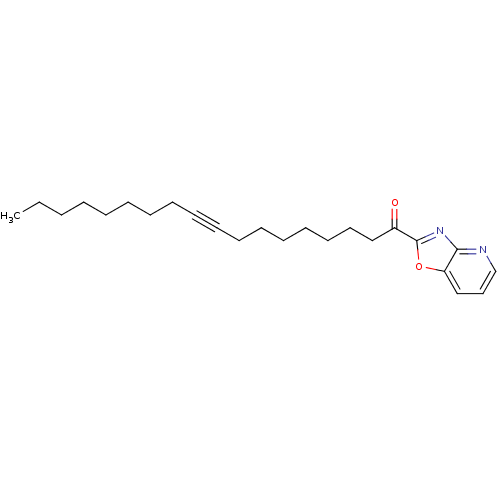 (1-Oxazolo[4,5-b]pyridin-2-yl-octadec-9-yn-1-one | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory constant determined against recombinant Fatty-acid amide hydrolase from rat expressed in Escherichia coli | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161518 (1-(oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50161518 (1-(oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory constant determined against recombinant Fatty-acid amide hydrolase from rat expressed in Escherichia coli | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM23316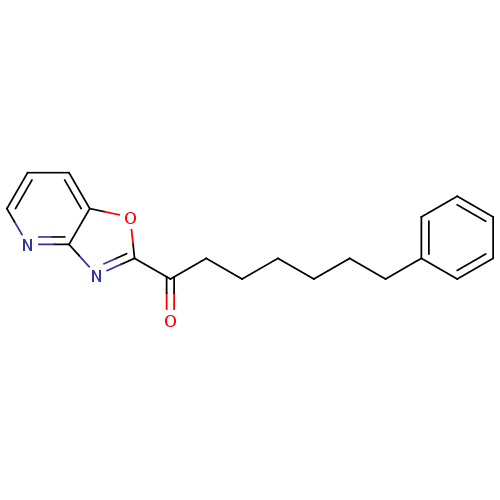 (7-phenyl-1-{pyrido[2,3-d][1,3]oxazol-2-yl}heptan-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM23316 (7-phenyl-1-{pyrido[2,3-d][1,3]oxazol-2-yl}heptan-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory constant determined against recombinant Fatty-acid amide hydrolase from rat expressed in Escherichia coli | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161528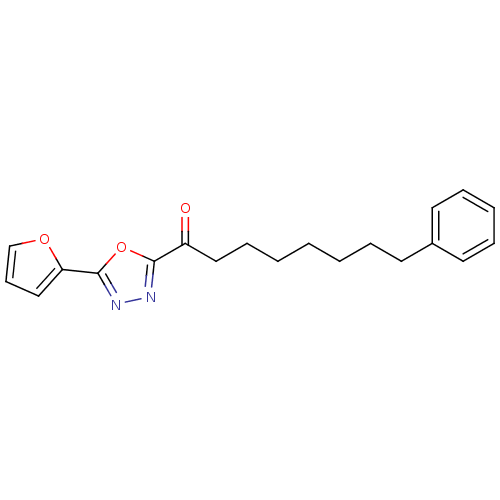 (8-Phenyl-1-(5-pyridin-2-yl-[1,3,4]oxadiazol-2-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23079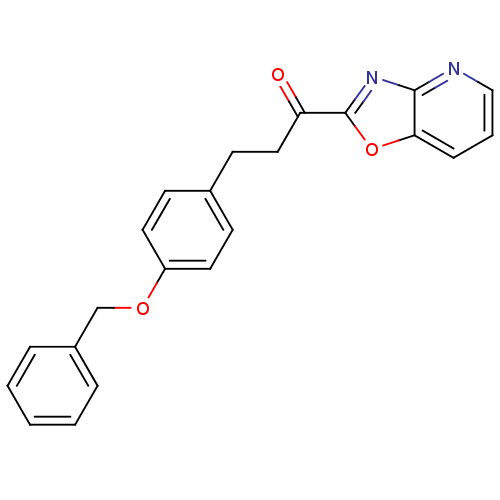 (3-[4-(benzyloxy)phenyl]-1-{pyrido[2,3-d][1,3]oxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | -53.8 | 0.300 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161520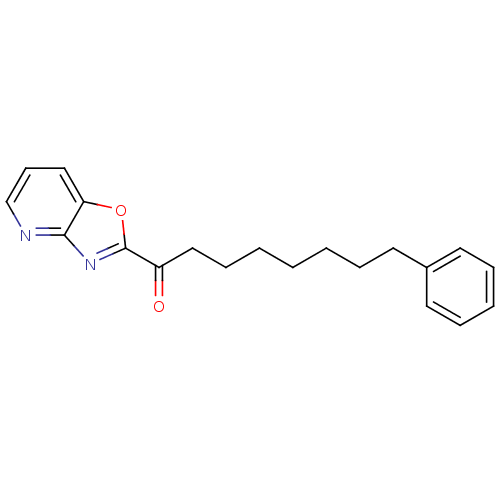 (1-(oxazolo[4,5-b]pyridin-2-yl)-8-phenyloctan-1-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23069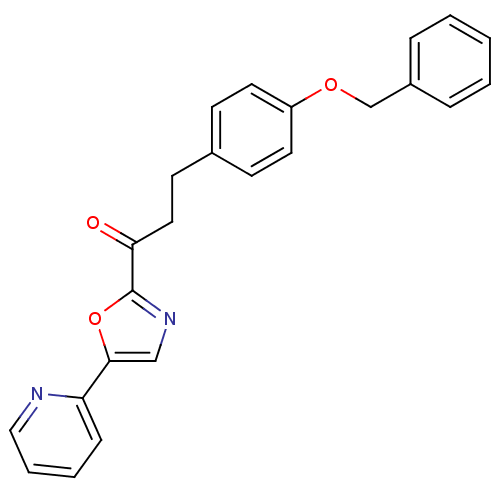 (3-[4-(benzyloxy)phenyl]-1-[5-(pyridin-2-yl)-1,3-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | -53.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161525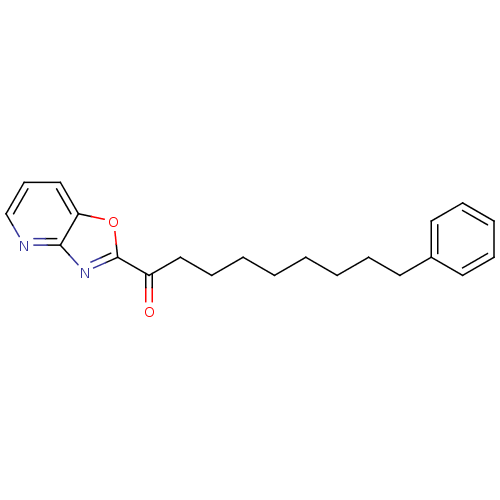 (1-(oxazolo[4,5-b]pyridin-2-yl)-9-phenylnonan-1-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161511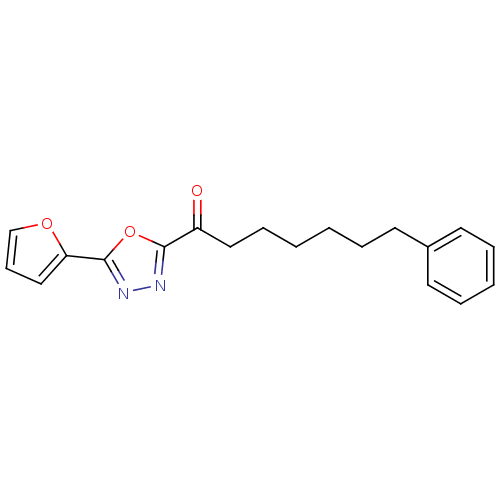 (1-(5-(Furan-2-yl)-1,3,4-oxadiazol-2-yl)-heptan-1-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23079 (3-[4-(benzyloxy)phenyl]-1-{pyrido[2,3-d][1,3]oxazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23078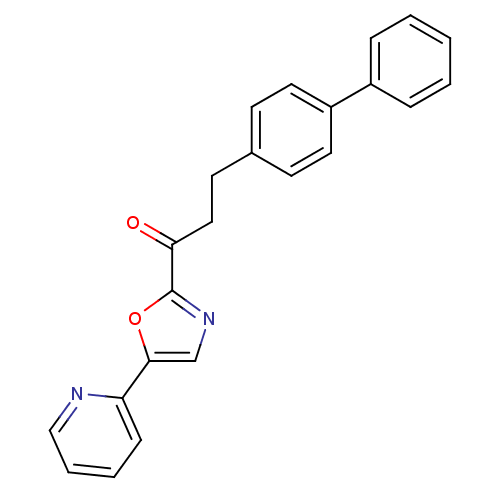 (3-(4-phenylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | -52.1 | 0.700 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161513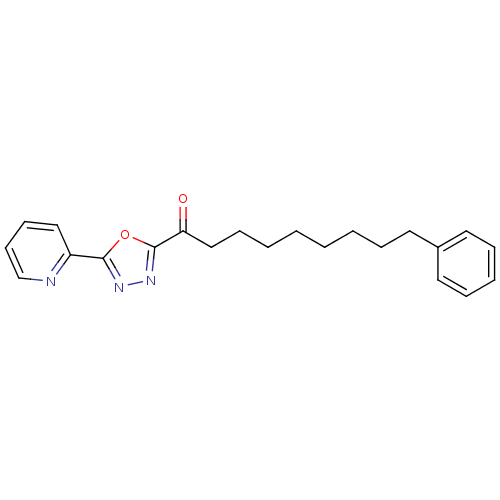 (7-Phenyl-1-(5-pyridin-2-yl-[1,3,4]oxadiazol-2-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161513 (7-Phenyl-1-(5-pyridin-2-yl-[1,3,4]oxadiazol-2-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23061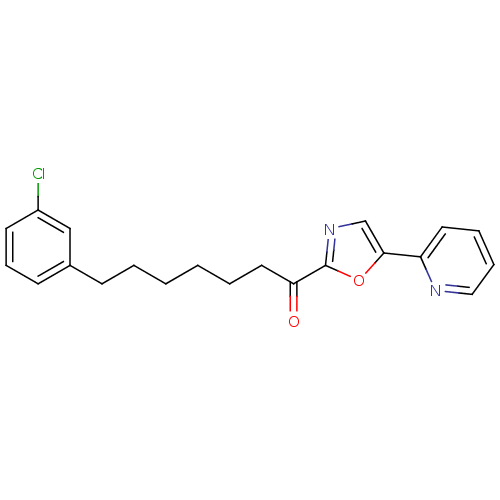 (7-(3-chlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | -51.6 | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23063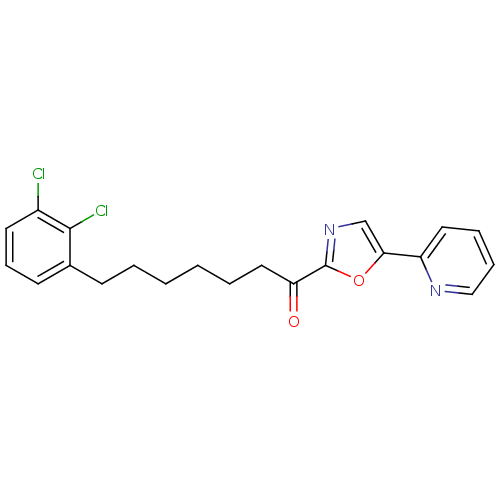 (7-(2,3-dichlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | -51.6 | 7 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23055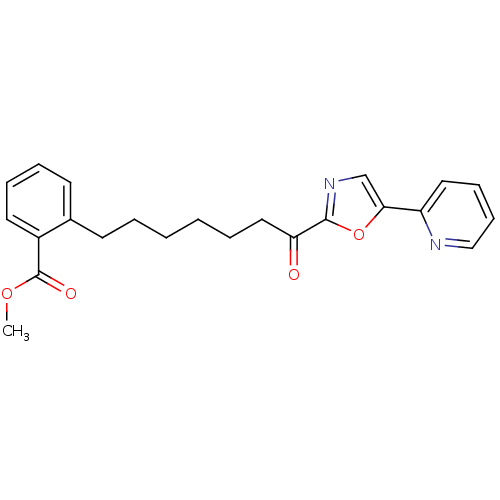 (alpha-ketooxazole, 5aa | methyl 2-{7-oxo-7-[5-(pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -50.9 | 0.400 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23073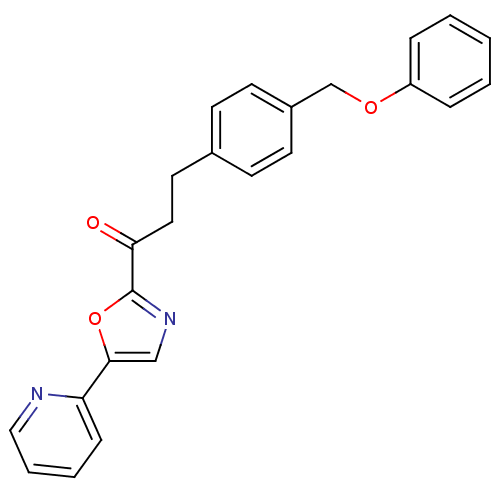 (3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -51.4 | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23053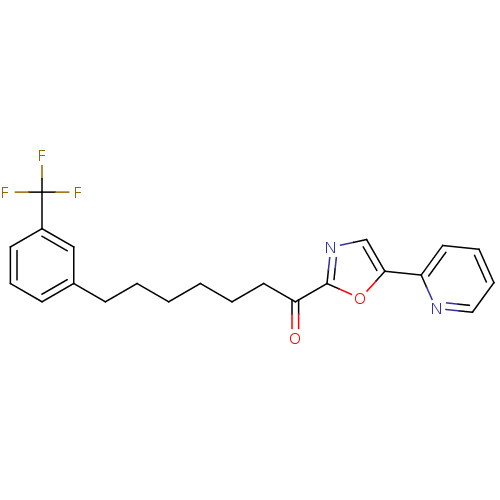 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-[3-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -50.9 | 20 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161524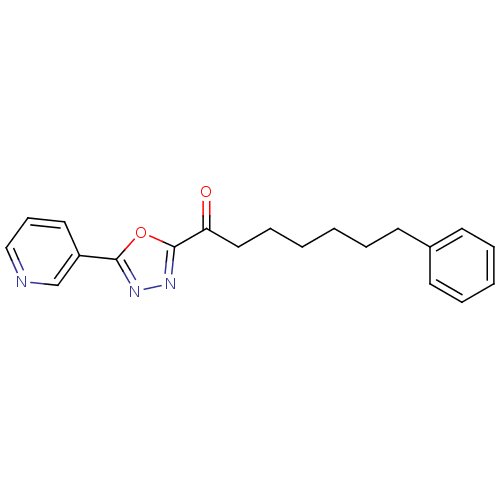 (7-Phenyl-1-(5-pyridin-3-yl-[1,3,4]oxadiazol-2-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161514 (7-Phenyl-1-(5-pyridin-4-yl-[1,3,4]oxadiazol-2-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23069 (3-[4-(benzyloxy)phenyl]-1-[5-(pyridin-2-yl)-1,3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | -50.7 | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23065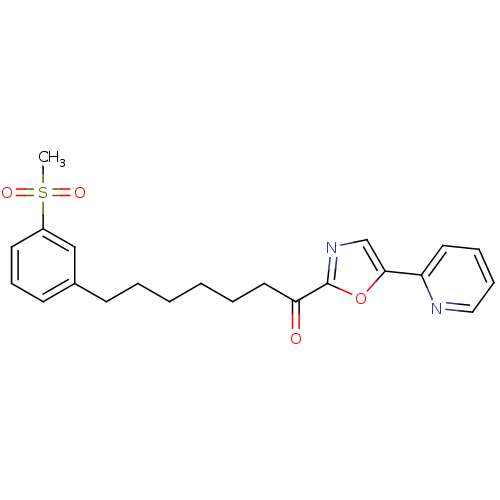 (7-(3-methanesulfonylphenyl)-1-[5-(pyridin-2-yl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | -50.7 | 4 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23057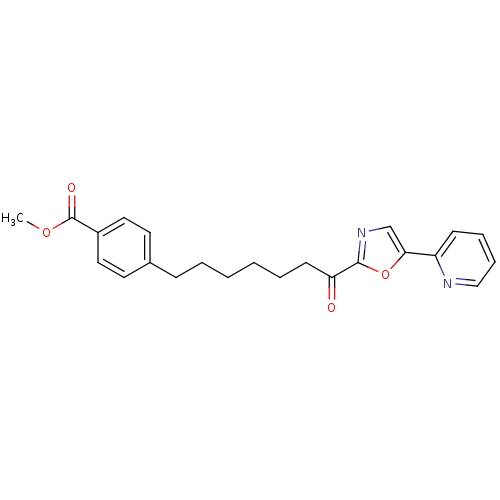 (alpha-ketooxazole, 5cc | methyl 4-{7-oxo-7-[5-(pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | -49.9 | 0.240 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23045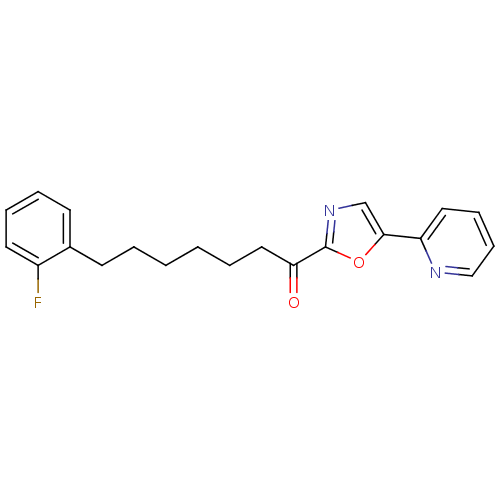 (7-(2-fluorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | -49.6 | 30 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23056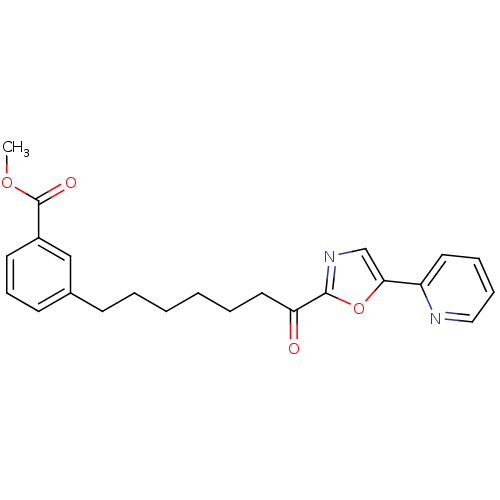 (alpha-ketooxazole, 5bb | methyl 3-{7-oxo-7-[5-(pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | -49.3 | 0.400 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23060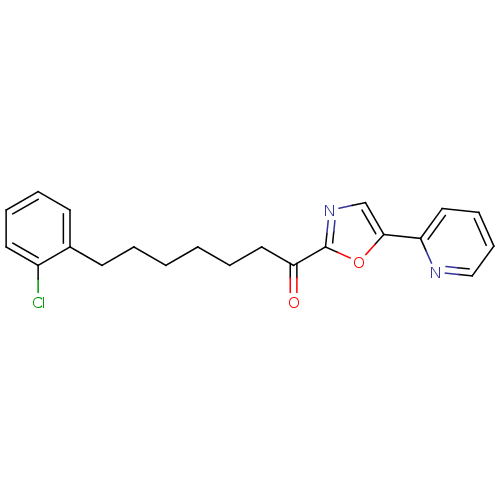 (7-(2-chlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | -49.8 | 20 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23075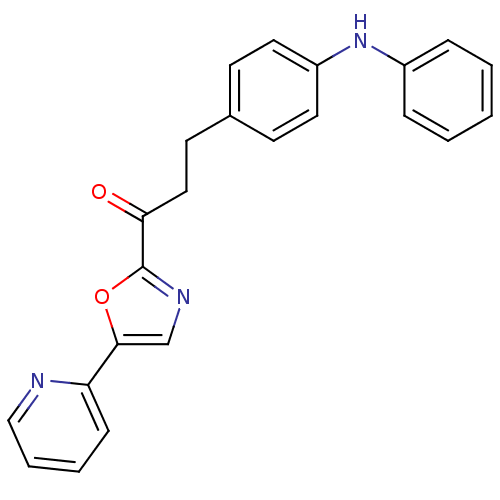 (3-[4-(phenylamino)phenyl]-1-[5-(pyridin-2-yl)-1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | -49.7 | 5 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23077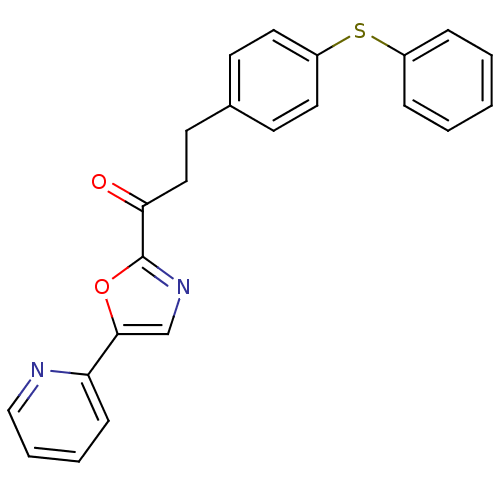 (3-[4-(phenylsulfanyl)phenyl]-1-[5-(pyridin-2-yl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | -49.4 | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23046 (7-(3-fluorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | -48.9 | 10 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50163165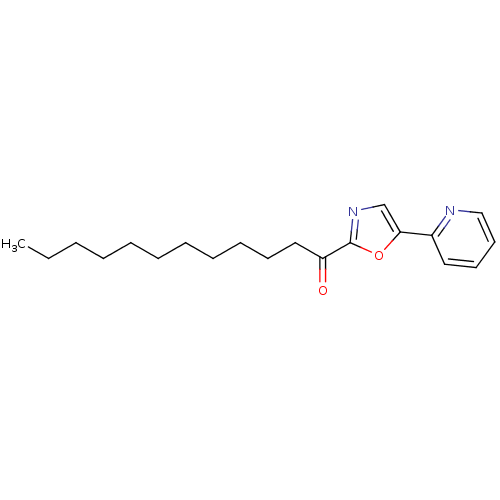 (1-(5-(pyridin-2-yl)oxazol-2-yl)dodecan-1-one | 1-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory constant determined against recombinant Fatty-acid amide hydrolase from rat expressed in Escherichia coli | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50100865 ((Z)-1-(oxazolo[4,5-b]pyridin-2-yl)octadec-9-en-1-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50163177 (7-Phenyl-1-(5-pyrimidin-4-yl-oxazol-2-yl)-heptan-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory constant determined against recombinant Fatty-acid amide hydrolase from rat expressed in Escherichia coli | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23043 (alpha-ketooxazole, 5o | tert-butyl N-(3-{7-oxo-7-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | -48.7 | n/a | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23039 (7-(3-methoxyphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | -48.6 | 20 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23050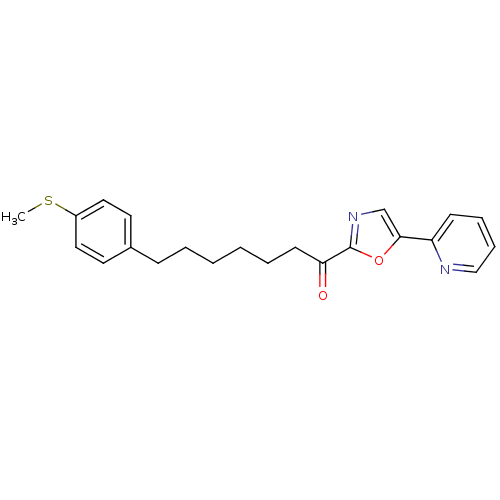 (7-[4-(methylsulfanyl)phenyl]-1-[5-(pyridin-2-yl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | -48.6 | 20 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23030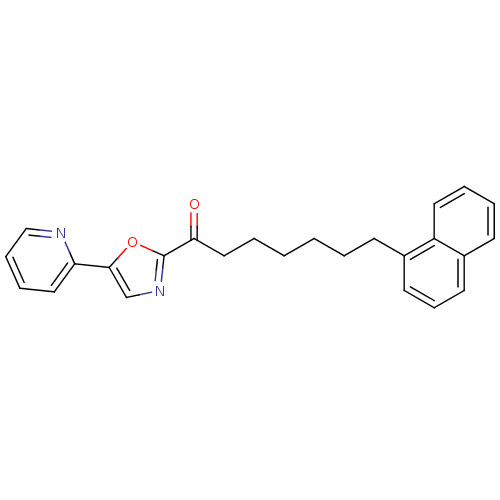 (7-(naphthalen-1-yl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | -48.5 | 10 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23062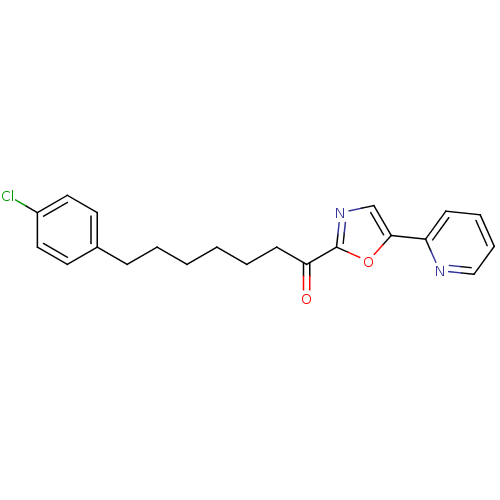 (7-(4-chlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | -48.9 | 30 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23037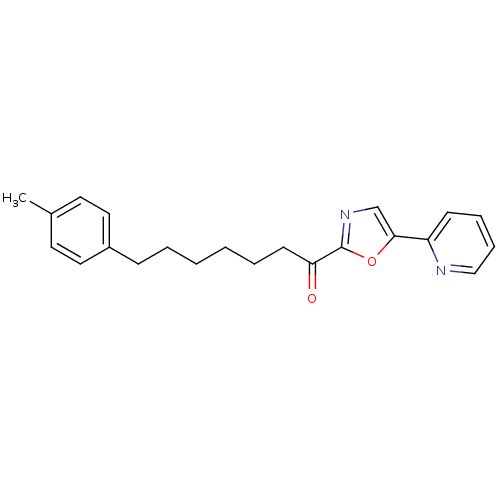 (7-(4-methylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | -48.3 | 40 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23078 (3-(4-phenylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | -48.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23042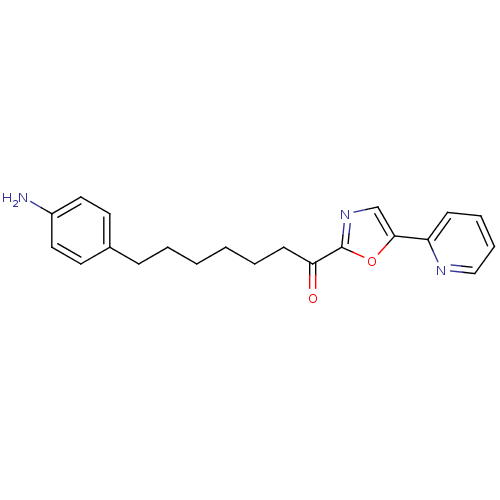 (7-(4-aminophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.2 | 4 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23034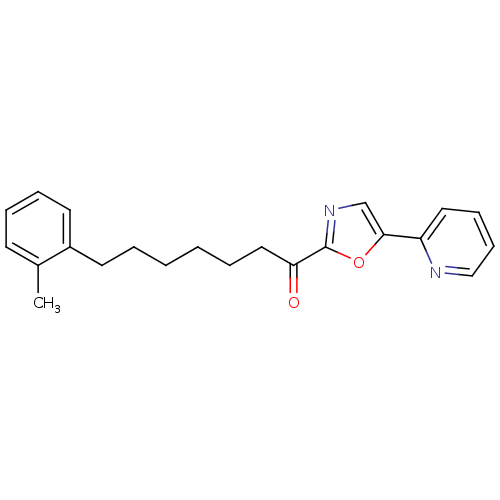 (7-(2-methylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.2 | 30 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50157120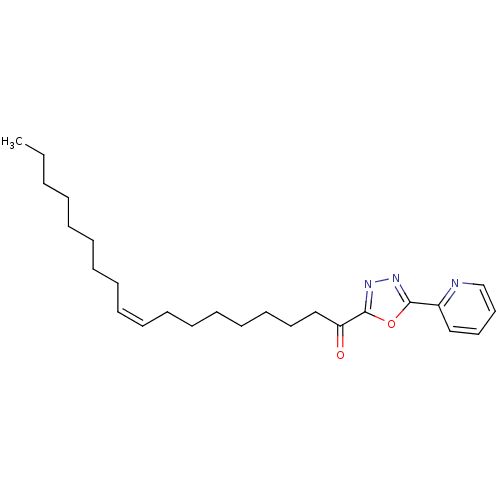 ((9Z)-1-(5-pyridin-2-yl-1,3,4-oxadiazol-2-yl)octade...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23117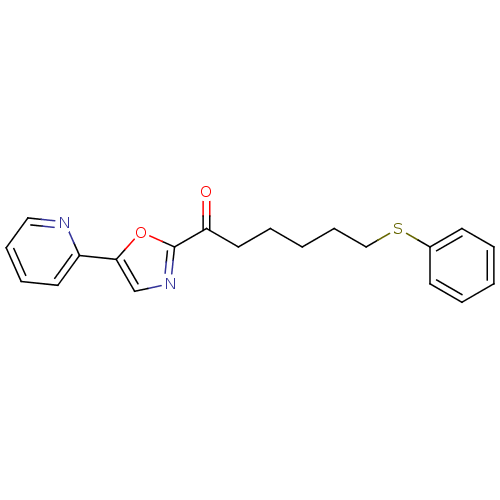 (6-(phenylsulfanyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50157120 ((9Z)-1-(5-pyridin-2-yl-1,3,4-oxadiazol-2-yl)octade...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity against purified rat Fatty-acid amide hydrolase (FAAH) expressed in Escherichia coli | Bioorg Med Chem Lett 15: 103-6 (2004) Article DOI: 10.1016/j.bmcl.2004.10.025 BindingDB Entry DOI: 10.7270/Q2MC8ZGW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23076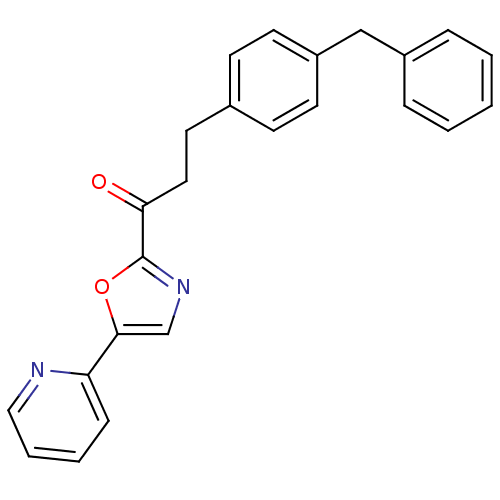 (3-(4-benzylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | -48.5 | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23047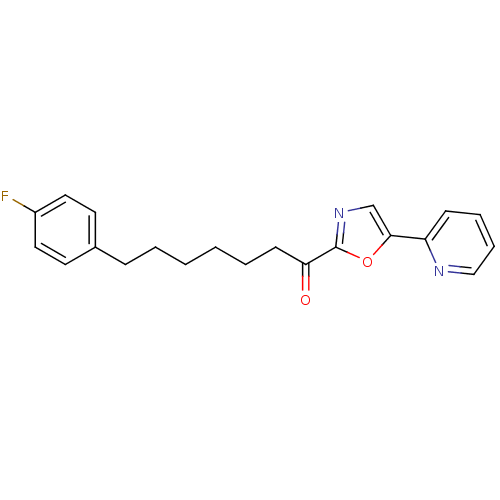 (7-(4-fluorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | -48.0 | 20 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23087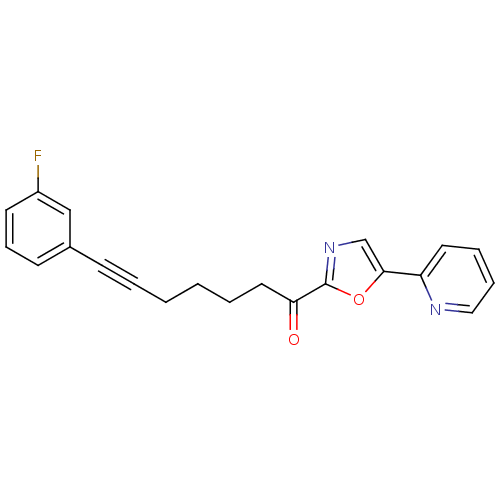 (7-(3-fluorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | -48.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23048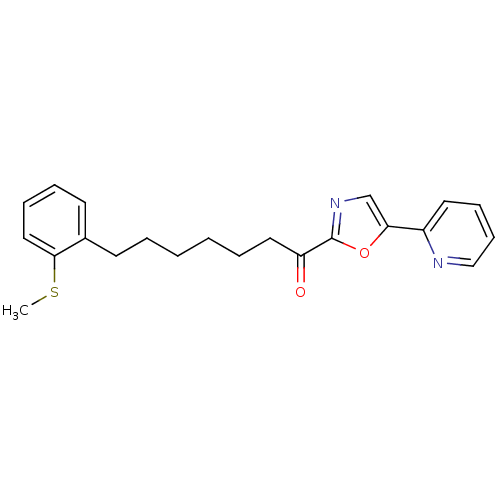 (7-[2-(methylsulfanyl)phenyl]-1-[5-(pyridin-2-yl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30 | -47.9 | 30 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 426 total ) | Next | Last >> |