

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM446832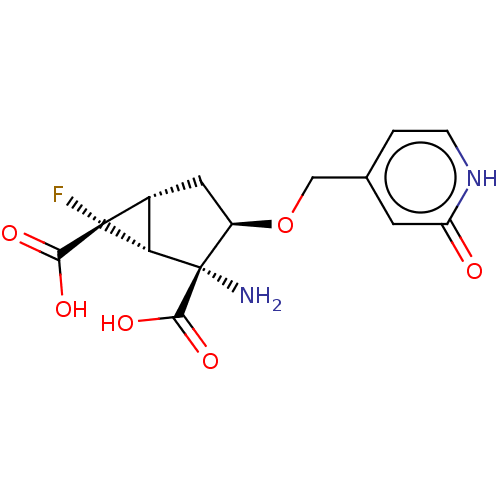 ((1R,2R,3R,5R,6R)-2-amino-6-fluoro-3-[(2-oxo-1,2- d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.35 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM446712 ((1R,2R,3R,5R,6R)-2-amino-6-fluoro-3-[(1R)-1-(4- fl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.04 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM446712 ((1R,2R,3R,5R,6R)-2-amino-6-fluoro-3-[(1R)-1-(4- fl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.22 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM446818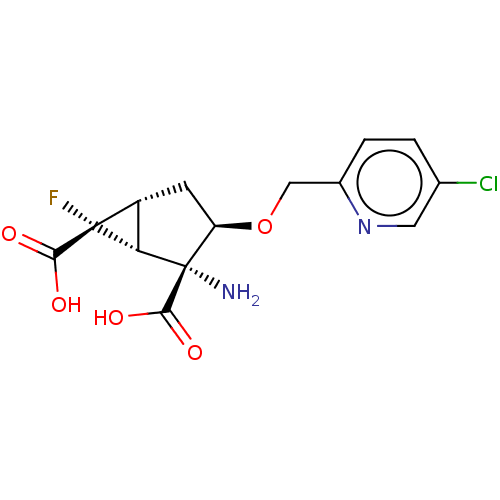 ((1R,2R,3R,5R,6R)-2-amino-3-[(5-chloropyridin-2- yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM446701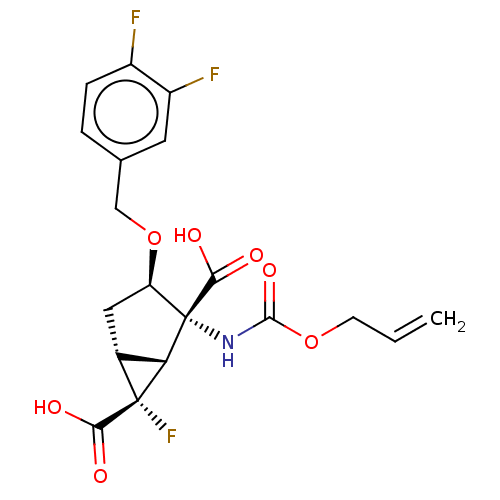 (US10689327, Compound (II)-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM446836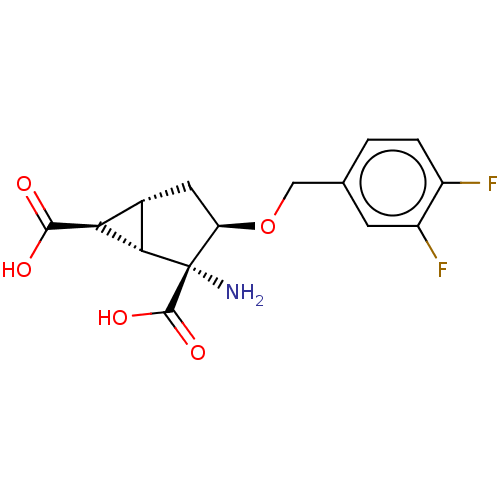 ((1S,2R,3R,5R,6S)-2-amino-3-[(3,4- difluorophenyl)m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM446784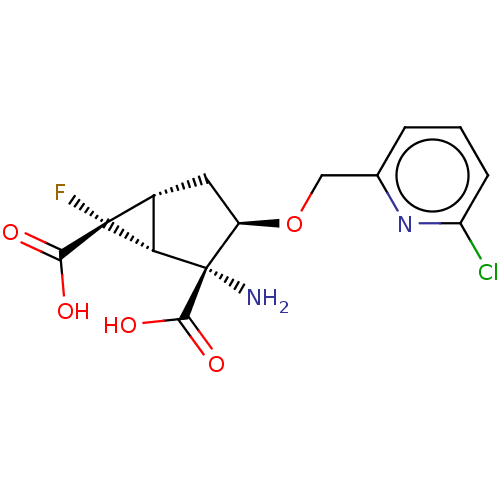 ((1R,2R,3R,5R,6R)-2-amino-3-[(6-chloropyridin-2- yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM446701 (US10689327, Compound (II)-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM446718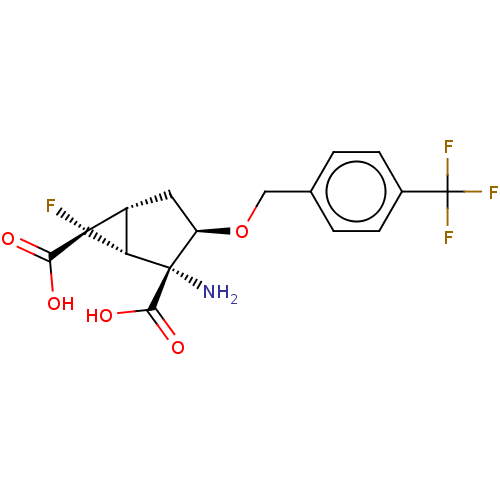 ((1R,2R,3R,5R,6R)-2-amino-6-fluoro-3-{[4- (triflour...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23.7 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM446828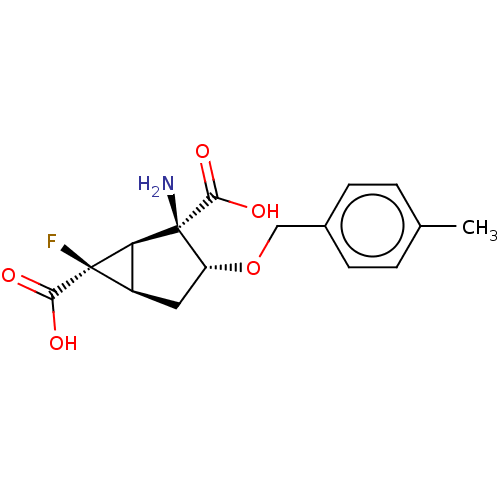 ((1R,2R,3R,5R,6R)-2-amino-6-fluoro-3-[(4- methylphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136699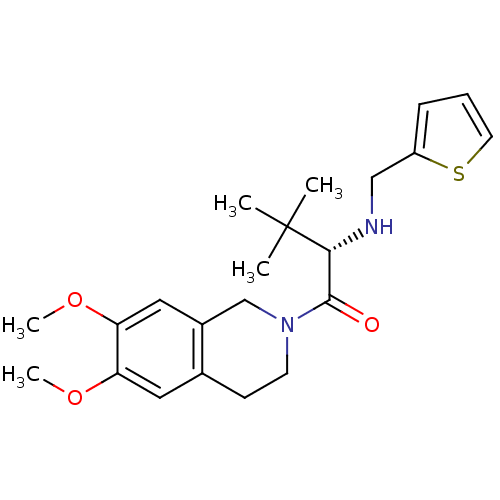 ((S)-1-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM446739 ((1R,2R,3R,5R,6R)-2-amino-6-fluoro-3-[(3- fluorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136711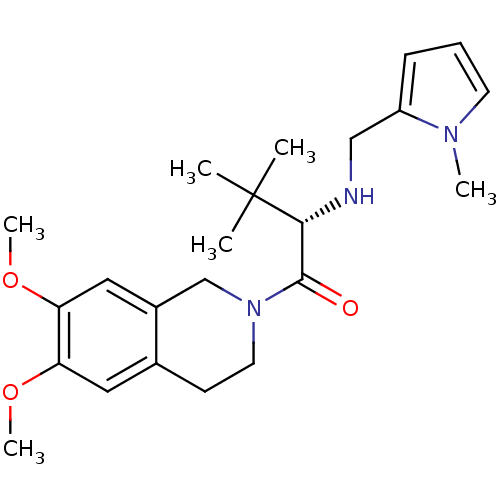 ((S)-1-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM446818 ((1R,2R,3R,5R,6R)-2-amino-3-[(5-chloropyridin-2- yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136714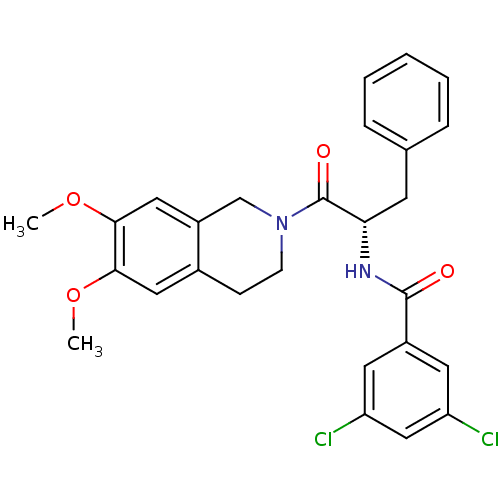 ((S)-3,5-dichloro-N-(1-(6,7-dimethoxy-3,4-dihydrois...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM446836 ((1S,2R,3R,5R,6S)-2-amino-3-[(3,4- difluorophenyl)m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM446784 ((1R,2R,3R,5R,6R)-2-amino-3-[(6-chloropyridin-2- yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM446857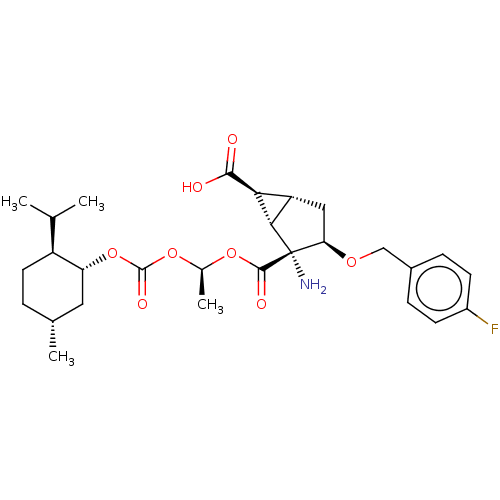 (US10689327, Compound (II)-15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32.7 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136718 ((S)-1-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136720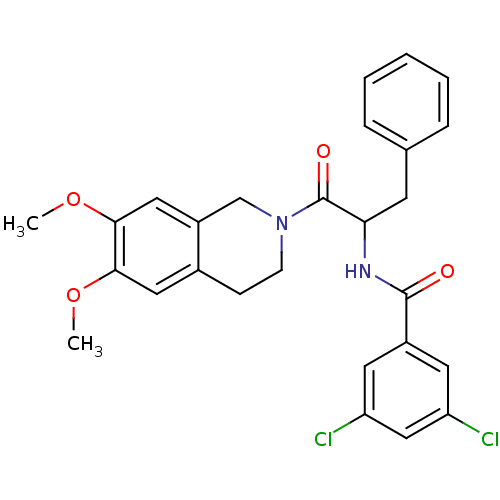 (CHEMBL343551 | N-[1-Benzyl-2-(6,7-dimethoxy-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM446699 (US10689327, Compound (II)-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM446699 (US10689327, Compound (II)-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM446832 ((1R,2R,3R,5R,6R)-2-amino-6-fluoro-3-[(2-oxo-1,2- d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136694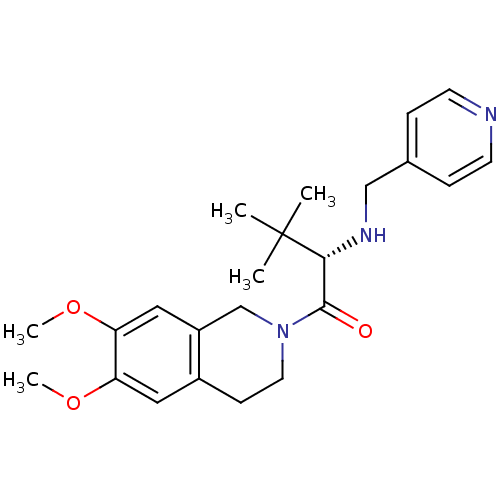 ((S)-1-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM446817 ((1R,2R,3R,5R,6R)-2-amino-3-[(6-chloropyridin-3- yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49.7 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM446817 ((1R,2R,3R,5R,6R)-2-amino-3-[(6-chloropyridin-3- yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM446828 ((1R,2R,3R,5R,6R)-2-amino-6-fluoro-3-[(4- methylphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 54.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136693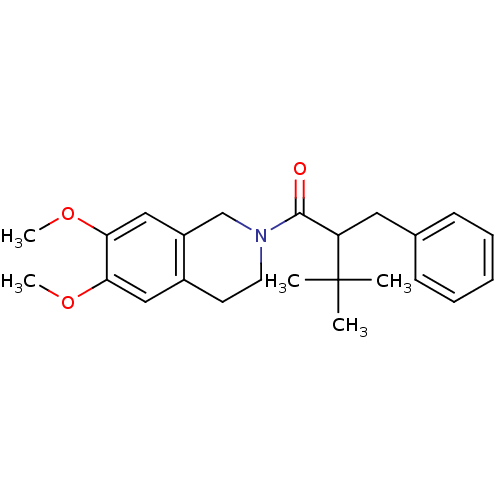 ((+/-)-2-benzyl-1-(6,7-dimethoxy-3,4-dihydroisoquin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136701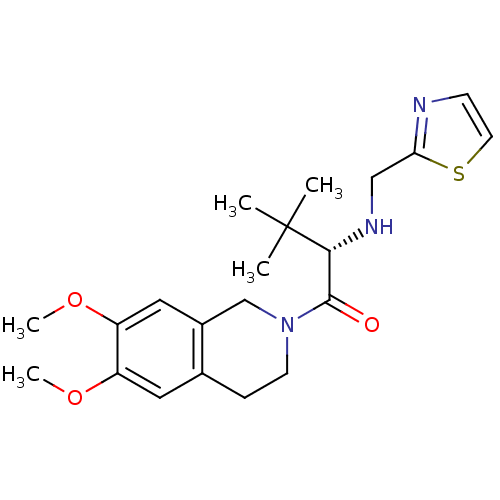 ((S)-1-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM446857 (US10689327, Compound (II)-15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50151462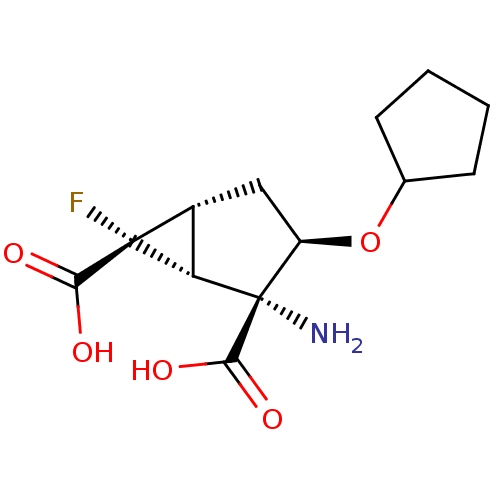 ((1R,2R,3R,5R,6R)-2-Amino-3-cyclopentyloxy-6-fluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 62.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136703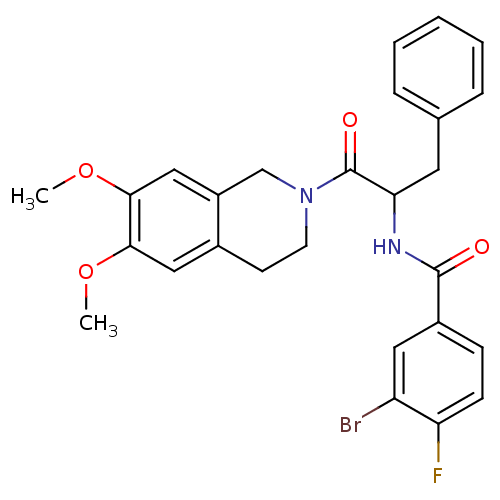 ((+/-)-3-bromo-N-(1-(6,7-dimethoxy-3,4-dihydroisoqu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM446702 (US10689327, Compound (II)-3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 75.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM446739 ((1R,2R,3R,5R,6R)-2-amino-6-fluoro-3-[(3- fluorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 75.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50151462 ((1R,2R,3R,5R,6R)-2-Amino-3-cyclopentyloxy-6-fluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 97.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM446756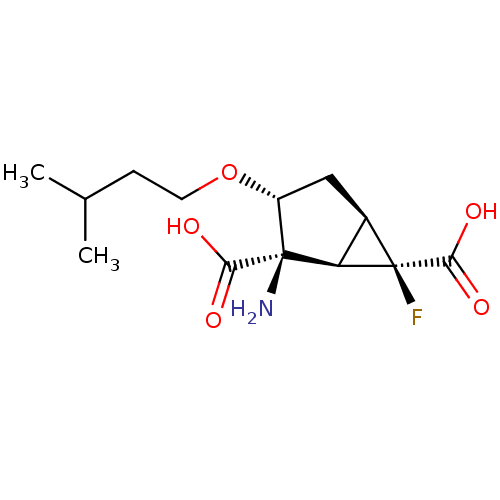 ((1R,2R,3R,5R,6R)-2-amino-6-fluoro-3-(3- methylbuto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 98.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136698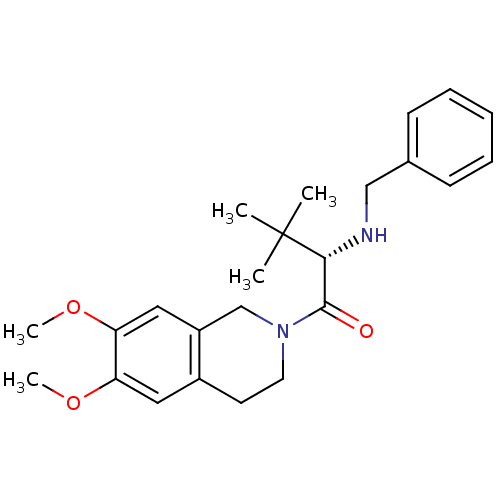 ((S)-2-(benzylamino)-1-(6,7-dimethoxy-3,4-dihydrois...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM446756 ((1R,2R,3R,5R,6R)-2-amino-6-fluoro-3-(3- methylbuto...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136710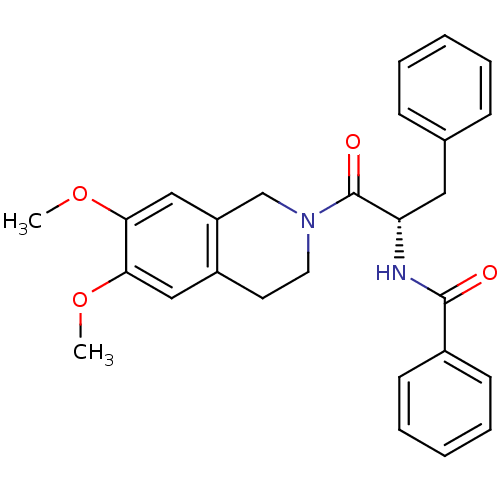 ((S)-N-(1-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136719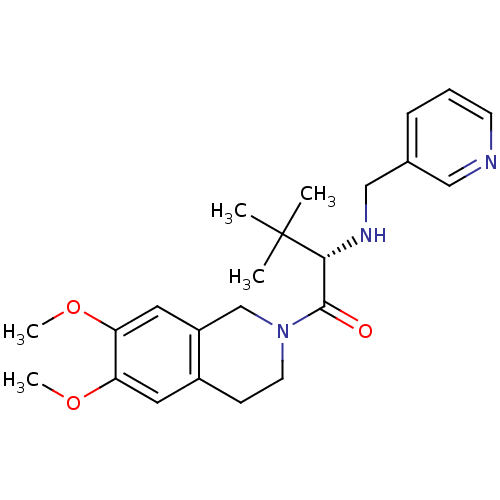 ((S)-1-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM446718 ((1R,2R,3R,5R,6R)-2-amino-6-fluoro-3-{[4- (triflour...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM446702 (US10689327, Compound (II)-3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 321 | n/a | n/a | n/a | n/a | n/a | n/a |
TAISHO PHARMACEUTICAL CO., LTD. US Patent | Assay Description CHO cells stably expressing human metabotropic glutamate receptors mGluR2 and mGluR3 were cultured at 37° C. under 5% CO2 using a Dulbecco's modi... | US Patent US10689327 (2020) BindingDB Entry DOI: 10.7270/Q2FR00NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136702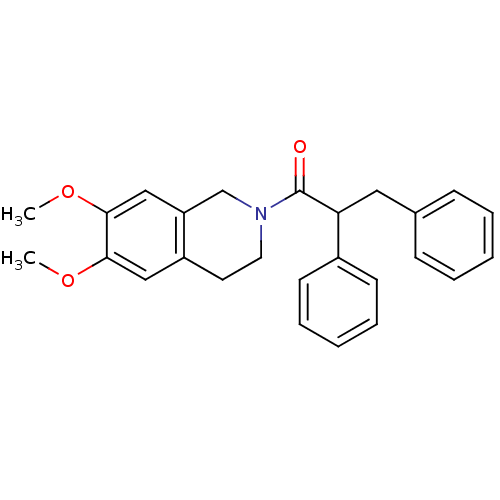 ((+/-)-1-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 395 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136705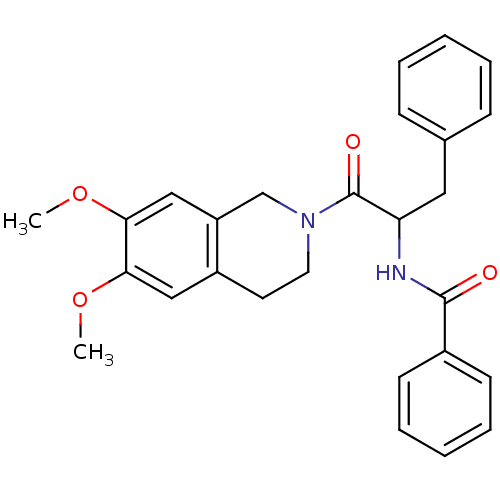 ((+/-)-N-(1-(6,7-dimethoxy-3,4-dihydroisoquinolin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136708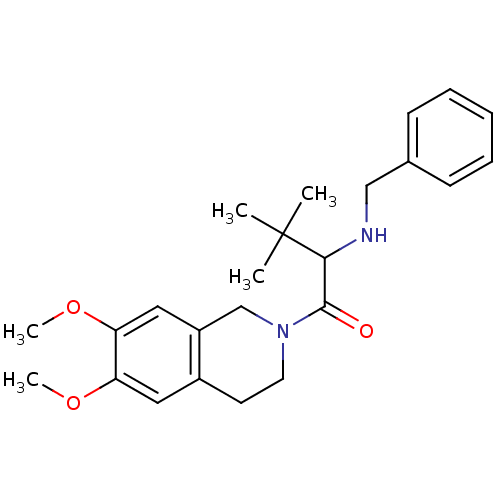 ((+/-)-2-(benzylamino)-1-(6,7-dimethoxy-3,4-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50136699 ((S)-1-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-1 receptor (hOX1R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136695 ((S)-1-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136717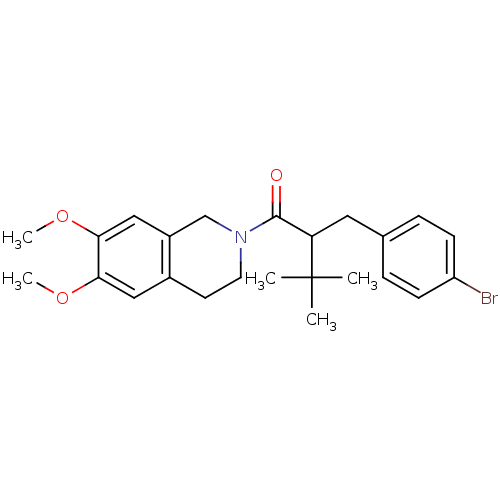 ((+/-)-2-(4-bromobenzyl)-1-(6,7-dimethoxy-3,4-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50136704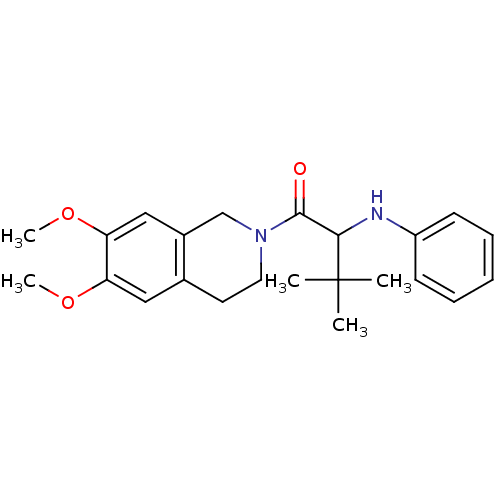 ((+/-)-1-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-2 receptor (hOX2R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50136714 ((S)-3,5-dichloro-N-(1-(6,7-dimethoxy-3,4-dihydrois...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human orexin-1 receptor (hOX1R) | Bioorg Med Chem Lett 13: 4497-9 (2003) BindingDB Entry DOI: 10.7270/Q20R9NT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 105 total ) | Next | Last >> |