 Found 355 hits with Last Name = 'haynes' and Initial = 'n'
Found 355 hits with Last Name = 'haynes' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100437
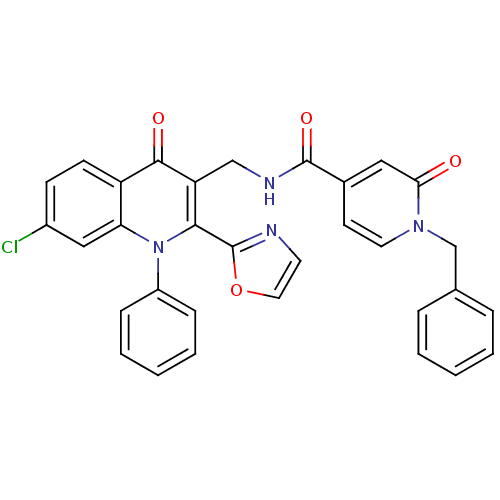
(US8501732, I-1)Show SMILES Clc1ccc2c(c1)n(-c1ccccc1)c(-c1ncco1)c(CNC(=O)c1ccn(Cc3ccccc3)c(=O)c1)c2=O Show InChI InChI=1S/C32H23ClN4O4/c33-23-11-12-25-27(18-23)37(24-9-5-2-6-10-24)29(32-34-14-16-41-32)26(30(25)39)19-35-31(40)22-13-15-36(28(38)17-22)20-21-7-3-1-4-8-21/h1-18H,19-20H2,(H,35,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100549
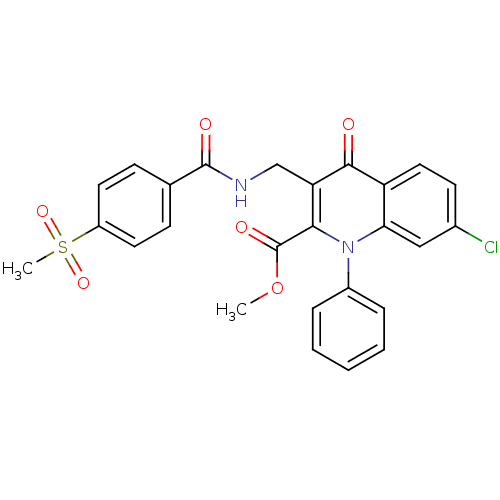
(US8501732, I-109)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(cc2)S(C)(=O)=O)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C26H21ClN2O6S/c1-35-26(32)23-21(15-28-25(31)16-8-11-19(12-9-16)36(2,33)34)24(30)20-13-10-17(27)14-22(20)29(23)18-6-4-3-5-7-18/h3-14H,15H2,1-2H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100550
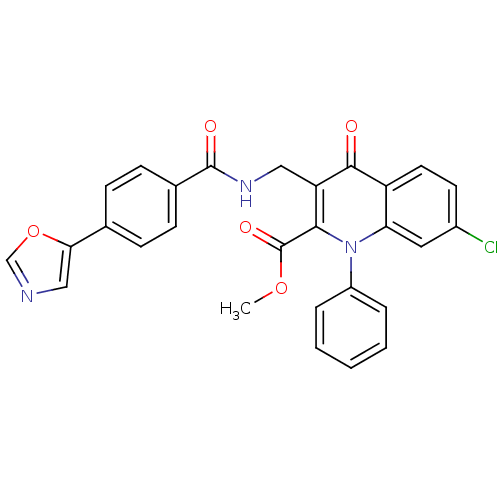
(US8501732, I-110)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(cc2)-c2cnco2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C28H20ClN3O5/c1-36-28(35)25-22(14-31-27(34)18-9-7-17(8-10-18)24-15-30-16-37-24)26(33)21-12-11-19(29)13-23(21)32(25)20-5-3-2-4-6-20/h2-13,15-16H,14H2,1H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100445
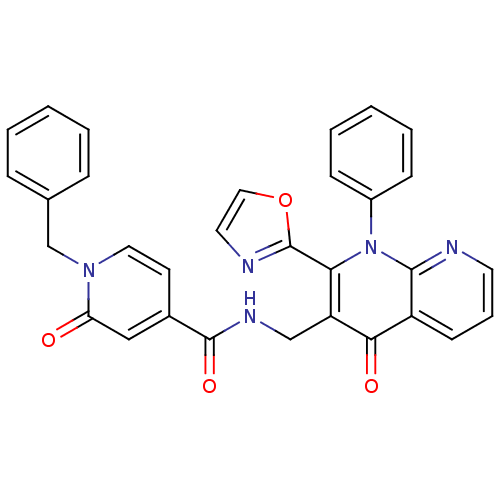
(US8501732, I-9)Show SMILES O=C(NCc1c(-c2ncco2)n(-c2ccccc2)c2ncccc2c1=O)c1ccn(Cc2ccccc2)c(=O)c1 Show InChI InChI=1S/C31H23N5O4/c37-26-18-22(13-16-35(26)20-21-8-3-1-4-9-21)30(39)34-19-25-27(31-33-15-17-40-31)36(23-10-5-2-6-11-23)29-24(28(25)38)12-7-14-32-29/h1-18H,19-20H2,(H,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018759
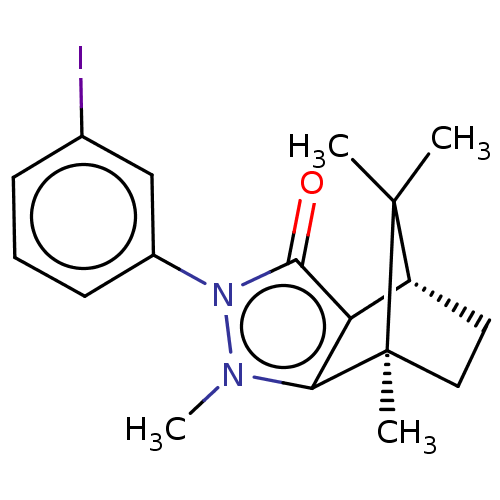
(CHEMBL3291350)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1cccc(I)c1)n3C)C2(C)C |r| Show InChI InChI=1S/C18H21IN2O/c1-17(2)13-8-9-18(17,3)15-14(13)16(22)21(20(15)4)12-7-5-6-11(19)10-12/h5-7,10,13H,8-9H2,1-4H3/t13-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018760
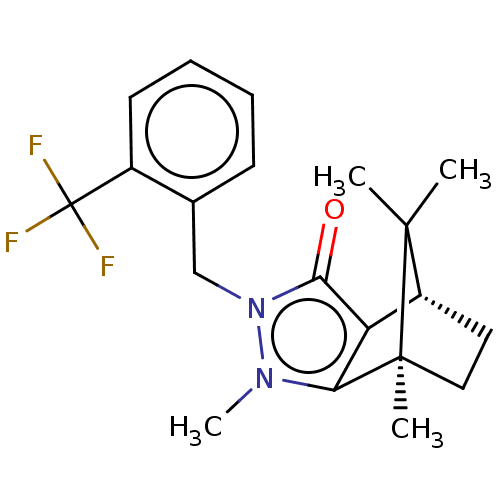
(CHEMBL3291357)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(Cc1ccccc1C(F)(F)F)n3C)C2(C)C |r| Show InChI InChI=1S/C20H23F3N2O/c1-18(2)14-9-10-19(18,3)16-15(14)17(26)25(24(16)4)11-12-7-5-6-8-13(12)20(21,22)23/h5-8,14H,9-11H2,1-4H3/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100609
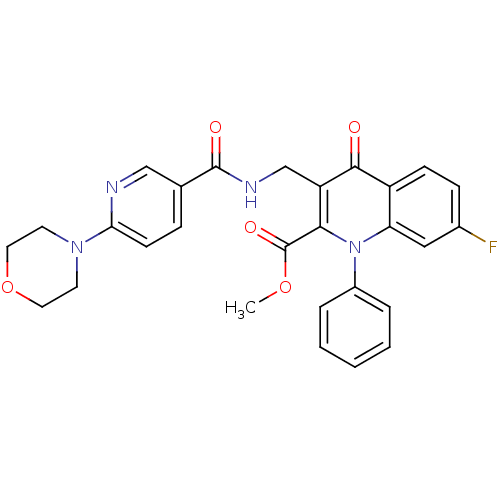
(US8501732, I-169)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(nc2)N2CCOCC2)c(=O)c2ccc(F)cc2n1-c1ccccc1 Show InChI InChI=1S/C28H25FN4O5/c1-37-28(36)25-22(17-31-27(35)18-7-10-24(30-16-18)32-11-13-38-14-12-32)26(34)21-9-8-19(29)15-23(21)33(25)20-5-3-2-4-6-20/h2-10,15-16H,11-14,17H2,1H3,(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018761
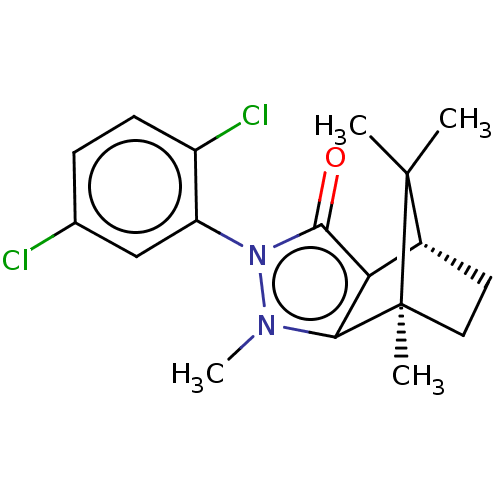
(CHEMBL3291348)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1cc(Cl)ccc1Cl)n3C)C2(C)C |r,wU:4.4,1.0,(16.87,-14.9,;16.87,-13.4,;18.16,-12.66,;18.16,-11.16,;16.87,-10.41,;16.45,-8.98,;15.58,-11.16,;15.58,-12.66,;14.16,-13.12,;13.7,-14.54,;13.28,-11.91,;11.73,-11.9,;10.97,-13.24,;9.43,-13.25,;8.66,-14.58,;8.65,-11.9,;9.43,-10.56,;10.97,-10.57,;11.75,-9.23,;14.16,-10.7,;13.68,-9.23,;17.73,-11.9,;19.22,-12.76,;19.22,-11.04,)| Show InChI InChI=1S/C18H20Cl2N2O/c1-17(2)11-7-8-18(17,3)15-14(11)16(23)22(21(15)4)13-9-10(19)5-6-12(13)20/h5-6,9,11H,7-8H2,1-4H3/t11-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100551
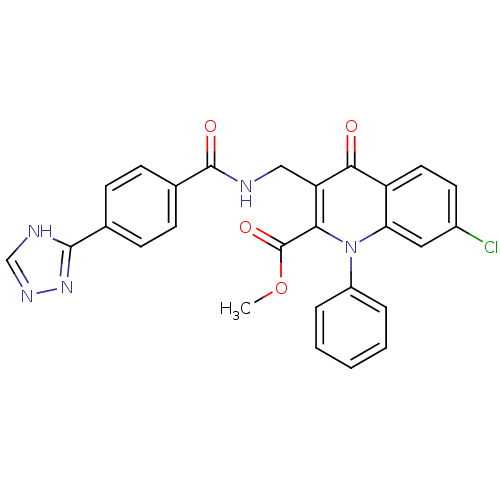
(US8501732, I-111)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(cc2)-c2nnc[nH]2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C27H20ClN5O4/c1-37-27(36)23-21(14-29-26(35)17-9-7-16(8-10-17)25-30-15-31-32-25)24(34)20-12-11-18(28)13-22(20)33(23)19-5-3-2-4-6-19/h2-13,15H,14H2,1H3,(H,29,35)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100573
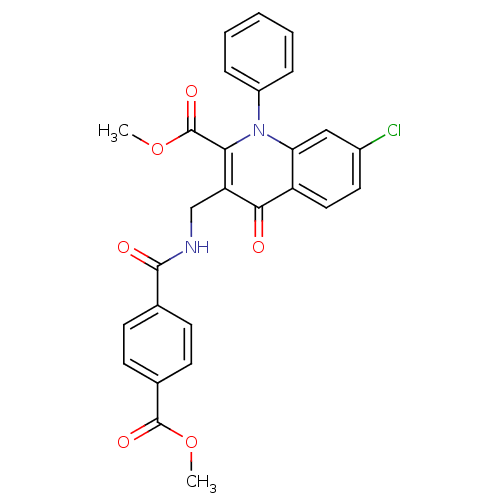
(US8501732, I-133)Show SMILES COC(=O)c1ccc(cc1)C(=O)NCc1c(C(=O)OC)n(-c2ccccc2)c2cc(Cl)ccc2c1=O Show InChI InChI=1S/C27H21ClN2O6/c1-35-26(33)17-10-8-16(9-11-17)25(32)29-15-21-23(27(34)36-2)30(19-6-4-3-5-7-19)22-14-18(28)12-13-20(22)24(21)31/h3-14H,15H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100552
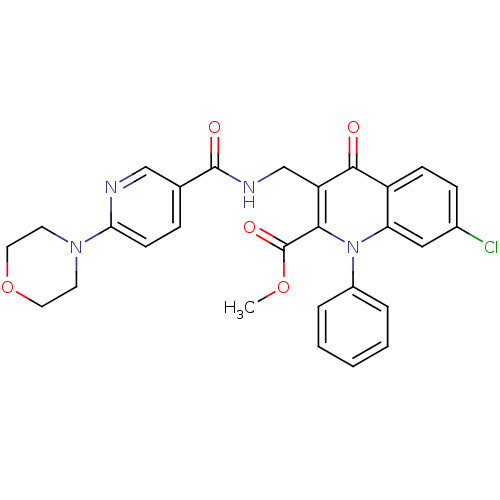
(US8501732, I-112)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(nc2)N2CCOCC2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C28H25ClN4O5/c1-37-28(36)25-22(17-31-27(35)18-7-10-24(30-16-18)32-11-13-38-14-12-32)26(34)21-9-8-19(29)15-23(21)33(25)20-5-3-2-4-6-20/h2-10,15-16H,11-14,17H2,1H3,(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018793
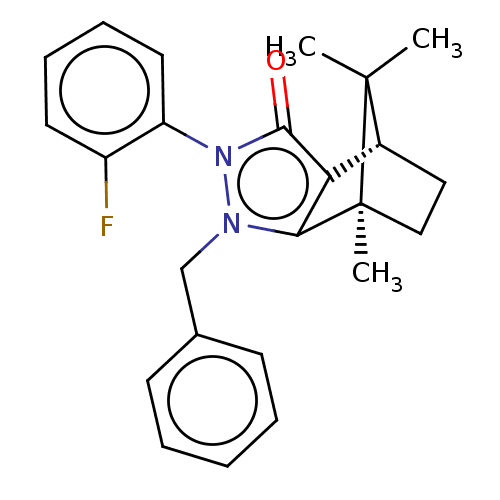
(CHEMBL3291344)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3Cc1ccccc1)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.96,-10.25,;20.96,-8.75,;19.67,-8,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.95,-10.71,;16.5,-12.13,;16.08,-9.5,;14.53,-9.5,;13.77,-10.83,;12.23,-10.84,;11.45,-9.49,;12.23,-8.16,;13.77,-8.16,;14.55,-6.83,;16.96,-8.29,;16.48,-6.82,;17.51,-5.68,;19.01,-6,;20.04,-4.85,;19.56,-3.38,;18.04,-3.07,;17.02,-4.22,;20.53,-9.49,;22.02,-10.35,;22.02,-8.63,)| Show InChI InChI=1S/C24H25FN2O/c1-23(2)17-13-14-24(23,3)21-20(17)22(28)27(19-12-8-7-11-18(19)25)26(21)15-16-9-5-4-6-10-16/h4-12,17H,13-15H2,1-3H3/t17-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018761

(CHEMBL3291348)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1cc(Cl)ccc1Cl)n3C)C2(C)C |r,wU:4.4,1.0,(16.87,-14.9,;16.87,-13.4,;18.16,-12.66,;18.16,-11.16,;16.87,-10.41,;16.45,-8.98,;15.58,-11.16,;15.58,-12.66,;14.16,-13.12,;13.7,-14.54,;13.28,-11.91,;11.73,-11.9,;10.97,-13.24,;9.43,-13.25,;8.66,-14.58,;8.65,-11.9,;9.43,-10.56,;10.97,-10.57,;11.75,-9.23,;14.16,-10.7,;13.68,-9.23,;17.73,-11.9,;19.22,-12.76,;19.22,-11.04,)| Show InChI InChI=1S/C18H20Cl2N2O/c1-17(2)11-7-8-18(17,3)15-14(11)16(23)22(21(15)4)13-9-10(19)5-6-12(13)20/h5-6,9,11H,7-8H2,1-4H3/t11-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100553
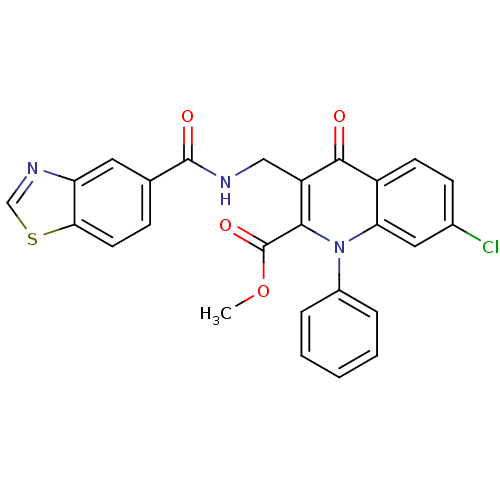
(US8501732, I-113)Show SMILES COC(=O)c1c(CNC(=O)c2ccc3scnc3c2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C26H18ClN3O4S/c1-34-26(33)23-19(13-28-25(32)15-7-10-22-20(11-15)29-14-35-22)24(31)18-9-8-16(27)12-21(18)30(23)17-5-3-2-4-6-17/h2-12,14H,13H2,1H3,(H,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100448
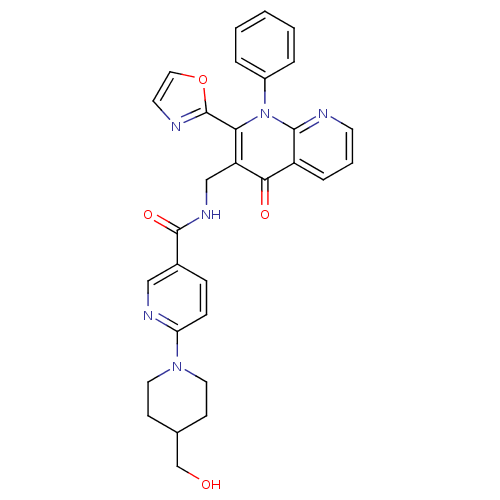
(US8501732, I-12)Show SMILES OCC1CCN(CC1)c1ccc(cn1)C(=O)NCc1c(-c2ncco2)n(-c2ccccc2)c2ncccc2c1=O Show InChI InChI=1S/C30H28N6O4/c37-19-20-10-14-35(15-11-20)25-9-8-21(17-33-25)29(39)34-18-24-26(30-32-13-16-40-30)36(22-5-2-1-3-6-22)28-23(27(24)38)7-4-12-31-28/h1-9,12-13,16-17,20,37H,10-11,14-15,18-19H2,(H,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100574
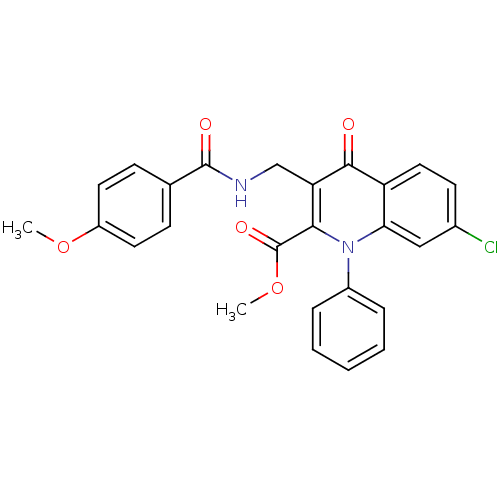
(US8501732, I-134)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(OC)cc2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C26H21ClN2O5/c1-33-19-11-8-16(9-12-19)25(31)28-15-21-23(26(32)34-2)29(18-6-4-3-5-7-18)22-14-17(27)10-13-20(22)24(21)30/h3-14H,15H2,1-2H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100438

(US8501732, I-2)Show SMILES Clc1ccc2c(c1)n(-c1ccccc1)c(-c1ncco1)c(CNC(=O)c1ccnc(c1)N1CCOCC1)c2=O Show InChI InChI=1S/C29H24ClN5O4/c30-20-6-7-22-24(17-20)35(21-4-2-1-3-5-21)26(29-32-10-13-39-29)23(27(22)36)18-33-28(37)19-8-9-31-25(16-19)34-11-14-38-15-12-34/h1-10,13,16-17H,11-12,14-15,18H2,(H,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100554
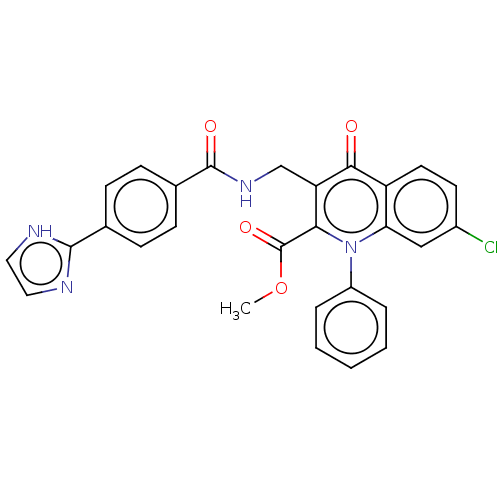
(US8501732, I-114)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(cc2)-c2ncc[nH]2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C28H21ClN4O4/c1-37-28(36)24-22(16-32-27(35)18-9-7-17(8-10-18)26-30-13-14-31-26)25(34)21-12-11-19(29)15-23(21)33(24)20-5-3-2-4-6-20/h2-15H,16H2,1H3,(H,30,31)(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018759

(CHEMBL3291350)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1cccc(I)c1)n3C)C2(C)C |r| Show InChI InChI=1S/C18H21IN2O/c1-17(2)13-8-9-18(17,3)15-14(13)16(22)21(20(15)4)12-7-5-6-11(19)10-12/h5-7,10,13H,8-9H2,1-4H3/t13-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100575
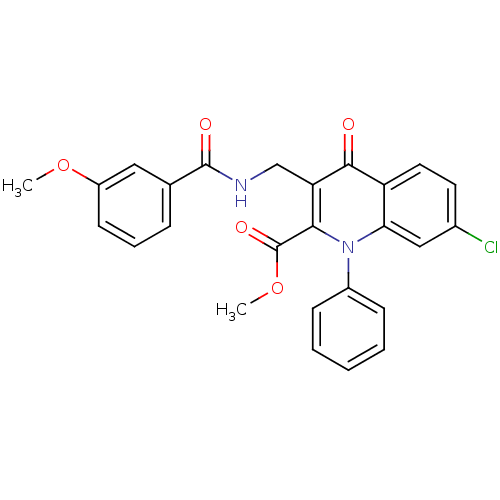
(US8501732, I-135)Show SMILES COC(=O)c1c(CNC(=O)c2cccc(OC)c2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C26H21ClN2O5/c1-33-19-10-6-7-16(13-19)25(31)28-15-21-23(26(32)34-2)29(18-8-4-3-5-9-18)22-14-17(27)11-12-20(22)24(21)30/h3-14H,15H2,1-2H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100556
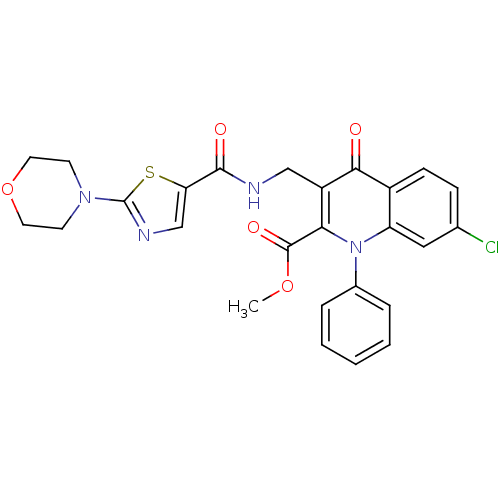
(US8501732, I-116)Show SMILES COC(=O)c1c(CNC(=O)c2cnc(s2)N2CCOCC2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C26H23ClN4O5S/c1-35-25(34)22-19(14-28-24(33)21-15-29-26(37-21)30-9-11-36-12-10-30)23(32)18-8-7-16(27)13-20(18)31(22)17-5-3-2-4-6-17/h2-8,13,15H,9-12,14H2,1H3,(H,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100555
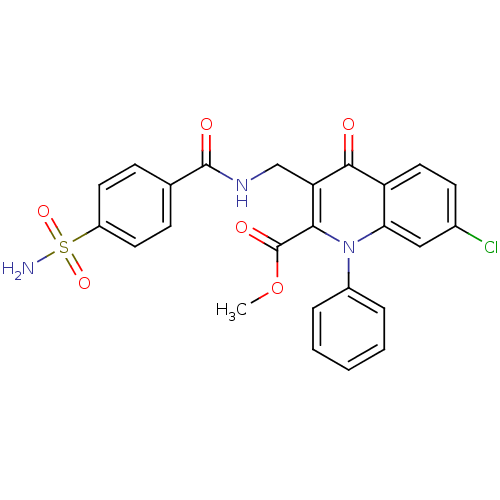
(US8501732, I-115)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(cc2)S(N)(=O)=O)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C25H20ClN3O6S/c1-35-25(32)22-20(14-28-24(31)15-7-10-18(11-8-15)36(27,33)34)23(30)19-12-9-16(26)13-21(19)29(22)17-5-3-2-4-6-17/h2-13H,14H2,1H3,(H,28,31)(H2,27,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018795
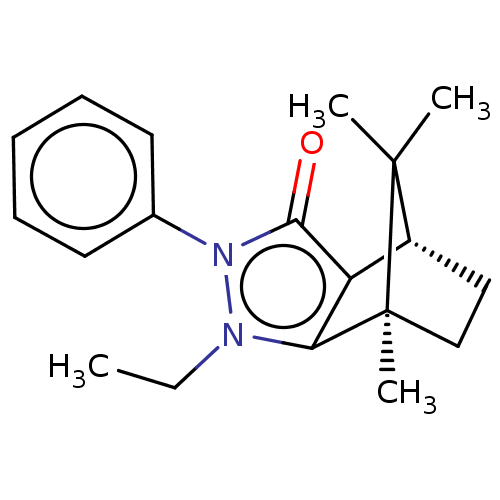
(CHEMBL3291339)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1)n3CC)C2(C)C |r| Show InChI InChI=1S/C19H24N2O/c1-5-20-16-15(14-11-12-19(16,4)18(14,2)3)17(22)21(20)13-9-7-6-8-10-13/h6-10,14H,5,11-12H2,1-4H3/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100439
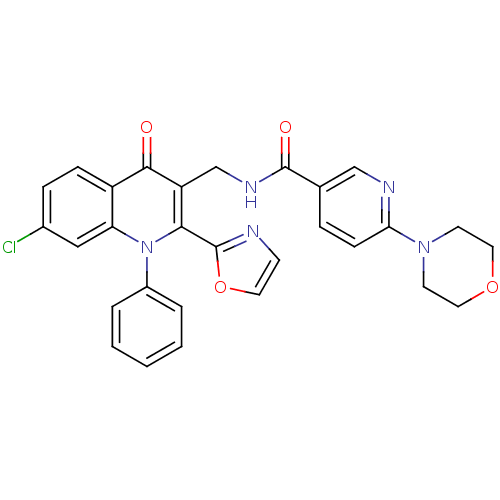
(US8501732, I-3)Show SMILES Clc1ccc2c(c1)n(-c1ccccc1)c(-c1ncco1)c(CNC(=O)c1ccc(nc1)N1CCOCC1)c2=O Show InChI InChI=1S/C29H24ClN5O4/c30-20-7-8-22-24(16-20)35(21-4-2-1-3-5-21)26(29-31-10-13-39-29)23(27(22)36)18-33-28(37)19-6-9-25(32-17-19)34-11-14-38-15-12-34/h1-10,13,16-17H,11-12,14-15,18H2,(H,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018769
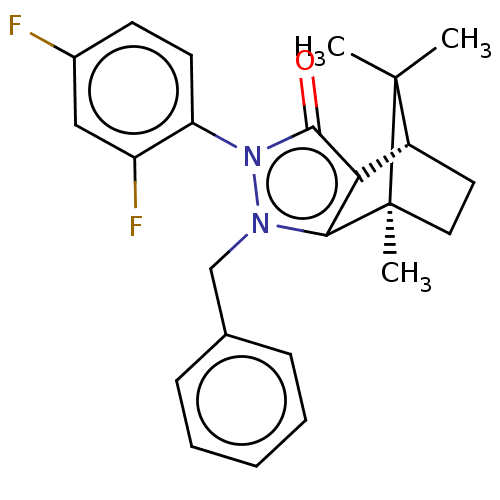
(CHEMBL3291346)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccc(F)cc1F)n3Cc1ccccc1)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.96,-10.25,;20.96,-8.75,;19.67,-8,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.95,-10.71,;16.5,-12.13,;16.08,-9.5,;14.53,-9.5,;13.77,-10.83,;12.23,-10.84,;11.45,-9.49,;9.91,-9.49,;12.23,-8.16,;13.77,-8.16,;14.55,-6.83,;16.96,-8.29,;16.48,-6.82,;17.51,-5.68,;19.01,-6,;20.04,-4.85,;19.56,-3.38,;18.04,-3.07,;17.02,-4.22,;20.53,-9.49,;22.02,-10.35,;22.02,-8.63,)| Show InChI InChI=1S/C24H24F2N2O/c1-23(2)17-11-12-24(23,3)21-20(17)22(29)28(19-10-9-16(25)13-18(19)26)27(21)14-15-7-5-4-6-8-15/h4-10,13,17H,11-12,14H2,1-3H3/t17-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018797
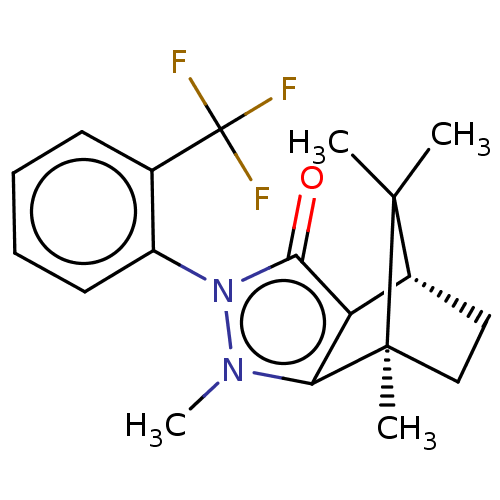
(CHEMBL3291345)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1C(F)(F)F)n3C)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;13.37,-6.66,;14.46,-5.57,;11.88,-6.27,;12.97,-5.16,;16.96,-8.3,;16.48,-6.83,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C19H21F3N2O/c1-17(2)12-9-10-18(17,3)15-14(12)16(25)24(23(15)4)13-8-6-5-7-11(13)19(20,21)22/h5-8,12H,9-10H2,1-4H3/t12-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018760

(CHEMBL3291357)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(Cc1ccccc1C(F)(F)F)n3C)C2(C)C |r| Show InChI InChI=1S/C20H23F3N2O/c1-18(2)14-9-10-19(18,3)16-15(14)17(26)25(24(16)4)11-12-7-5-6-8-13(12)20(21,22)23/h5-8,14H,9-11H2,1-4H3/t14-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100610
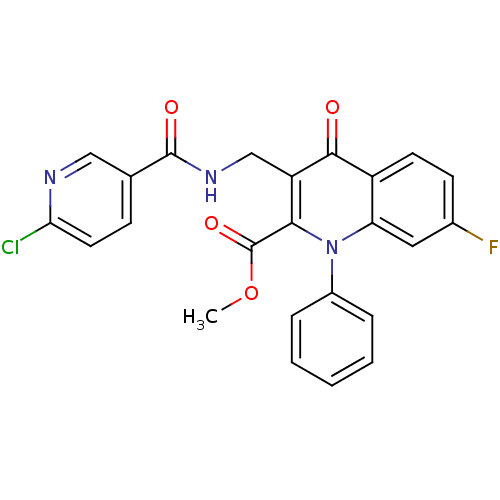
(US8501732, I-170)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(Cl)nc2)c(=O)c2ccc(F)cc2n1-c1ccccc1 Show InChI InChI=1S/C24H17ClFN3O4/c1-33-24(32)21-18(13-28-23(31)14-7-10-20(25)27-12-14)22(30)17-9-8-15(26)11-19(17)29(21)16-5-3-2-4-6-16/h2-12H,13H2,1H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100557
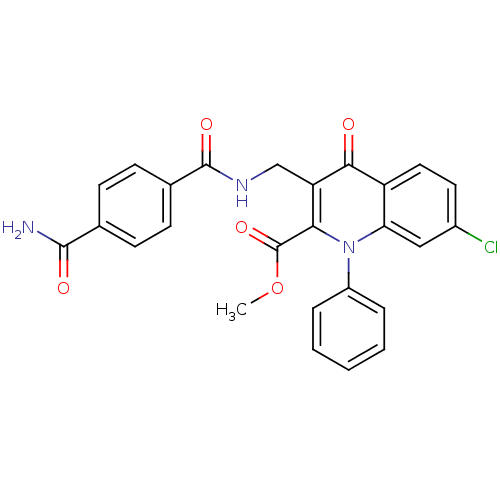
(US8501732, I-117)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(cc2)C(N)=O)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C26H20ClN3O5/c1-35-26(34)22-20(14-29-25(33)16-9-7-15(8-10-16)24(28)32)23(31)19-12-11-17(27)13-21(19)30(22)18-5-3-2-4-6-18/h2-13H,14H2,1H3,(H2,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018794
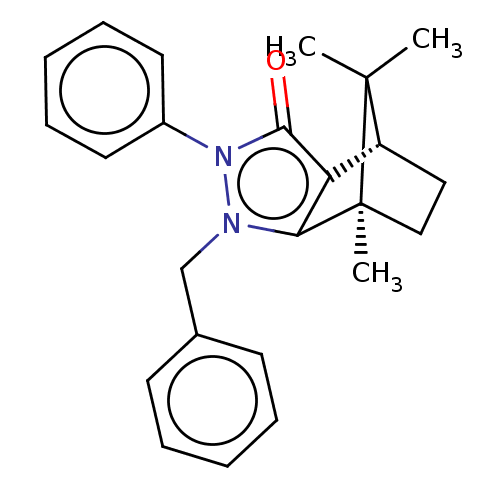
(CHEMBL3291340)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1)n3Cc1ccccc1)C2(C)C |r| Show InChI InChI=1S/C24H26N2O/c1-23(2)19-14-15-24(23,3)21-20(19)22(27)26(18-12-8-5-9-13-18)25(21)16-17-10-6-4-7-11-17/h4-13,19H,14-16H2,1-3H3/t19-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100502

(US8501732, I-62)Show SMILES CC(C)(C)OC(=O)N1CC2CC1CN2c1ccc(cn1)C(=O)NCc1cn(-c2ccccc2)c2cc(Cl)ccc2c1=O |THB:5:7:12.13:10,14:13:7.8:10| Show InChI InChI=1S/C32H32ClN5O4/c1-32(2,3)42-31(41)38-19-24-14-25(38)18-37(24)28-12-9-20(15-34-28)30(40)35-16-21-17-36(23-7-5-4-6-8-23)27-13-22(33)10-11-26(27)29(21)39/h4-13,15,17,24-25H,14,16,18-19H2,1-3H3,(H,35,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100558
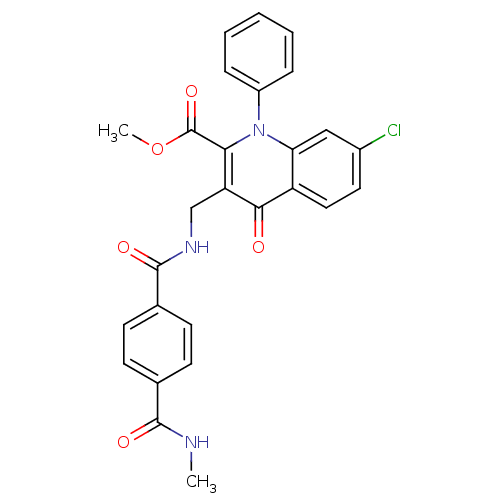
(US8501732, I-118)Show SMILES CNC(=O)c1ccc(cc1)C(=O)NCc1c(C(=O)OC)n(-c2ccccc2)c2cc(Cl)ccc2c1=O Show InChI InChI=1S/C27H22ClN3O5/c1-29-25(33)16-8-10-17(11-9-16)26(34)30-15-21-23(27(35)36-2)31(19-6-4-3-5-7-19)22-14-18(28)12-13-20(22)24(21)32/h3-14H,15H2,1-2H3,(H,29,33)(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018793

(CHEMBL3291344)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3Cc1ccccc1)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.96,-10.25,;20.96,-8.75,;19.67,-8,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.95,-10.71,;16.5,-12.13,;16.08,-9.5,;14.53,-9.5,;13.77,-10.83,;12.23,-10.84,;11.45,-9.49,;12.23,-8.16,;13.77,-8.16,;14.55,-6.83,;16.96,-8.29,;16.48,-6.82,;17.51,-5.68,;19.01,-6,;20.04,-4.85,;19.56,-3.38,;18.04,-3.07,;17.02,-4.22,;20.53,-9.49,;22.02,-10.35,;22.02,-8.63,)| Show InChI InChI=1S/C24H25FN2O/c1-23(2)17-13-14-24(23,3)21-20(17)22(28)27(19-12-8-7-11-18(19)25)26(21)15-16-9-5-4-6-10-16/h4-12,17H,13-15H2,1-3H3/t17-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50392983
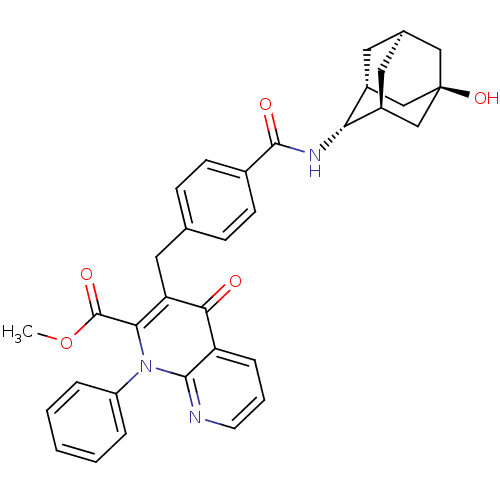
(CHEMBL2152383)Show SMILES COC(=O)c1c(Cc2ccc(cc2)C(=O)N[C@H]2[C@H]3C[C@H]4C[C@@H]2C[C@](O)(C4)C3)c(=O)c2cccnc2n1-c1ccccc1 |r,TLB:26:23:20:16.17.18,THB:18:17:22:20.19.25,18:19:16.17.26:22,15:16:22:20.19.25,24:23:20:16.17.18| Show InChI InChI=1S/C34H33N3O5/c1-42-33(40)29-27(30(38)26-8-5-13-35-31(26)37(29)25-6-3-2-4-7-25)16-20-9-11-22(12-10-20)32(39)36-28-23-14-21-15-24(28)19-34(41,17-21)18-23/h2-13,21,23-24,28,41H,14-19H2,1H3,(H,36,39)/t21-,23-,24+,28-,34- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 after 30 mins by TR-FRET assay |
ACS Med Chem Lett 3: 764-768 (2012)
Article DOI: 10.1021/ml300175c
BindingDB Entry DOI: 10.7270/Q22808P8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100590

(US8501732, I-150)Show SMILES COC(=O)c1c(CNC(=O)c2ccccc2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C25H19ClN2O4/c1-32-25(31)22-20(15-27-24(30)16-8-4-2-5-9-16)23(29)19-13-12-17(26)14-21(19)28(22)18-10-6-3-7-11-18/h2-14H,15H2,1H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100576
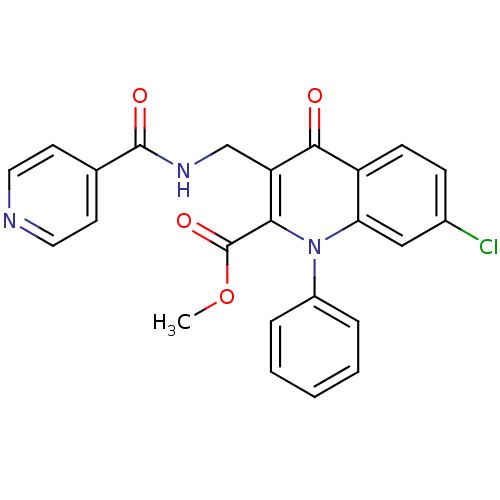
(US8501732, I-136)Show SMILES COC(=O)c1c(CNC(=O)c2ccncc2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C24H18ClN3O4/c1-32-24(31)21-19(14-27-23(30)15-9-11-26-12-10-15)22(29)18-8-7-16(25)13-20(18)28(21)17-5-3-2-4-6-17/h2-13H,14H2,1H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100577

(US8501732, I-137)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(F)c(F)c2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C25H17ClF2N2O4/c1-34-25(33)22-18(13-29-24(32)14-7-10-19(27)20(28)11-14)23(31)17-9-8-15(26)12-21(17)30(22)16-5-3-2-4-6-16/h2-12H,13H2,1H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50392984
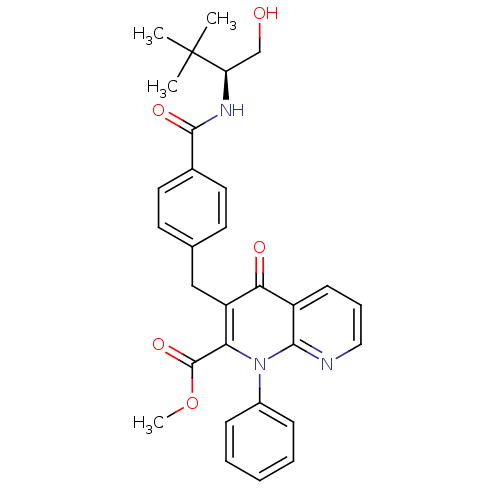
(CHEMBL2152384)Show SMILES COC(=O)c1c(Cc2ccc(cc2)C(=O)N[C@H](CO)C(C)(C)C)c(=O)c2cccnc2n1-c1ccccc1 |r| Show InChI InChI=1S/C30H31N3O5/c1-30(2,3)24(18-34)32-28(36)20-14-12-19(13-15-20)17-23-25(29(37)38-4)33(21-9-6-5-7-10-21)27-22(26(23)35)11-8-16-31-27/h5-16,24,34H,17-18H2,1-4H3,(H,32,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 after 30 mins by TR-FRET assay |
ACS Med Chem Lett 3: 764-768 (2012)
Article DOI: 10.1021/ml300175c
BindingDB Entry DOI: 10.7270/Q22808P8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018769

(CHEMBL3291346)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccc(F)cc1F)n3Cc1ccccc1)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.96,-10.25,;20.96,-8.75,;19.67,-8,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.95,-10.71,;16.5,-12.13,;16.08,-9.5,;14.53,-9.5,;13.77,-10.83,;12.23,-10.84,;11.45,-9.49,;9.91,-9.49,;12.23,-8.16,;13.77,-8.16,;14.55,-6.83,;16.96,-8.29,;16.48,-6.82,;17.51,-5.68,;19.01,-6,;20.04,-4.85,;19.56,-3.38,;18.04,-3.07,;17.02,-4.22,;20.53,-9.49,;22.02,-10.35,;22.02,-8.63,)| Show InChI InChI=1S/C24H24F2N2O/c1-23(2)17-11-12-24(23,3)21-20(17)22(29)28(19-10-9-16(25)13-18(19)26)27(21)14-15-7-5-4-6-8-15/h4-10,13,17H,11-12,14H2,1-3H3/t17-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018798
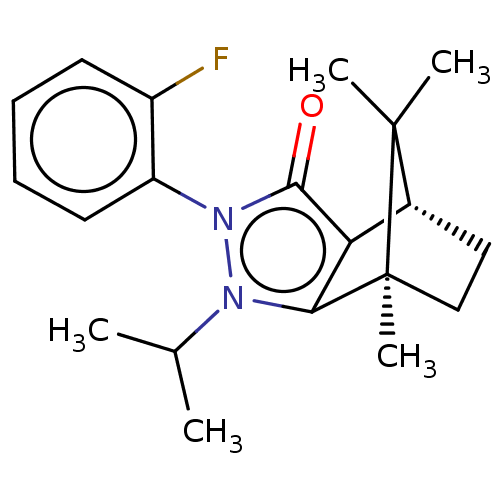
(CHEMBL3291342)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3C(C)C)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;14.55,-6.83,;16.96,-8.3,;16.48,-6.83,;17.51,-5.68,;15.7,-5.48,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C20H25FN2O/c1-12(2)22-17-16(13-10-11-20(17,5)19(13,3)4)18(24)23(22)15-9-7-6-8-14(15)21/h6-9,12-13H,10-11H2,1-5H3/t13-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018797

(CHEMBL3291345)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1C(F)(F)F)n3C)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;13.37,-6.66,;14.46,-5.57,;11.88,-6.27,;12.97,-5.16,;16.96,-8.3,;16.48,-6.83,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C19H21F3N2O/c1-17(2)12-9-10-18(17,3)15-14(12)16(25)24(23(15)4)13-8-6-5-7-11(13)19(20,21)22/h5-8,12H,9-10H2,1-4H3/t12-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100469
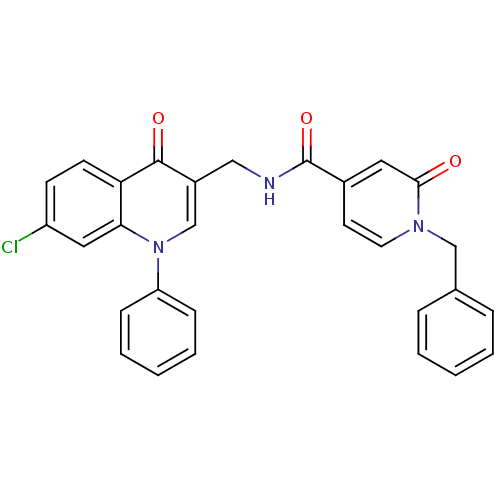
(US8501732, I-29)Show SMILES Clc1ccc2c(c1)n(cc(CNC(=O)c1ccn(Cc3ccccc3)c(=O)c1)c2=O)-c1ccccc1 Show InChI InChI=1S/C29H22ClN3O3/c30-23-11-12-25-26(16-23)33(24-9-5-2-6-10-24)19-22(28(25)35)17-31-29(36)21-13-14-32(27(34)15-21)18-20-7-3-1-4-8-20/h1-16,19H,17-18H2,(H,31,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100578
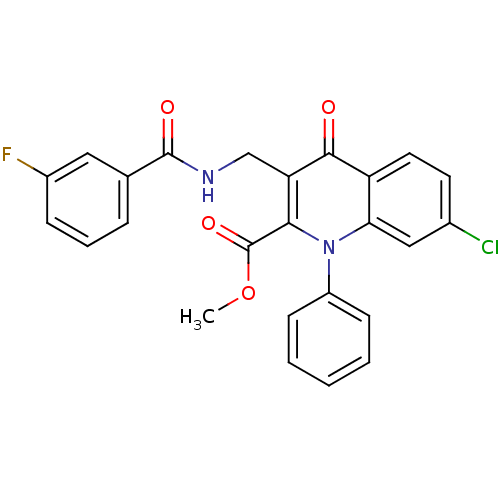
(US8501732, I-138)Show SMILES COC(=O)c1c(CNC(=O)c2cccc(F)c2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C25H18ClFN2O4/c1-33-25(32)22-20(14-28-24(31)15-6-5-7-17(27)12-15)23(30)19-11-10-16(26)13-21(19)29(22)18-8-3-2-4-9-18/h2-13H,14H2,1H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100579
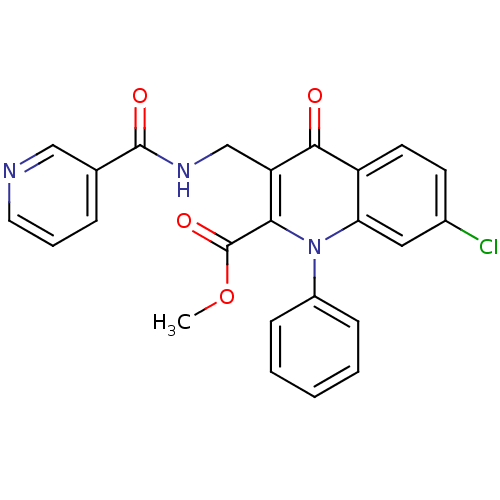
(US8501732, I-139)Show SMILES COC(=O)c1c(CNC(=O)c2cccnc2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C24H18ClN3O4/c1-32-24(31)21-19(14-27-23(30)15-6-5-11-26-13-15)22(29)18-10-9-16(25)12-20(18)28(21)17-7-3-2-4-8-17/h2-13H,14H2,1H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100559
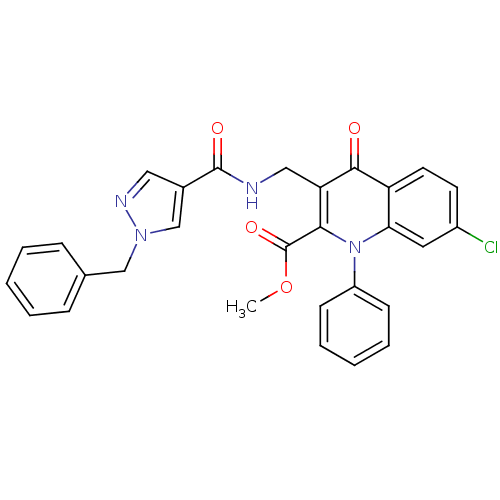
(US8501732, I-119)Show SMILES COC(=O)c1c(CNC(=O)c2cnn(Cc3ccccc3)c2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C29H23ClN4O4/c1-38-29(37)26-24(16-31-28(36)20-15-32-33(18-20)17-19-8-4-2-5-9-19)27(35)23-13-12-21(30)14-25(23)34(26)22-10-6-3-7-11-22/h2-15,18H,16-17H2,1H3,(H,31,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018799
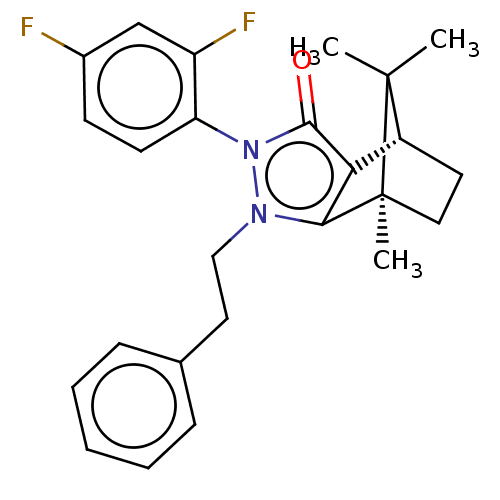
(CHEMBL3291347)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccc(F)cc1F)n3CCc1ccccc1)C2(C)C |r,wU:4.4,1.0,(16.88,-14.91,;16.88,-13.41,;18.18,-12.67,;18.18,-11.17,;16.89,-10.42,;16.46,-8.98,;15.59,-11.17,;15.59,-12.67,;14.17,-13.14,;13.71,-14.55,;13.29,-11.92,;11.75,-11.92,;10.98,-13.26,;9.44,-13.26,;8.66,-11.91,;7.11,-11.91,;9.44,-10.58,;10.98,-10.58,;11.76,-9.24,;14.17,-10.71,;13.69,-9.24,;14.72,-8.09,;14.24,-6.63,;15.26,-5.48,;14.77,-4.02,;13.26,-3.71,;12.23,-4.88,;12.73,-6.33,;17.75,-11.92,;19.24,-12.77,;19.24,-11.05,)| Show InChI InChI=1S/C25H26F2N2O/c1-24(2)18-11-13-25(24,3)22-21(18)23(30)29(20-10-9-17(26)15-19(20)27)28(22)14-12-16-7-5-4-6-8-16/h4-10,15,18H,11-14H2,1-3H3/t18-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018802
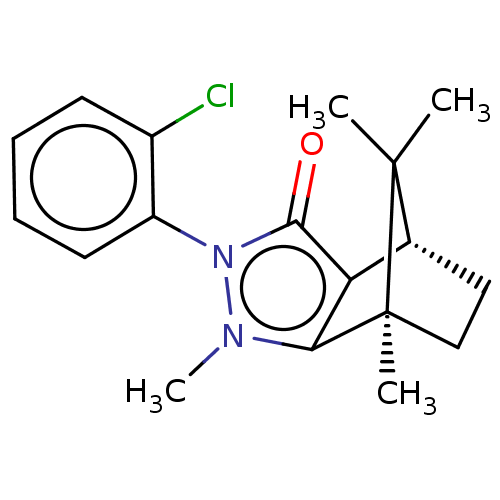
(CHEMBL3291354)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1Cl)n3C)C2(C)C |r,wU:4.4,1.0,(22.13,-16.72,;22.13,-15.22,;23.42,-14.48,;23.42,-12.98,;22.13,-12.23,;21.71,-10.79,;20.84,-12.98,;20.84,-14.48,;19.42,-14.94,;18.96,-16.36,;18.54,-13.73,;16.99,-13.72,;16.23,-12.39,;14.69,-12.38,;13.91,-13.72,;14.69,-15.07,;16.23,-15.06,;17.01,-16.4,;19.42,-12.52,;18.94,-11.05,;22.99,-13.72,;24.49,-14.58,;24.49,-12.86,)| Show InChI InChI=1S/C18H21ClN2O/c1-17(2)11-9-10-18(17,3)15-14(11)16(22)21(20(15)4)13-8-6-5-7-12(13)19/h5-8,11H,9-10H2,1-4H3/t11-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100443
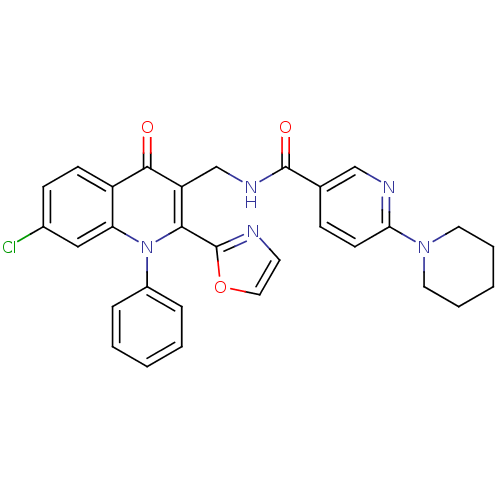
(US8501732, I-7)Show SMILES Clc1ccc2c(c1)n(-c1ccccc1)c(-c1ncco1)c(CNC(=O)c1ccc(nc1)N1CCCCC1)c2=O Show InChI InChI=1S/C30H26ClN5O3/c31-21-10-11-23-25(17-21)36(22-7-3-1-4-8-22)27(30-32-13-16-39-30)24(28(23)37)19-34-29(38)20-9-12-26(33-18-20)35-14-5-2-6-15-35/h1,3-4,7-13,16-18H,2,5-6,14-15,19H2,(H,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100440
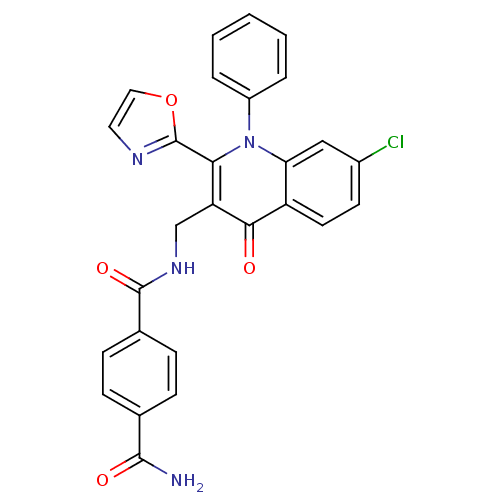
(US8501732, I-4)Show SMILES NC(=O)c1ccc(cc1)C(=O)NCc1c(-c2ncco2)n(-c2ccccc2)c2cc(Cl)ccc2c1=O Show InChI InChI=1S/C27H19ClN4O4/c28-18-10-11-20-22(14-18)32(19-4-2-1-3-5-19)23(27-30-12-13-36-27)21(24(20)33)15-31-26(35)17-8-6-16(7-9-17)25(29)34/h1-14H,15H2,(H2,29,34)(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM100580

(US8501732, I-140)Show SMILES COC(=O)c1c(CNC(=O)c2ccc(F)cc2)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C25H18ClFN2O4/c1-33-25(32)22-20(14-28-24(31)15-7-10-17(27)11-8-15)23(30)19-12-9-16(26)13-21(19)29(22)18-5-3-2-4-6-18/h2-13H,14H2,1H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
In vitro JNK1 assay. |
US Patent US8501732 (2013)
BindingDB Entry DOI: 10.7270/Q2RF5SP9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data