

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50278466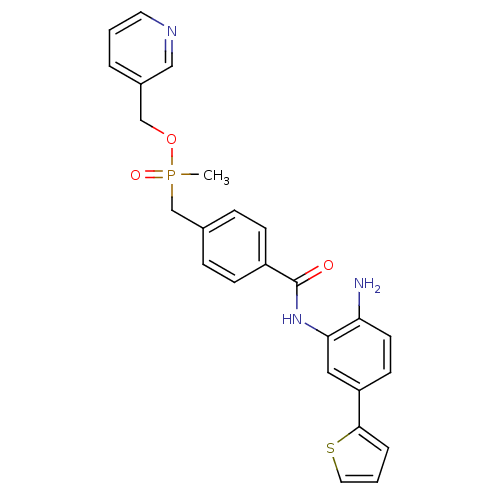 (CHEMBL470421 | pyridin-3-ylmethyl 4-(2-amino-5-(th...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HDAC1 | Bioorg Med Chem Lett 19: 2053-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.009 BindingDB Entry DOI: 10.7270/Q2QV3MDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172774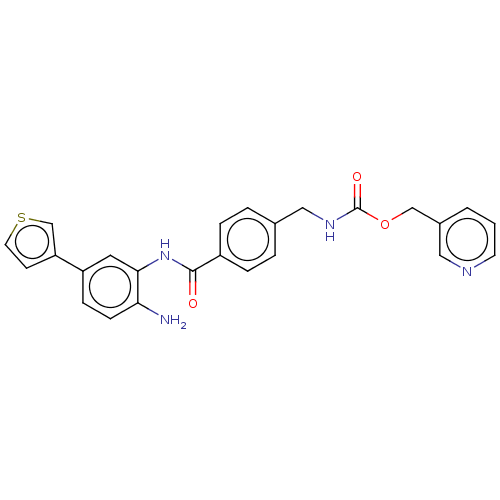 (US9096559, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50229193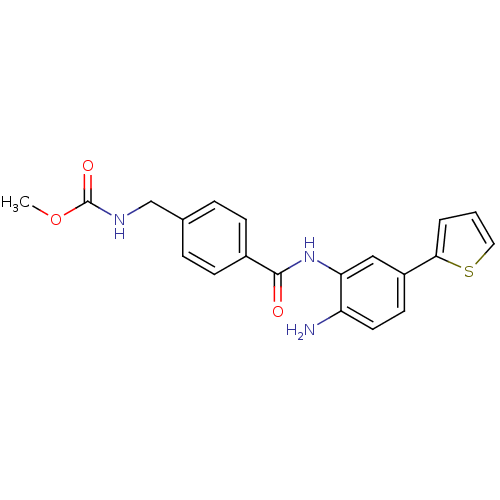 (CHEMBL400721 | US9096559, 17 | methyl 4-((2-amino-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50278467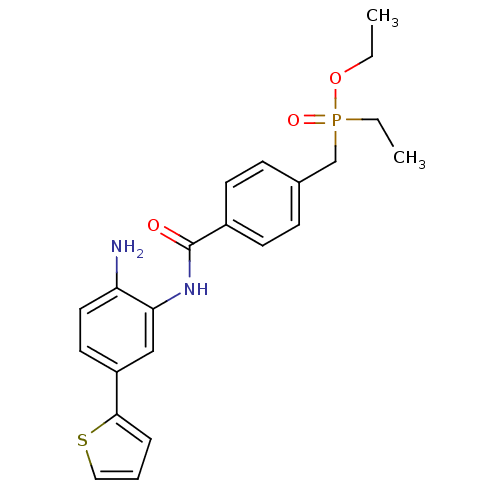 (CHEMBL511473 | ethyl 4-(2-amino-5-(thiophen-2-yl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HDAC1 | Bioorg Med Chem Lett 19: 2053-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.009 BindingDB Entry DOI: 10.7270/Q2QV3MDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50278377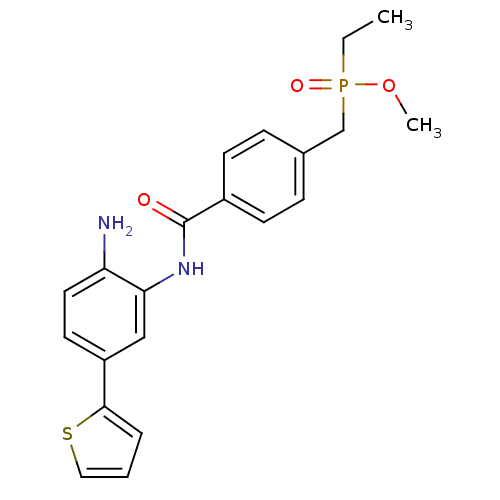 (CHEMBL472636 | methyl 4-(2-amino-5-(thiophen-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HDAC1 | Bioorg Med Chem Lett 19: 2053-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.009 BindingDB Entry DOI: 10.7270/Q2QV3MDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50278515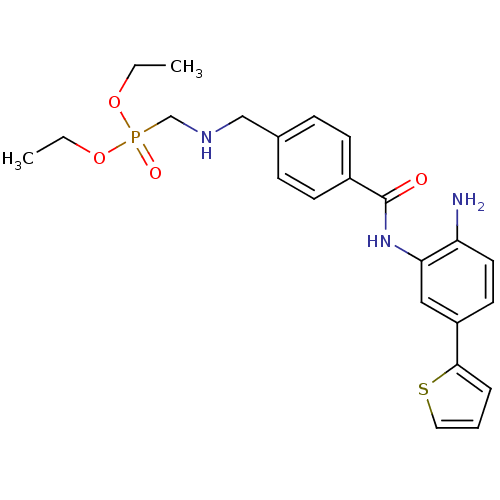 (CHEMBL470225 | diethyl(4-(2-amino-5-(thiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HDAC1 | Bioorg Med Chem Lett 19: 2053-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.009 BindingDB Entry DOI: 10.7270/Q2QV3MDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50229204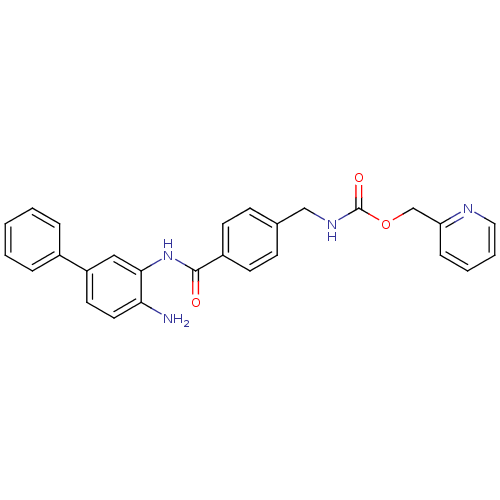 (CHEMBL253870 | US9096559, 14b | [4-(4-amino-biphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50278420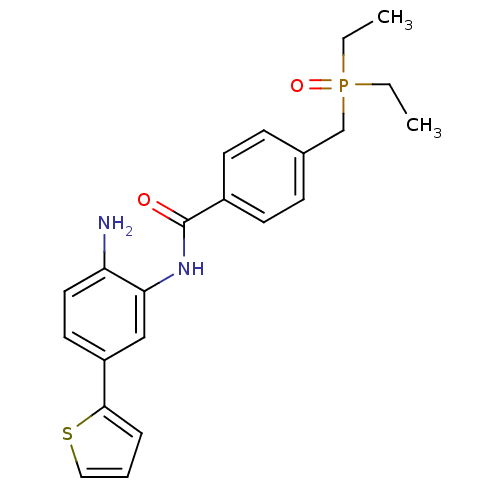 (CHEMBL513536 | N-(2-amino-5-(thiophen-2-yl)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HDAC1 | Bioorg Med Chem Lett 19: 2053-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.009 BindingDB Entry DOI: 10.7270/Q2QV3MDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172896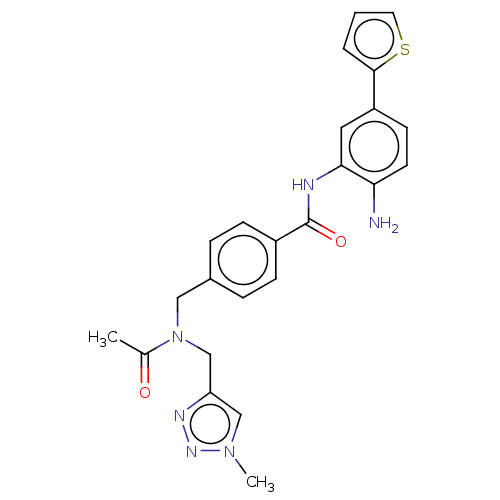 (US9096559, 123) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27762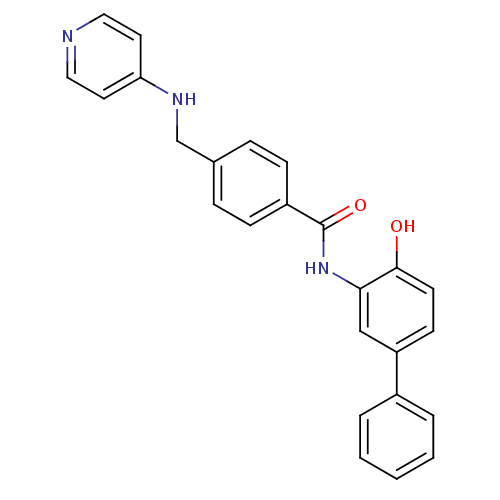 (N-(2-hydroxy-5-phenylphenyl)-4-[(pyridin-4-ylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172796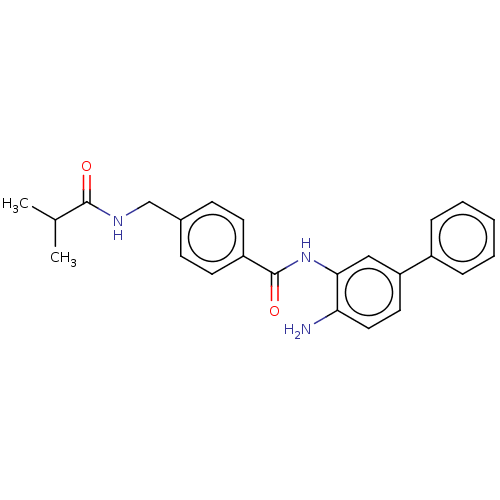 (US9096559, 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50229195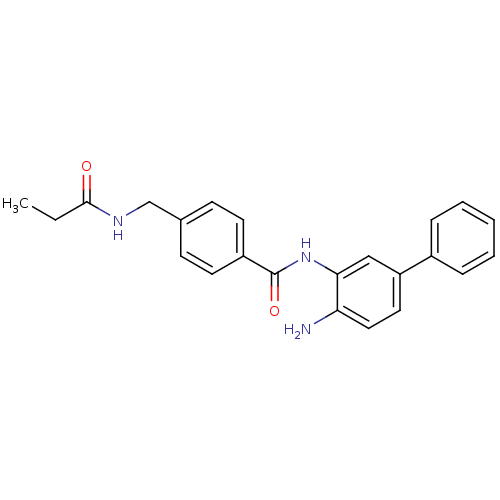 (CHEMBL398617 | N-(4-amino-biphenyl-3-yl)-4-(propio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50229201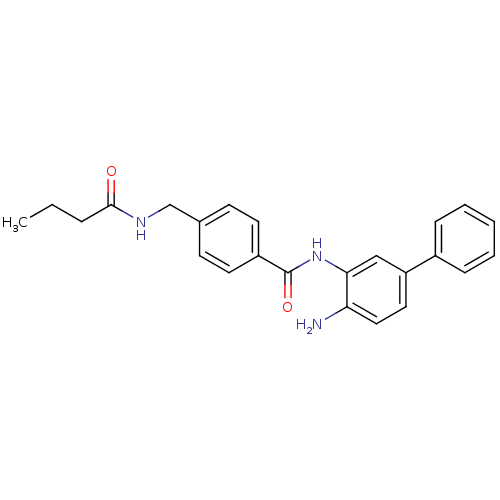 (CHEMBL253226 | N-(4-amino-biphenyl-3-yl)-4-(butyry...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172815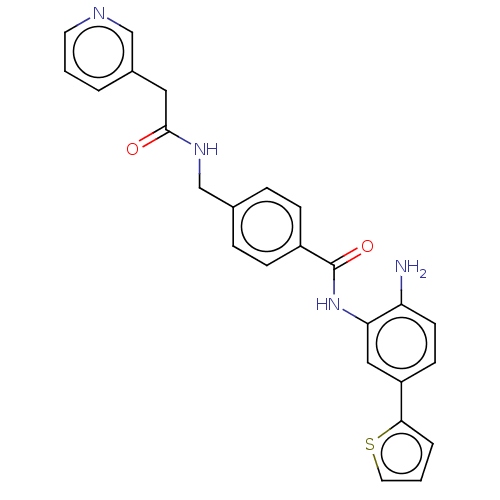 (US9096559, 49) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50229189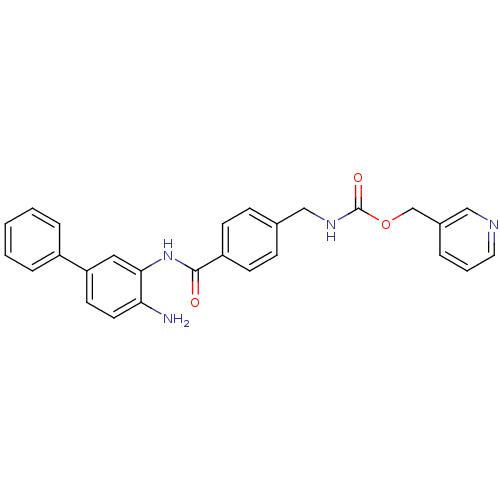 (CHEMBL253868 | US9096559, 2a | [4-(4-amino-bipheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27770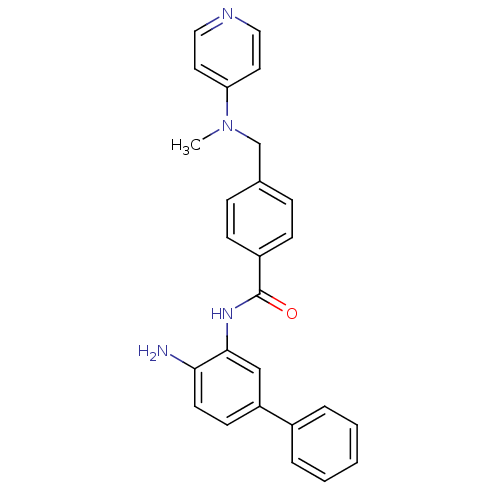 (N-(2-amino-5-phenylphenyl)-4-{[methyl(pyridin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27769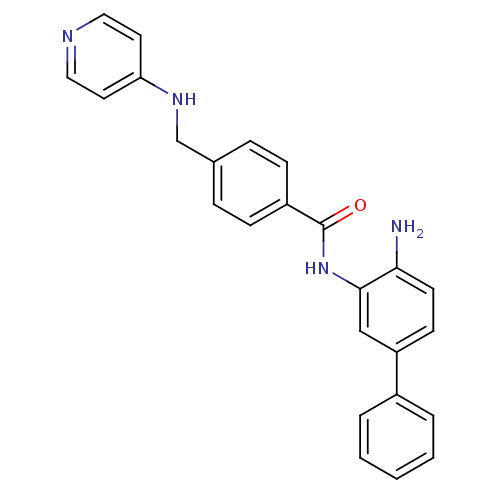 (N-(2-amino-5-phenylphenyl)-4-[(pyridin-4-ylamino)m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50229192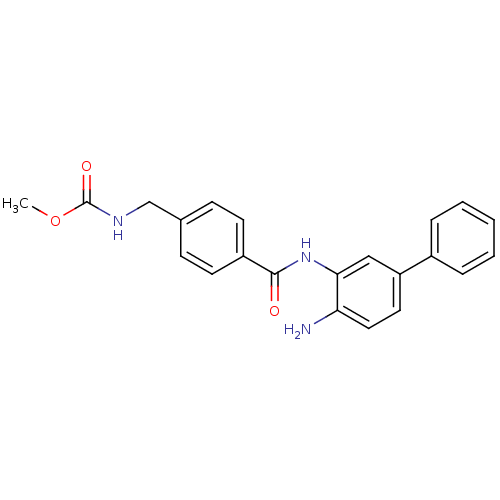 (CHEMBL398420 | US9096559, 14f | US9096559, 9 | [4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172804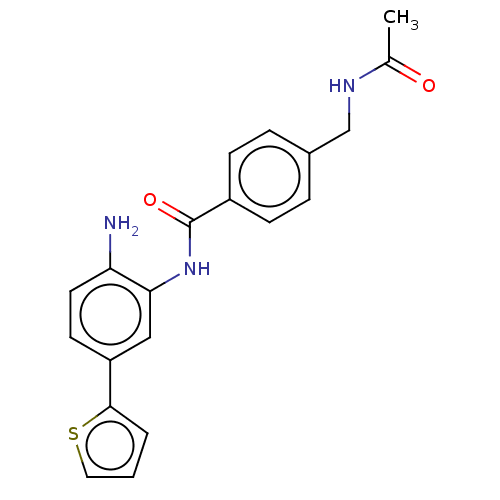 (US9096559, 36) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172809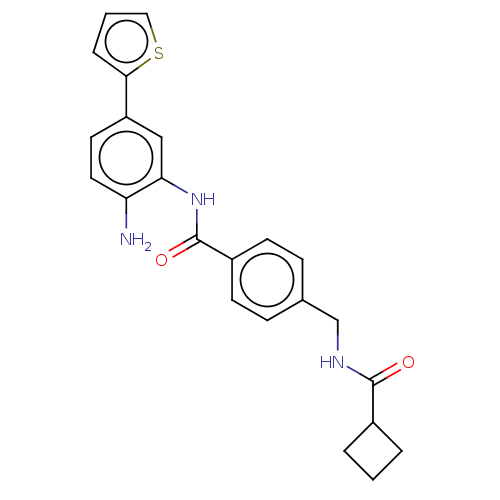 (US9096559, 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50229190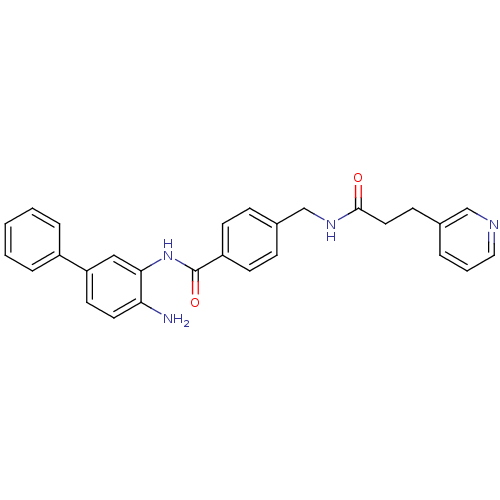 (CHEMBL253869 | N-(4-amino-biphenyl-3-yl)-4-[(3-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50278470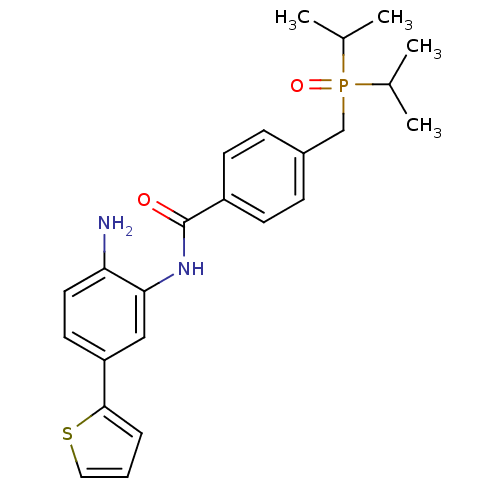 (CHEMBL513876 | N-(2-amino-5-(thiophen-2-yl)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HDAC1 | Bioorg Med Chem Lett 19: 2053-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.009 BindingDB Entry DOI: 10.7270/Q2QV3MDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50229197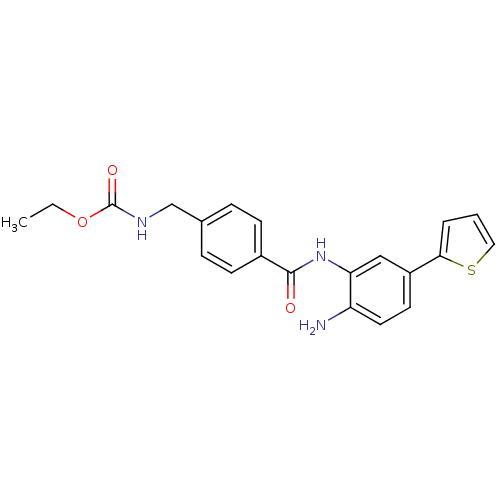 (CHEMBL399494 | US9096559, 16 | ethyl 4-((2-amino-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172819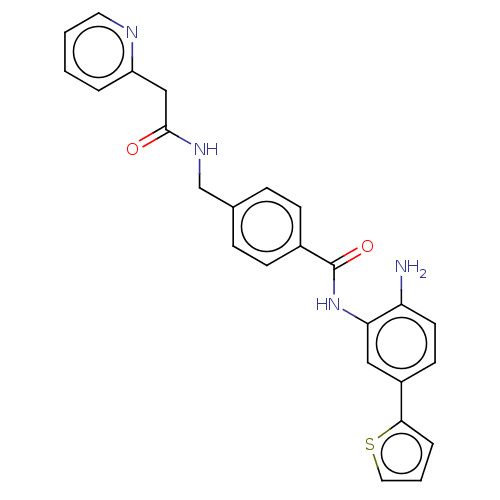 (US9096559, 54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50229200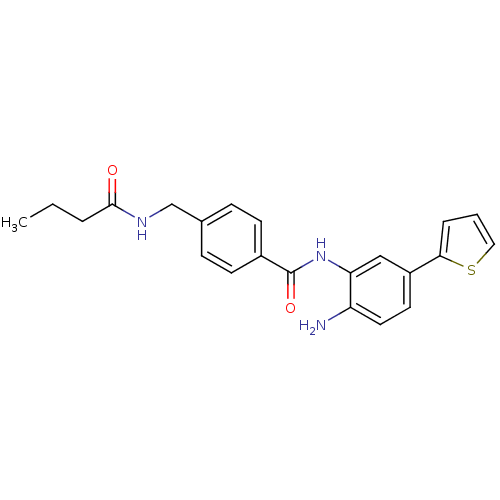 (CHEMBL251817 | N-(2-amino-5-(thiophen-2-yl)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50229194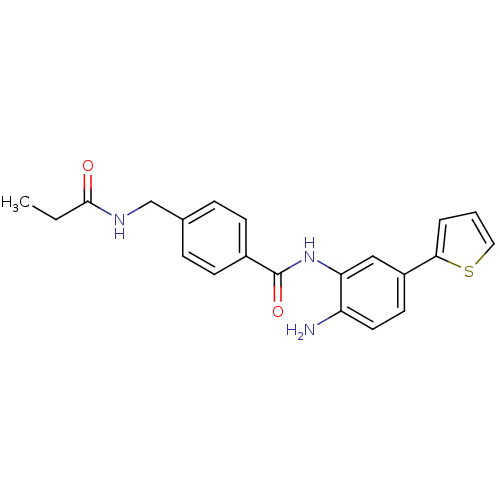 (CHEMBL251819 | N-(2-amino-5-(thiophen-2-yl)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172863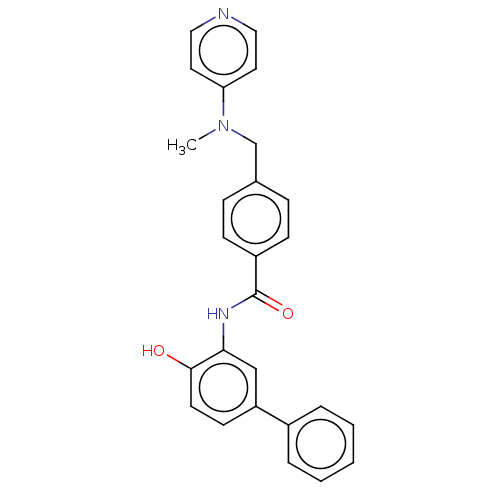 (US9096559, 103) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50229202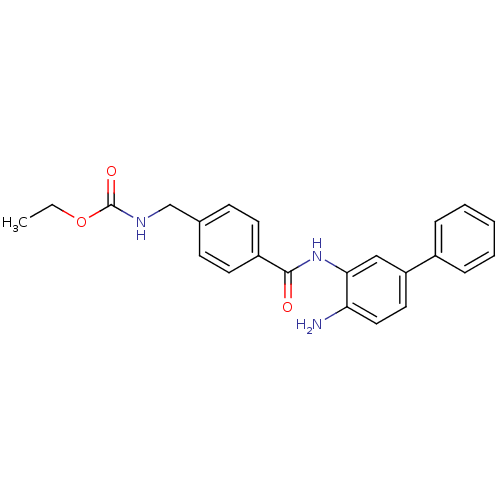 (CHEMBL400046 | US9096559, 10 | US9096559, 14e | [4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM27771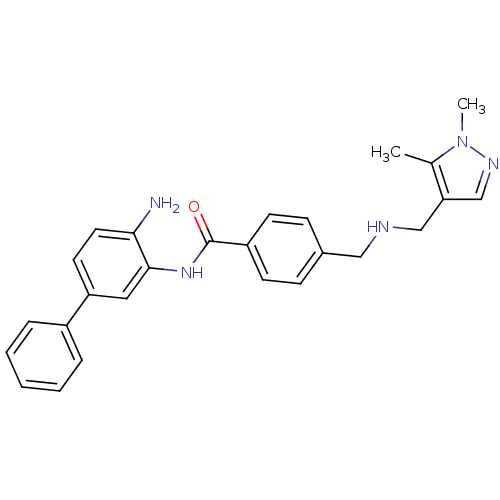 (N-(2-amino-5-phenylphenyl)-4-({[(1,5-dimethyl-1H-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172813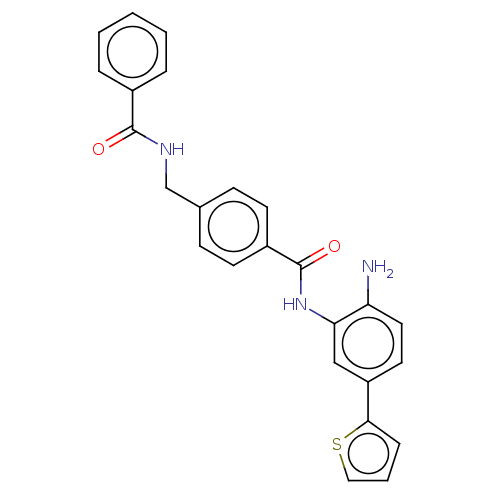 (US9096559, 48) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172821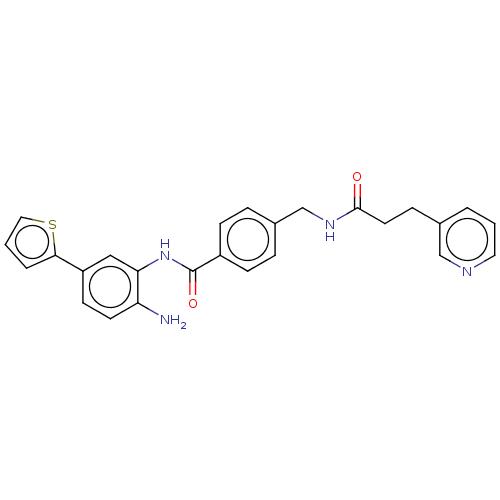 (US9096559, 56) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50278251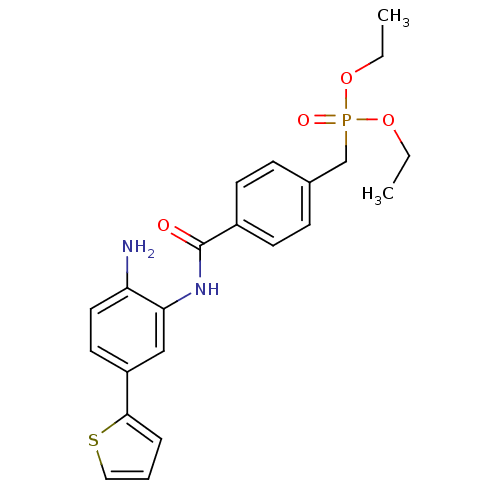 (CHEMBL471031 | diethyl 4-(2-amino-5-(thiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HDAC1 | Bioorg Med Chem Lett 19: 2053-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.009 BindingDB Entry DOI: 10.7270/Q2QV3MDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50278515 (CHEMBL470225 | diethyl(4-(2-amino-5-(thiophen-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HDAC2 | Bioorg Med Chem Lett 19: 2053-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.009 BindingDB Entry DOI: 10.7270/Q2QV3MDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172800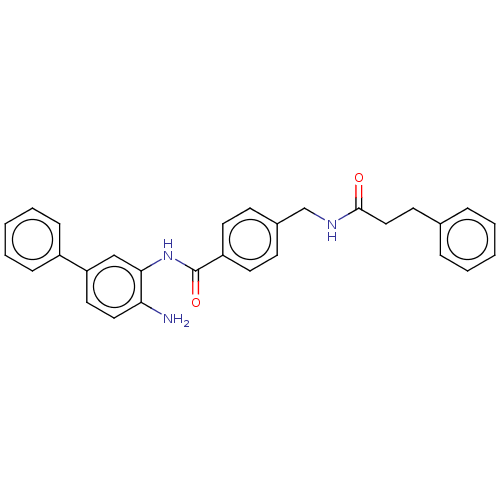 (US9096559, 31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172798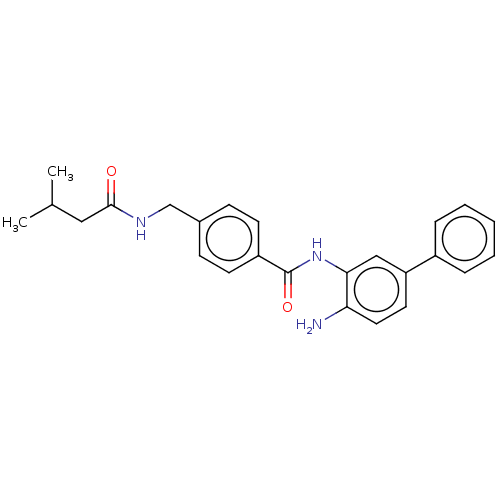 (US9096559, 29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50278308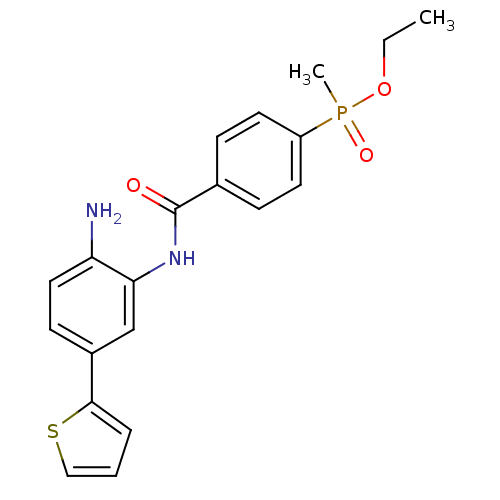 (CHEMBL469557 | ethyl 4-(2-amino-5-(thiophen-2-yl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HDAC1 | Bioorg Med Chem Lett 19: 2053-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.009 BindingDB Entry DOI: 10.7270/Q2QV3MDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172795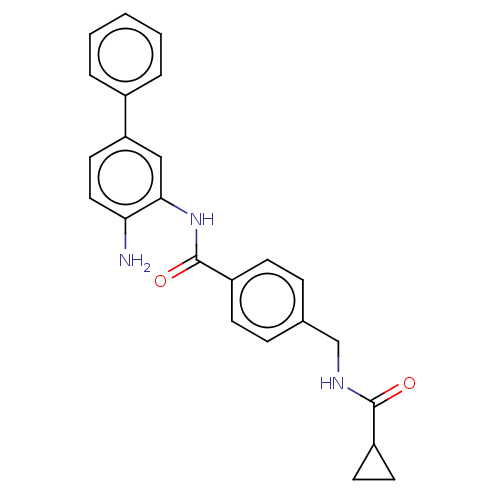 (US9096559, 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172805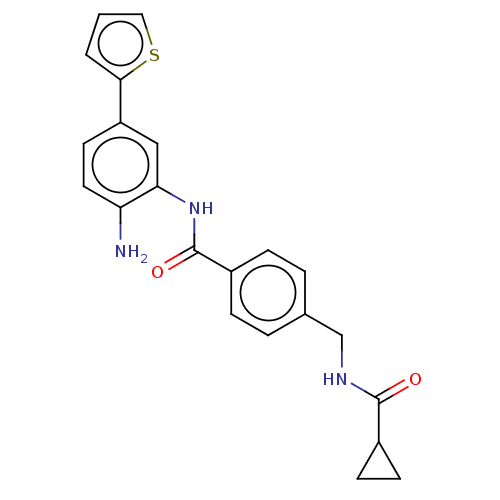 (US9096559, 39) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172833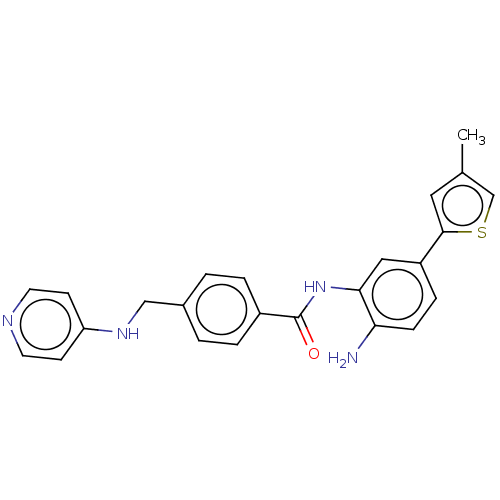 (US9096559, 69) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172818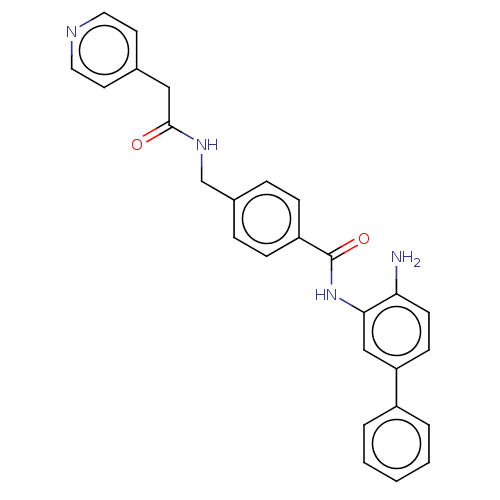 (US9096559, 52) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172808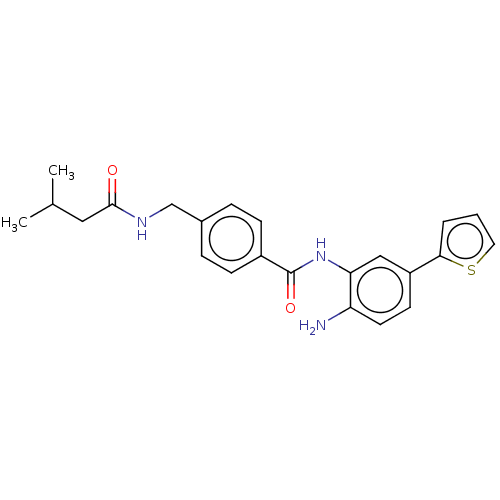 (US9096559, 42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172803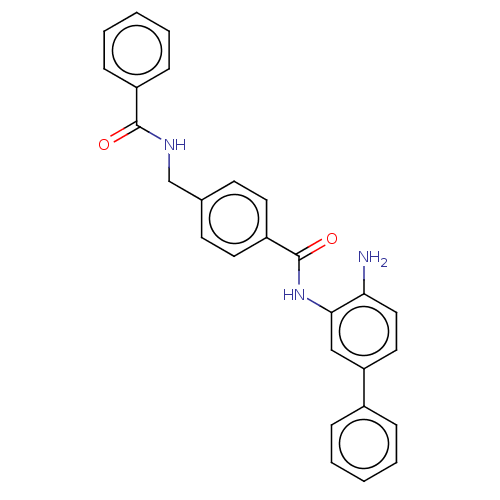 (US9096559, 14d | US9096559, 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50278468 (CHEMBL469377 | ethyl 4-(2-amino-5-(thiophen-2-yl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HDAC1 | Bioorg Med Chem Lett 19: 2053-8 (2009) Article DOI: 10.1016/j.bmcl.2009.02.009 BindingDB Entry DOI: 10.7270/Q2QV3MDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172895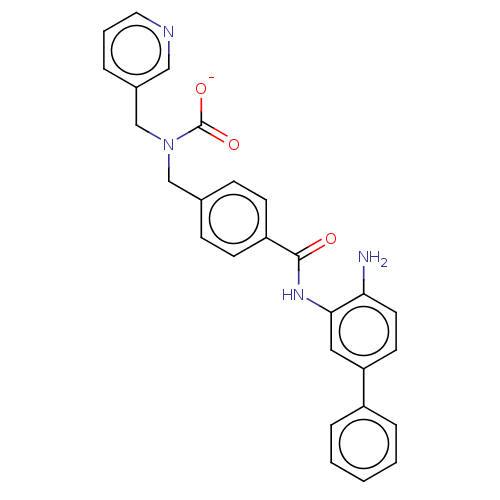 (US9096559, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172776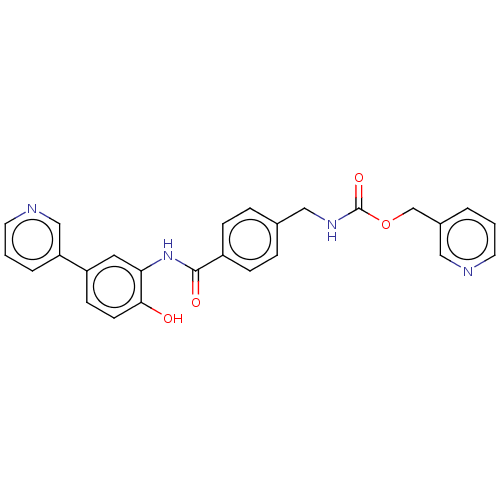 (US9096559, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50229190 (CHEMBL253869 | N-(4-amino-biphenyl-3-yl)-4-[(3-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172806 (US9096559, 40) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172807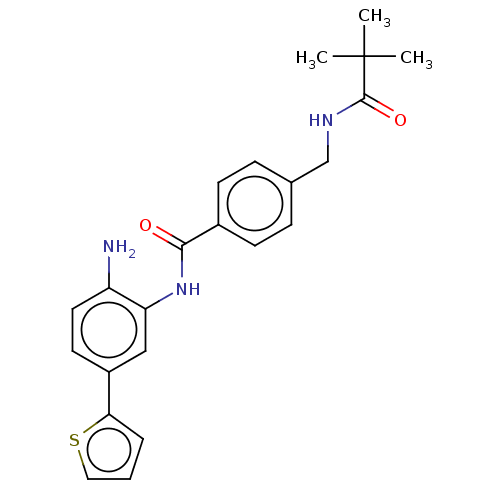 (US9096559, 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM172812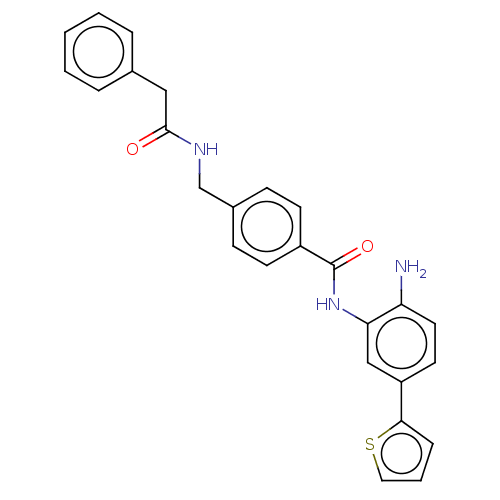 (US9096559, 47) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50229202 (CHEMBL400046 | US9096559, 10 | US9096559, 14e | [4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Novel compounds were tested for their ability to inhibit histone deacetylase, subtype 1 (HDAC1) using an in vitro deacetylation assay. The enzyme sou... | US Patent US9096559 (2015) BindingDB Entry DOI: 10.7270/Q2K07317 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 164 total ) | Next | Last >> |