

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426601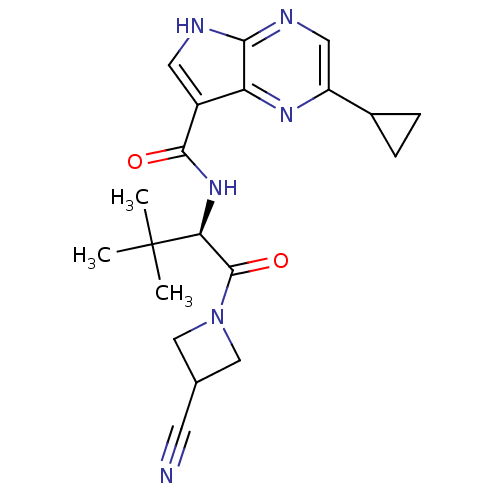 (CHEMBL2325895) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426599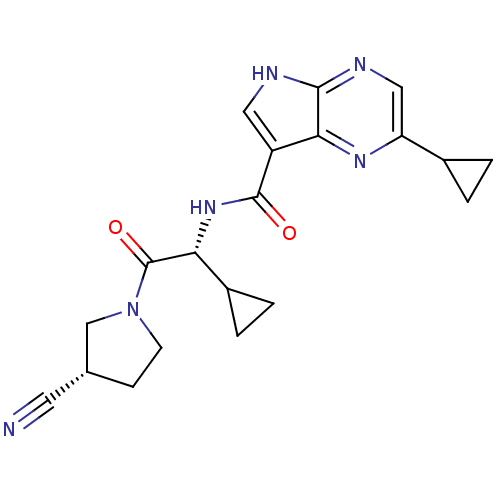 (CHEMBL2325898) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50426607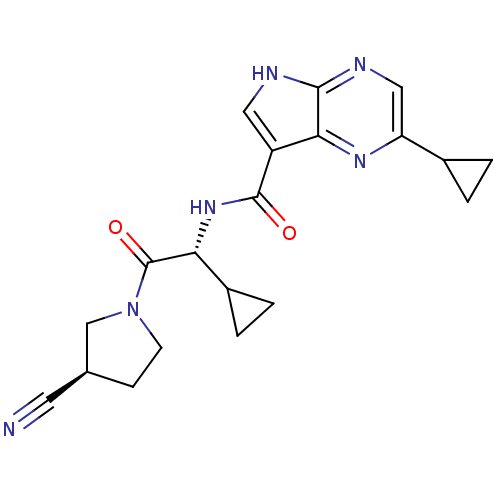 (CHEMBL2325897) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50426599 (CHEMBL2325898) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50426599 (CHEMBL2325898) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50426601 (CHEMBL2325895) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300504 (CHEMBL574455 | N-({3-[(5S)-5-tert-butyl-1-[(4-fluo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426606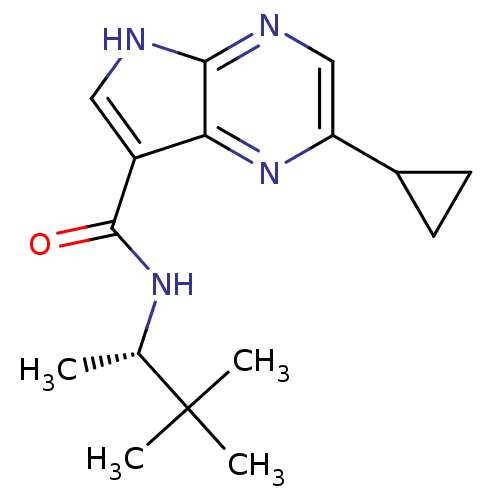 (CHEMBL2325903) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426604 (CHEMBL2325906) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301911 (CHEMBL583269 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300503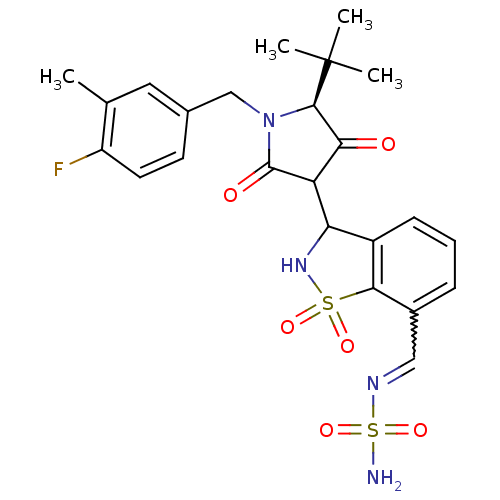 (CHEMBL582995 | N-({3-[(5S)-5-tert-butyl-1-[(4-fluo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300502 (CHEMBL572682 | N-({3-[(5S)-5-tert-butyl-1-[(4-fluo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300497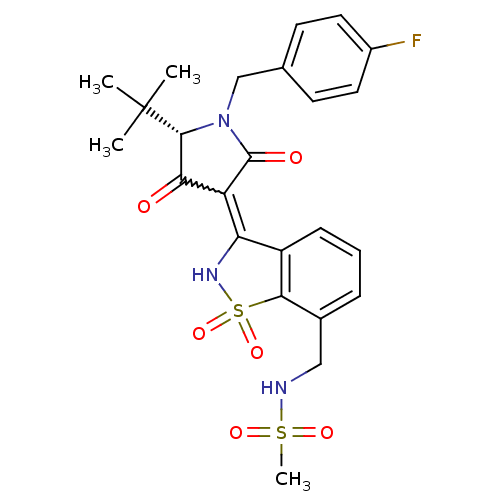 (CHEMBL578433 | N-({3-[(5S)-5-tert-butyl-1-(4-fluor...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301902 (CHEMBL571825 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50426601 (CHEMBL2325895) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300499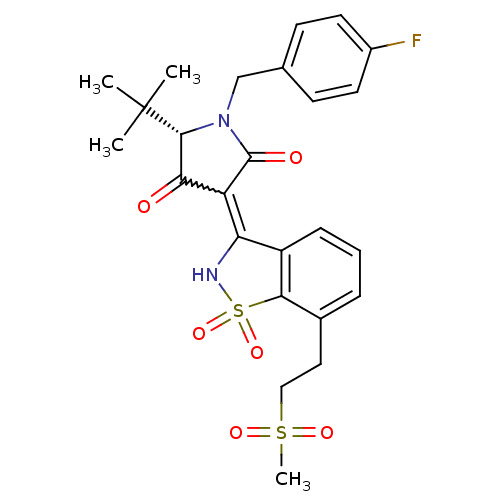 ((S)-5-tert-Butyl-1-(4-fluoro-benzyl)-4-hydroxy-3-[...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300498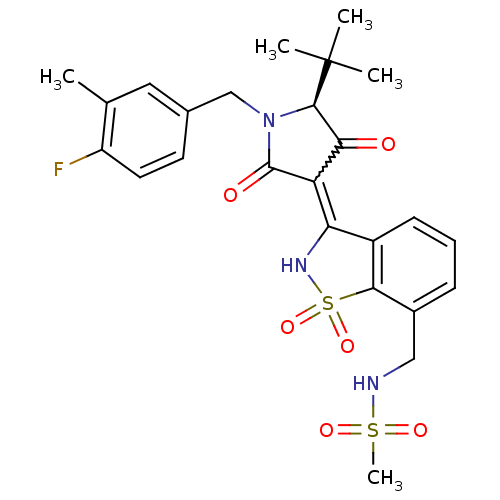 (CHEMBL575777 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301914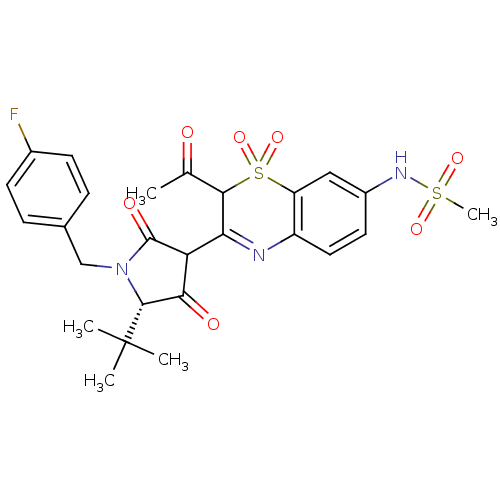 (CHEMBL570249 | N-{2-Acetyl-3-[(S)-5-tert-butyl-1-(...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301903 (CHEMBL569120 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301901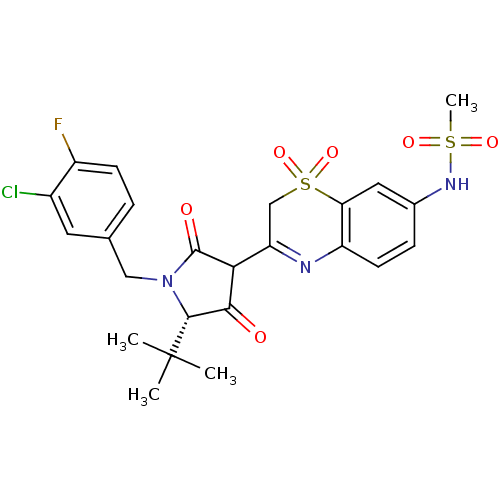 (CHEMBL570934 | N-{3-[(S)-5-tert-Butyl-1-(3-chloro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300505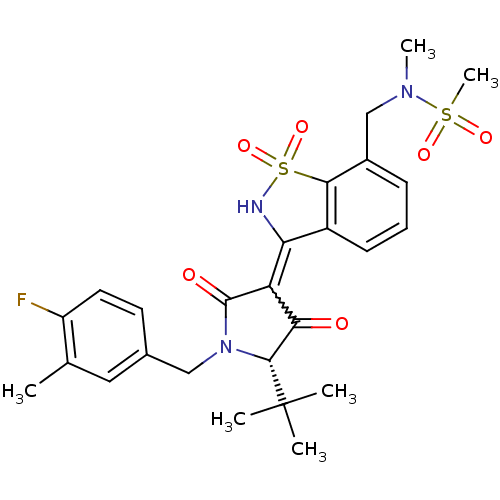 (CHEMBL572683 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300496 (CHEMBL577404 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301906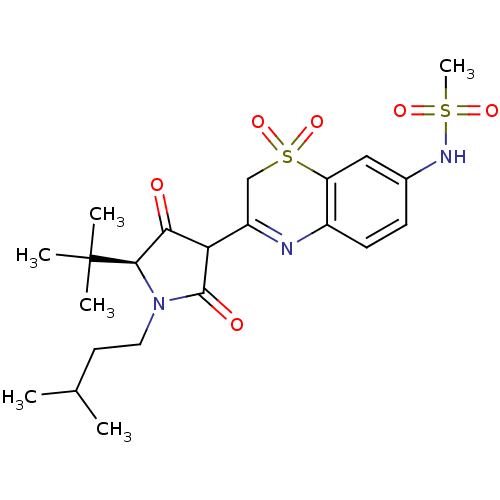 (CHEMBL571606 | N-{3-[(S)-5-tert-Butyl-4-hydroxy-1-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301913 (CHEMBL571564 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300501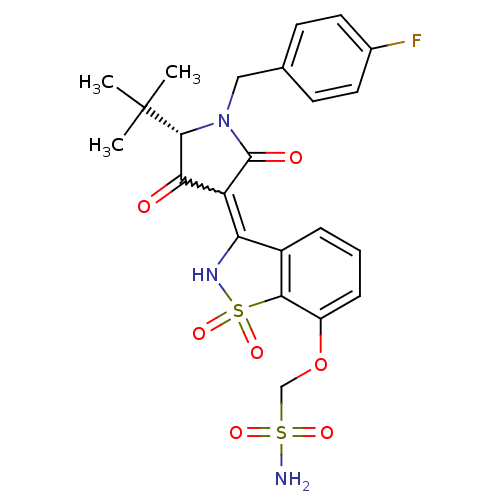 (CHEMBL573175 | {3-[(S)-5-tert-Butyl-1-(4-fluoro-be...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301900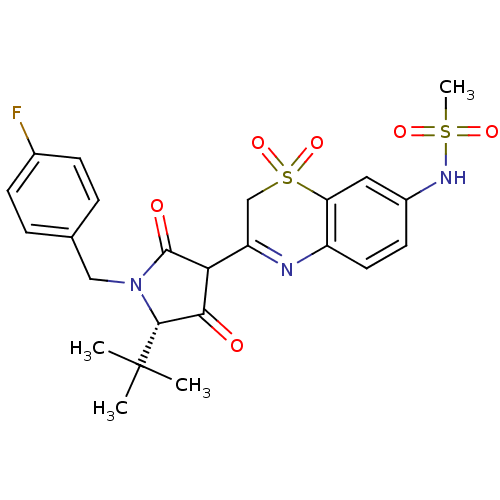 (CHEMBL568894 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426616 (CHEMBL2325899) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301907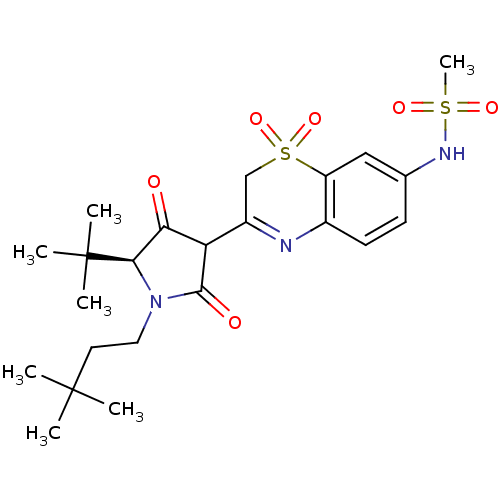 (CHEMBL572246 | N-{3-[(S)-5-tert-Butyl-1-(3,3-dimet...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301899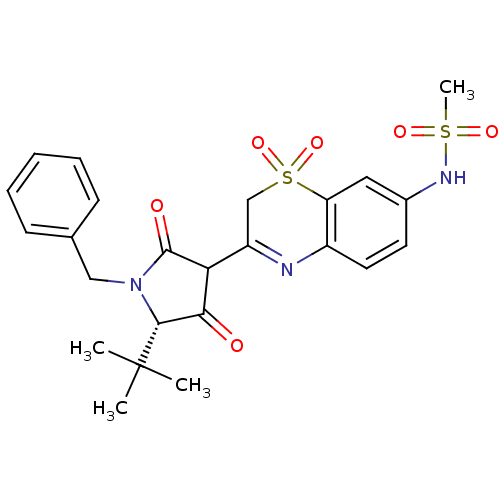 (CHEMBL585384 | N-[3-((S)-1-Benzyl-5-tert-butyl-4-h...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426602 (CHEMBL2325894) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426615 (CHEMBL2325900) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426603 (CHEMBL2325912) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300500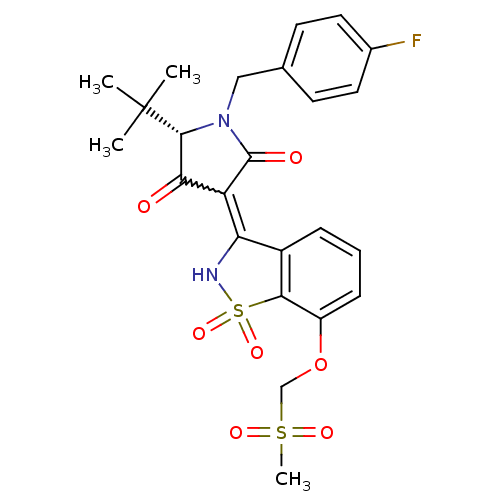 ((S)-5-tert-Butyl-1-(4-fluoro-benzyl)-4-hydroxy-3-(...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301916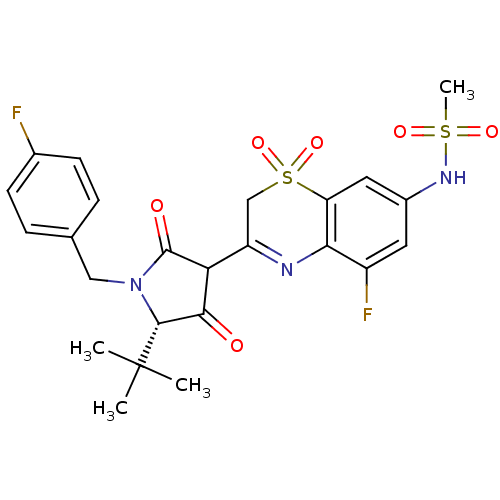 (CHEMBL585715 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426617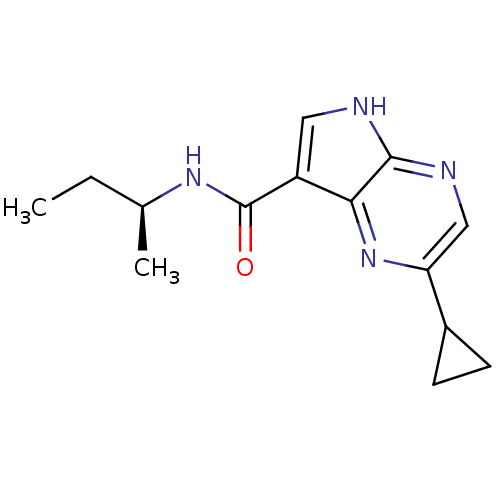 (CHEMBL2325902) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301912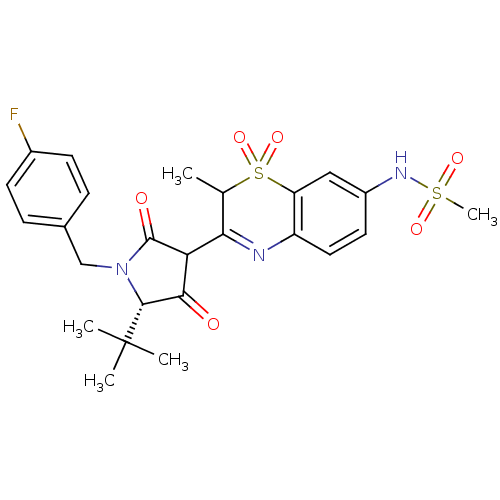 (CHEMBL584537 | N-{3-[(S)-5-tert-Butyl-1-(4-fluoro-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM30491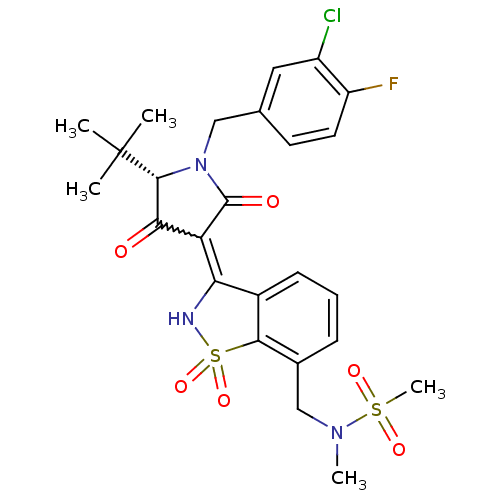 (1,1-dioxoisothiazole analog., 35) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301915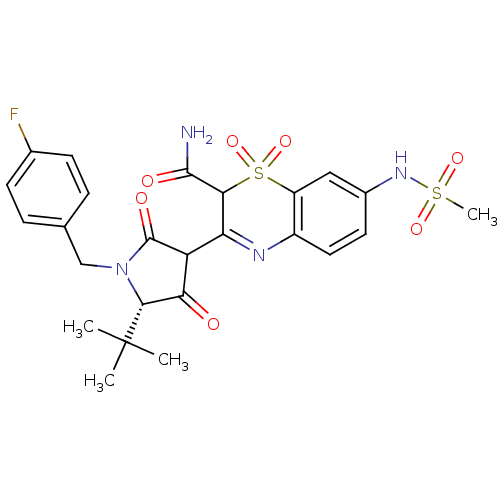 (3-[(S)-5-tert-Butyl-1-(4-fluoro-benzyl)-4-hydroxy-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50426604 (CHEMBL2325906) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50426606 (CHEMBL2325903) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50300506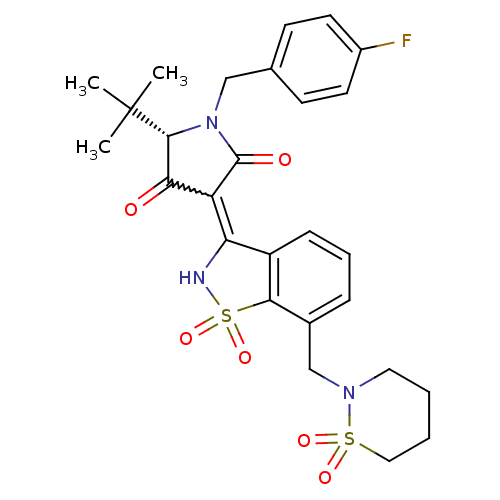 ((S)-5-tert-Butyl-3-[7-(1,1-dioxo-1lambda*6*-[1,2]t...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5652-6 (2009) Article DOI: 10.1016/j.bmcl.2009.08.022 BindingDB Entry DOI: 10.7270/Q2N016K0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301917 (CHEMBL585383 | N-{3-[(5S)-5-(1,1-dimethylpropyl)-1...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50301904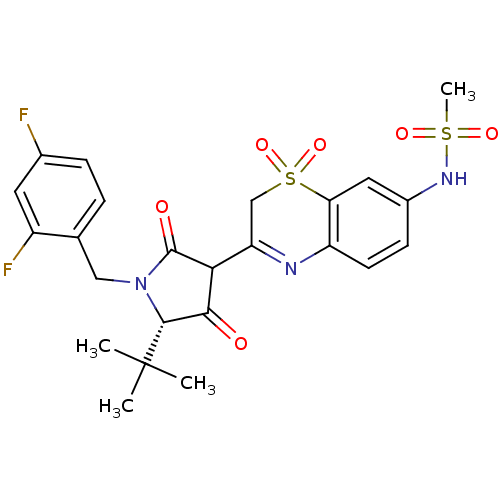 (CHEMBL569360 | N-{3-[(S)-5-tert-Butyl-1-(2,4-diflu...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Inhibition of HSV 1b con1 NS5B polymerase assessed as [3H]UTP incorporation into acid insoluble RNA product | Bioorg Med Chem Lett 19: 5648-51 (2009) Article DOI: 10.1016/j.bmcl.2009.08.023 BindingDB Entry DOI: 10.7270/Q2T72HJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426605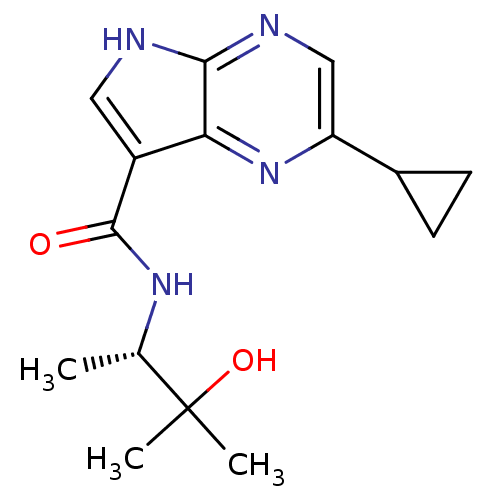 (CHEMBL2325904) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426614 (CHEMBL2325905) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50426615 (CHEMBL2325900) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50426603 (CHEMBL2325912) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 106 total ) | Next | Last >> |