

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029978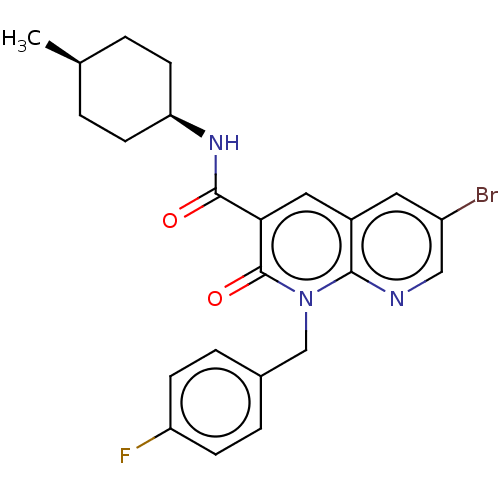 (CHEMBL3353441) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029963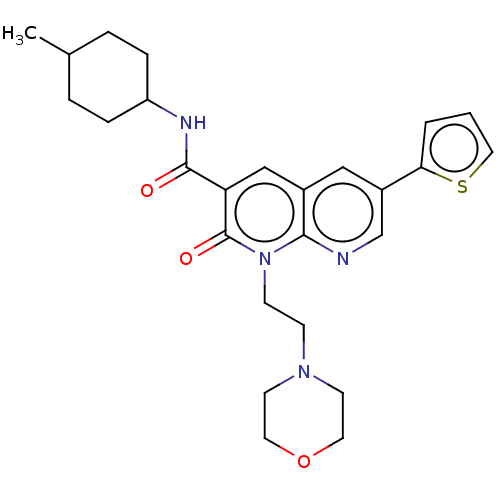 (CHEMBL3353452) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029958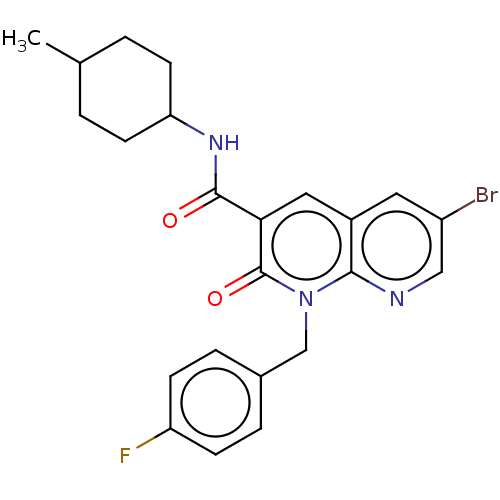 (CHEMBL3353439) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029962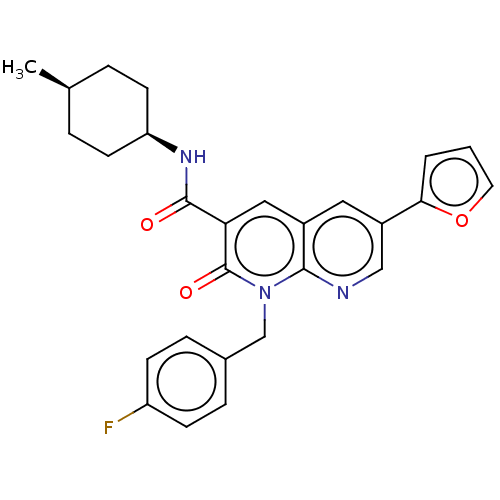 (CHEMBL3353450) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029965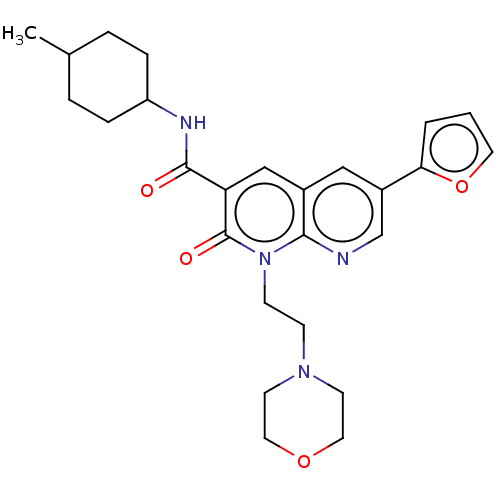 (CHEMBL3353454) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029989 (CHEMBL3353437) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50180022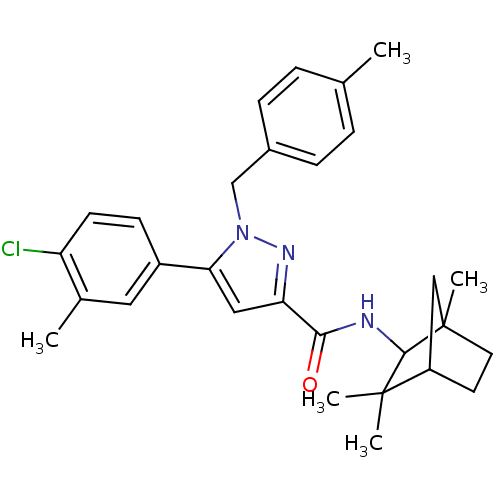 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029972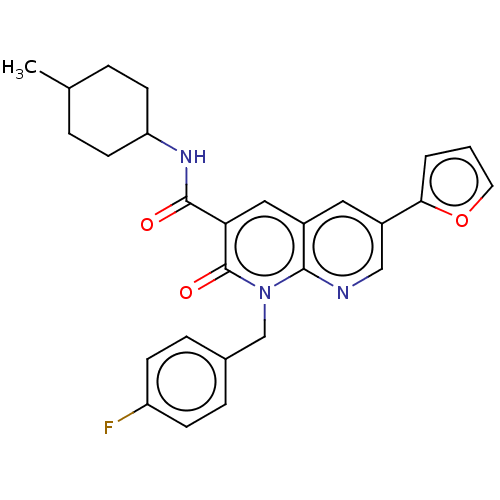 (CHEMBL3353448) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029964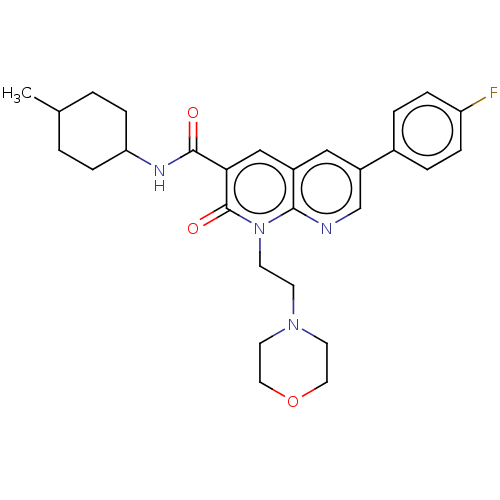 (CHEMBL3353453) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50258652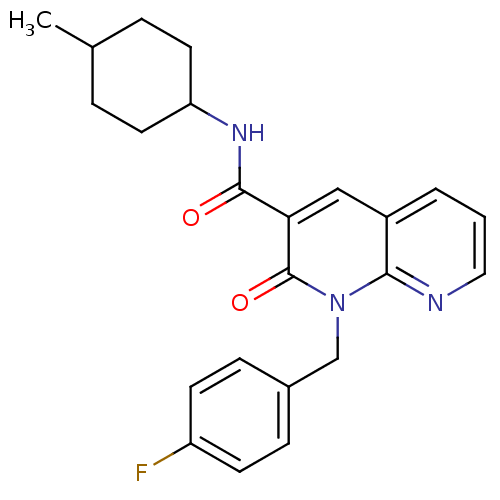 (CHEMBL466651 | N-(4-Methylcyclohexyl)-1-(p-fluorob...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50100130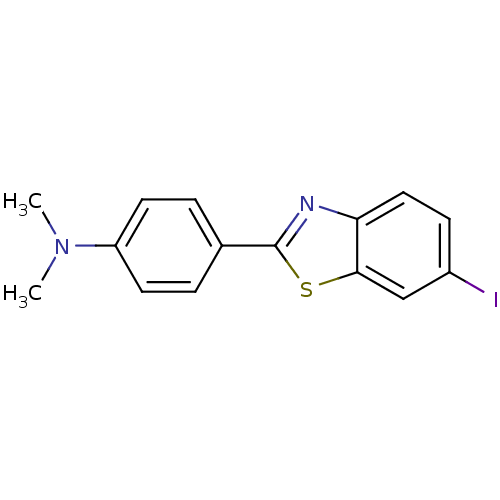 (4-(6-Iodobenzo[d]thiazol-2-yl)-N,N-dimethylaniline...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition constant against [125I]-7 (TZDM) binding to Amyloid beta 1-40 aggregates | J Med Chem 46: 237-43 (2003) Article DOI: 10.1021/jm020351j BindingDB Entry DOI: 10.7270/Q2WH2PBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029974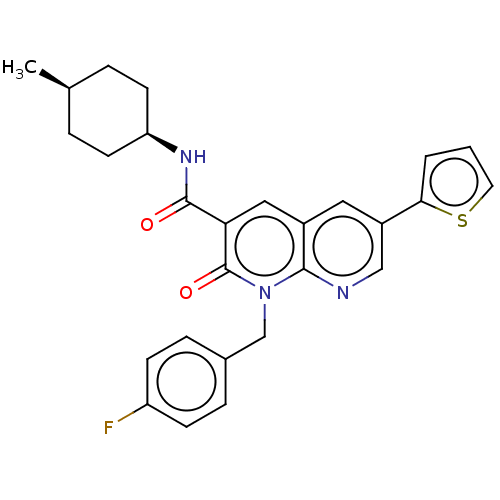 (CHEMBL3353446) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166904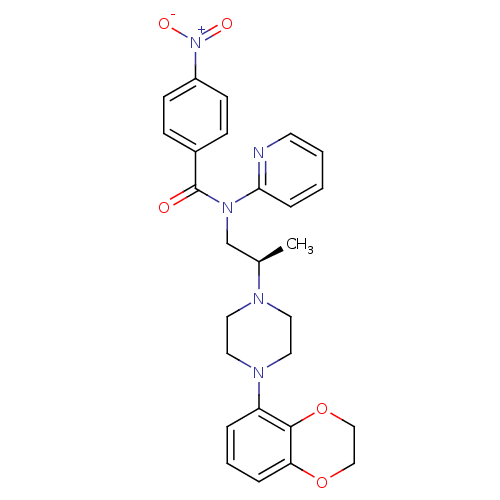 (CHEMBL193206 | N-{(R)-2-[4-(2,3-Dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029979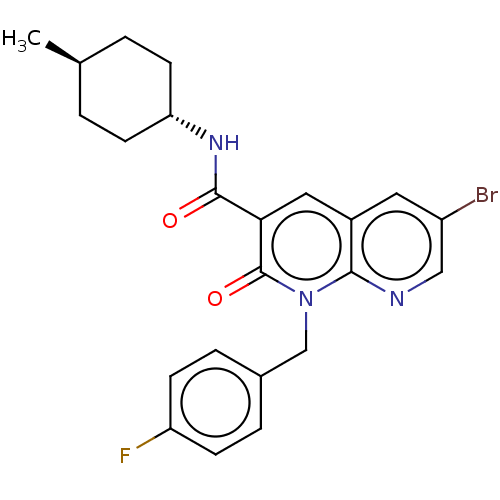 (CHEMBL3353440) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166901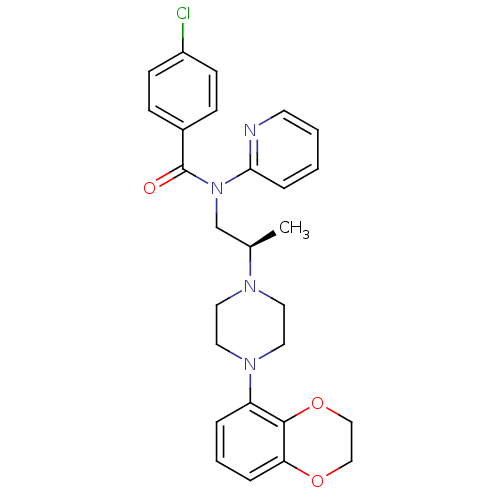 (4-Chloro-N-{(R)-2-[4-(2,3-dihydro-benzo[1,4]dioxin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029959 (CHEMBL3353442 | US11564928, Compound 61) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029928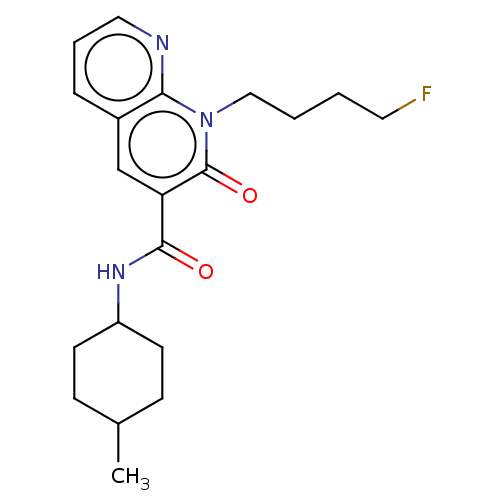 (CHEMBL3353436) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029960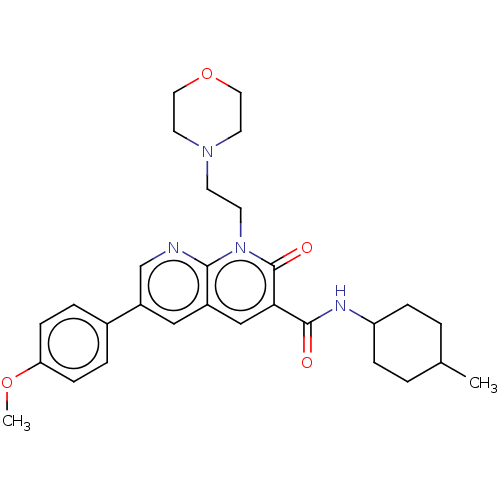 (CHEMBL3353451) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029980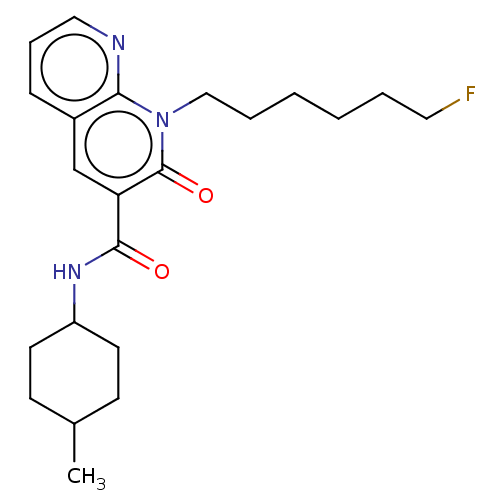 (CHEMBL3353438) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166903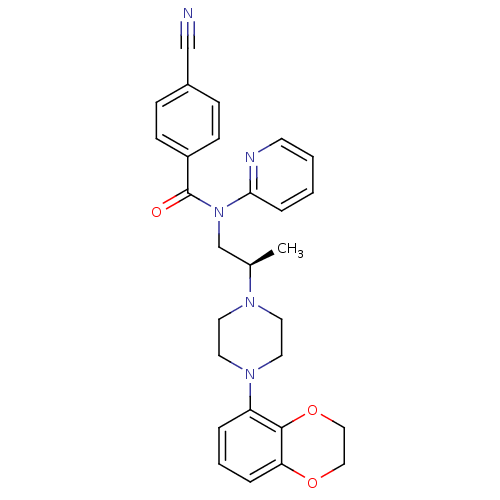 (4-Cyano-N-{(R)-2-[4-(2,3-dihydro-benzo[1,4]dioxin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human KOPR expressed in CHO cells | Bioorg Med Chem 17: 1370-80 (2009) Article DOI: 10.1016/j.bmc.2008.12.012 BindingDB Entry DOI: 10.7270/Q25H7G4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50258570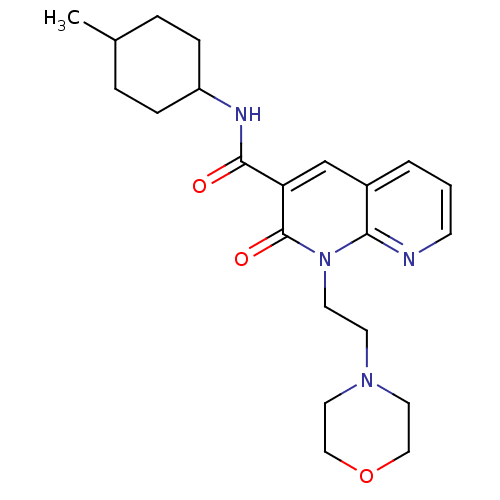 (CHEMBL466223 | N-(4-Methylcyclohexyl)-1-(2-morphol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029976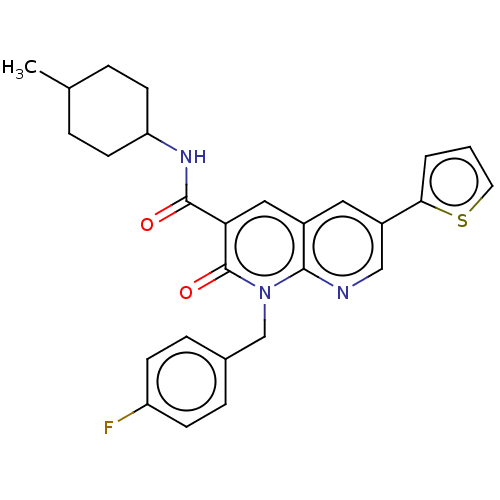 (CHEMBL3353444) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166899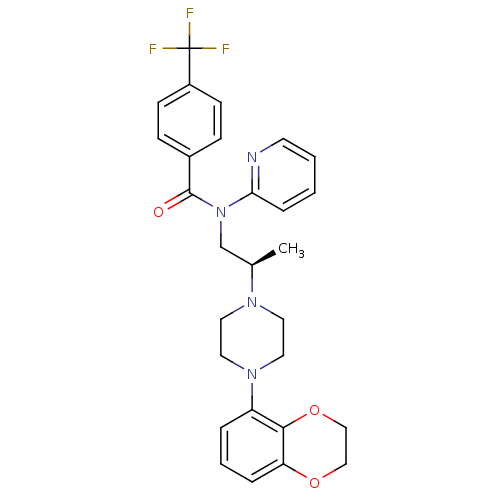 (CHEMBL435111 | N-{(R)-2-[4-(2,3-Dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35229 (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human norepinephrine transporter expressed in MDCK-Net6 cells | J Med Chem 51: 4038-49 (2008) Article DOI: 10.1021/jm8002262 BindingDB Entry DOI: 10.7270/Q2TB16PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029973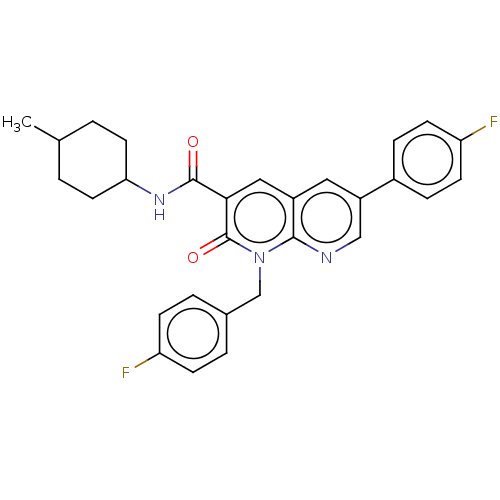 (CHEMBL3353447) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50159165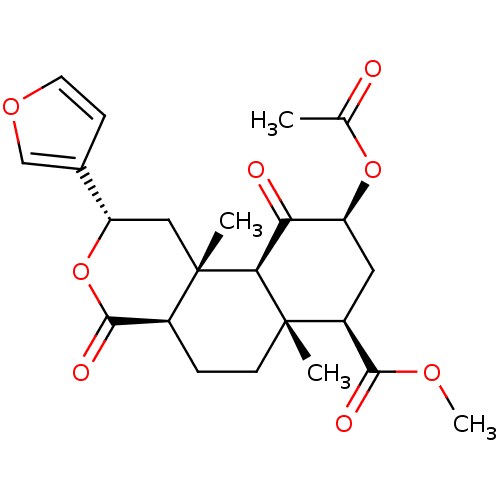 ((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human KOPR expressed in CHO cells | Bioorg Med Chem 17: 1370-80 (2009) Article DOI: 10.1016/j.bmc.2008.12.012 BindingDB Entry DOI: 10.7270/Q25H7G4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029967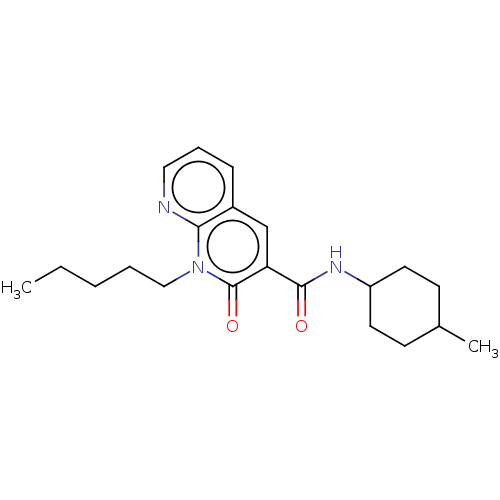 (CHEMBL3353424) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50216132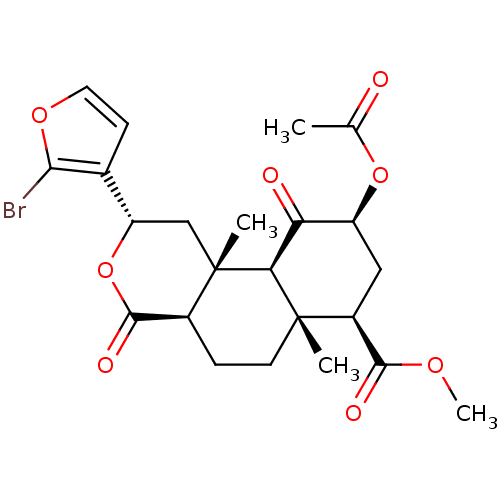 ((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human KOPR expressed in CHO cells | Bioorg Med Chem 17: 1370-80 (2009) Article DOI: 10.1016/j.bmc.2008.12.012 BindingDB Entry DOI: 10.7270/Q25H7G4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50180036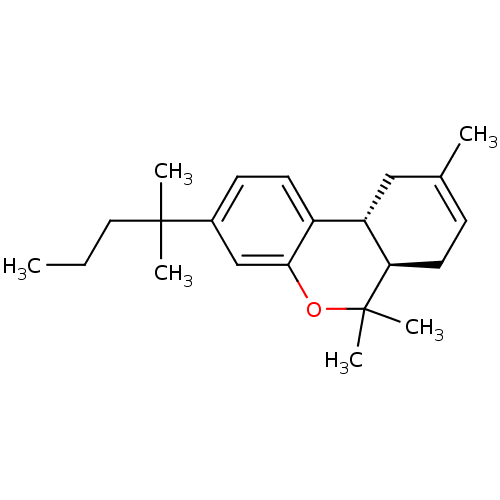 ((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166902 (CHEMBL195425 | N-{(S)-2-[4-(2,3-Dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029971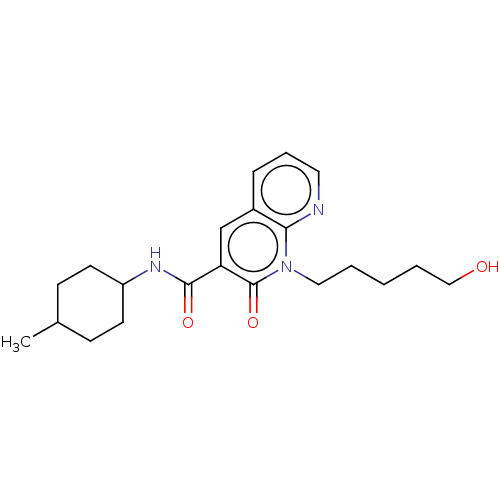 (CHEMBL3353428) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029977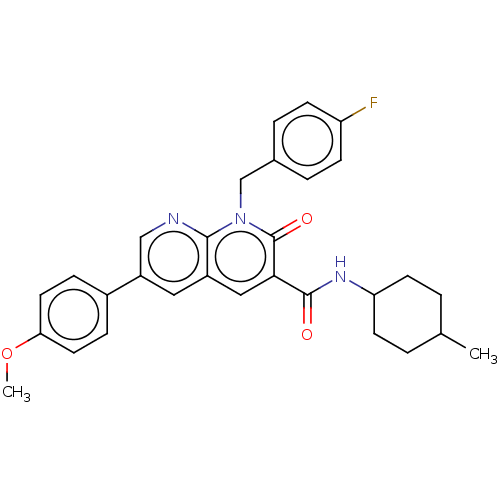 (CHEMBL3353443) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029966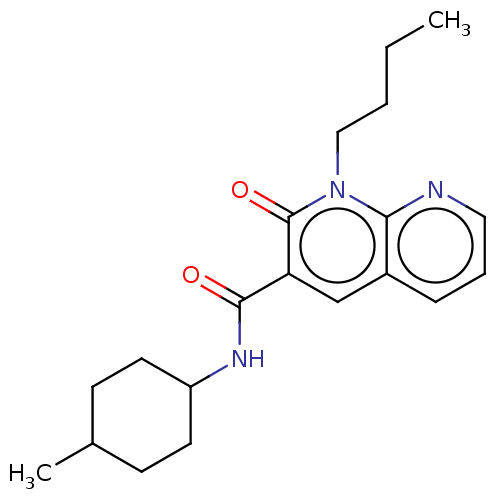 (CHEMBL3353423) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50367250 (3-DEAZAARISTEROMYCIN A | CHEMBL268272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against S-adenosyl-homocysteine hydrolase | J Med Chem 28: 471-7 (1985) BindingDB Entry DOI: 10.7270/Q21C1XF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166900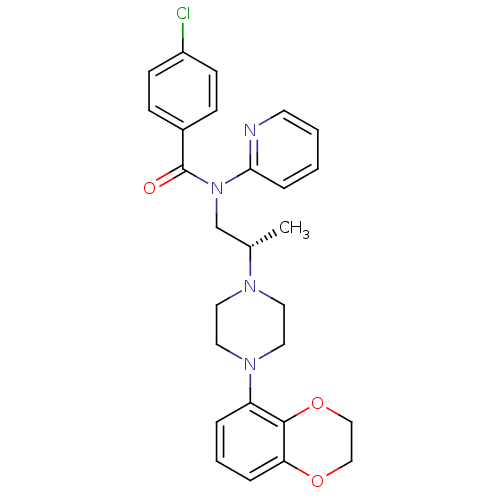 (4-Chloro-N-{(S)-2-[4-(2,3-dihydro-benzo[1,4]dioxin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50266336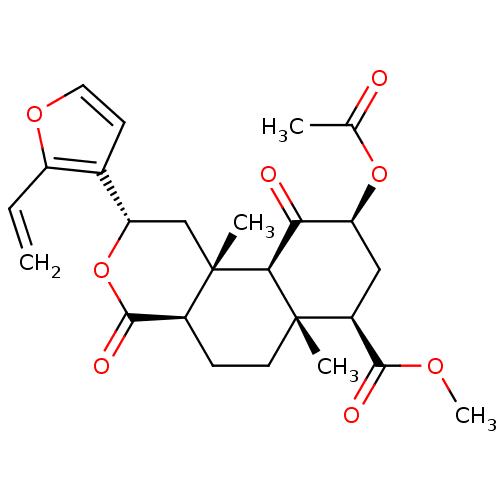 ((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-6a,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human KOPR expressed in CHO cells | Bioorg Med Chem 17: 1370-80 (2009) Article DOI: 10.1016/j.bmc.2008.12.012 BindingDB Entry DOI: 10.7270/Q25H7G4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166906 (CHEMBL365350 | N-{(S)-2-[4-(2,3-Dihydro-benzo[1,4]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50166905 (4-Cyano-N-{(S)-2-[4-(2,3-dihydro-benzo[1,4]dioxin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of 8-OH-DPAT from human 5-hydroxytryptamine 1A receptor expressed in chinese hamster ovary cells | J Med Chem 48: 3467-70 (2005) Article DOI: 10.1021/jm049493z BindingDB Entry DOI: 10.7270/Q2S1838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50122785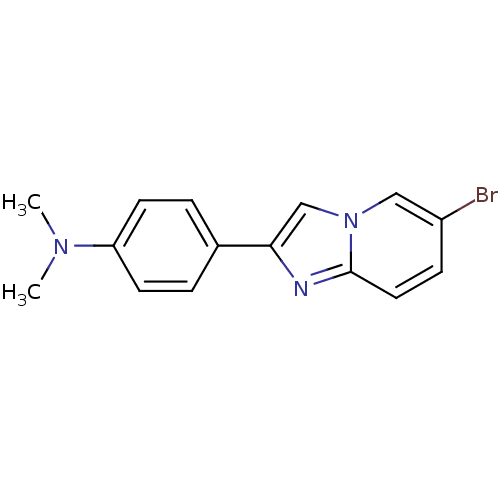 (4-(6-bromoH-imidazo[1,2-a]pyridin-2-yl)-N,N-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition constant against [125I]-7 (TZDM) binding to Amyloid beta 1-40 aggregates | J Med Chem 46: 237-43 (2003) Article DOI: 10.1021/jm020351j BindingDB Entry DOI: 10.7270/Q2WH2PBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50243500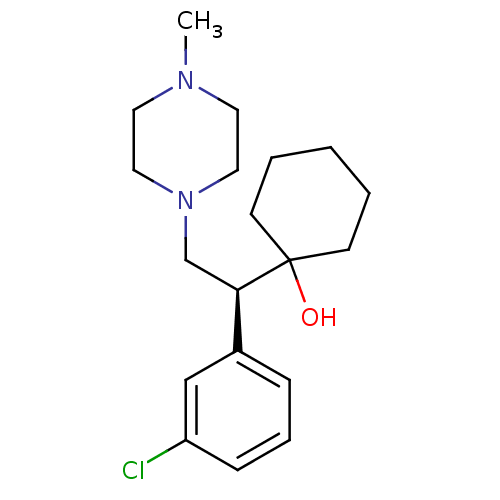 ((-)-1-[(1S)-1-(3-Chlorophenyl)-2-(4-methylpiperazi...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human norepinephrine transporter expressed in MDCK-Net6 cells | J Med Chem 51: 4038-49 (2008) Article DOI: 10.1021/jm8002262 BindingDB Entry DOI: 10.7270/Q2TB16PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50122787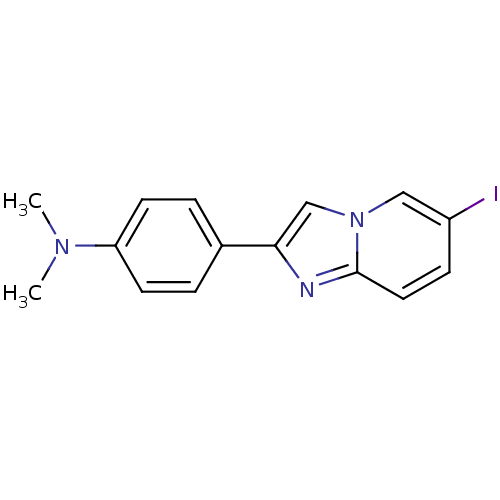 (2-(4'-dimethylaminophenyl)-6-iodoimidazo[1,2-a]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition constant against [125I]-7 (TZDM) binding to Amyloid beta 1-40 aggregates | J Med Chem 46: 237-43 (2003) Article DOI: 10.1021/jm020351j BindingDB Entry DOI: 10.7270/Q2WH2PBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50243503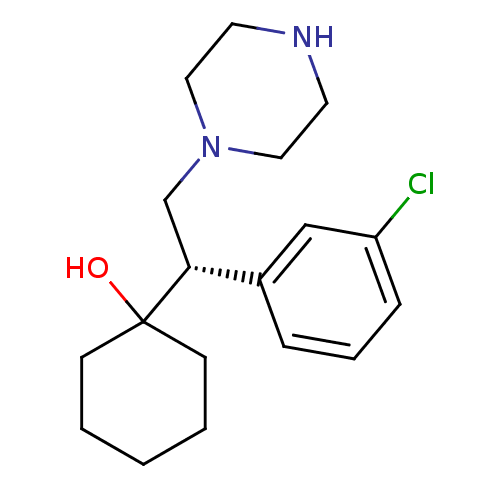 ((+)-1-[(1S)-1-(3-Chlorophenyl)-2-piperazin-1-yleth...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human norepinephrine transporter expressed in MDCK-Net6 cells | J Med Chem 51: 4038-49 (2008) Article DOI: 10.1021/jm8002262 BindingDB Entry DOI: 10.7270/Q2TB16PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50030093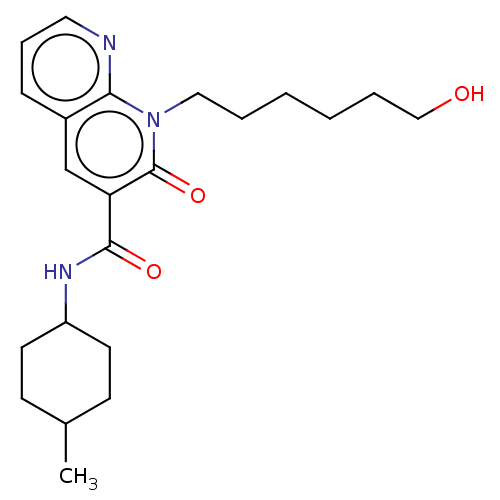 (CHEMBL3353429) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50243501 (1-[1-(3-Chlorophenyl)-2-piperazin-1-ylethyl]cycloh...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human norepinephrine transporter expressed in MDCK-Net6 cells | J Med Chem 51: 4038-49 (2008) Article DOI: 10.1021/jm8002262 BindingDB Entry DOI: 10.7270/Q2TB16PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50266418 ((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-((...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human KOPR expressed in CHO cells | Bioorg Med Chem 17: 1370-80 (2009) Article DOI: 10.1016/j.bmc.2008.12.012 BindingDB Entry DOI: 10.7270/Q25H7G4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50010884 (1-(2-(dimethylamino)-1-(3-(trifluoromethyl)phenyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human norepinephrine transporter expressed in MDCK-Net6 cells | J Med Chem 51: 4038-49 (2008) Article DOI: 10.1021/jm8002262 BindingDB Entry DOI: 10.7270/Q2TB16PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50029989 (CHEMBL3353437) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1913 total ) | Next | Last >> |