

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR (unknown origin) expressed in HEK cell membranes after 4 hrs by liquid scintillation counting ... | J Nat Prod 81: 1029-1035 (2018) Article DOI: 10.1021/acs.jnatprod.8b00062 BindingDB Entry DOI: 10.7270/Q2R49TF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 from guinea pig brain using [3H]U69,593 as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50007102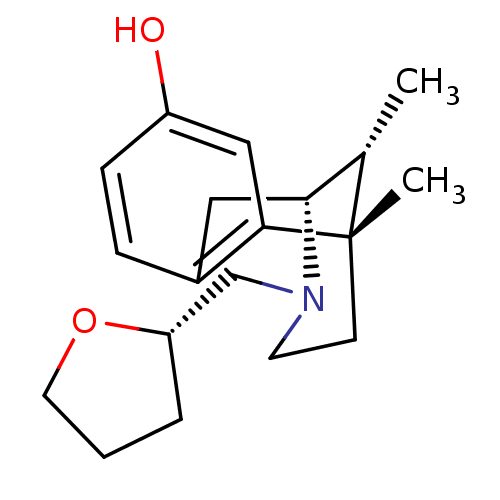 (6,11-Dimethyl-3-(tetrahydro-furan-2-ylmethyl)-1,2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 from guinea pig brain using [3H]U69,593 as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 from guinea pig brain using [3H]DAMGO as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50007102 (6,11-Dimethyl-3-(tetrahydro-furan-2-ylmethyl)-1,2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 from guinea pig brain using [3H]DAMGO as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50546246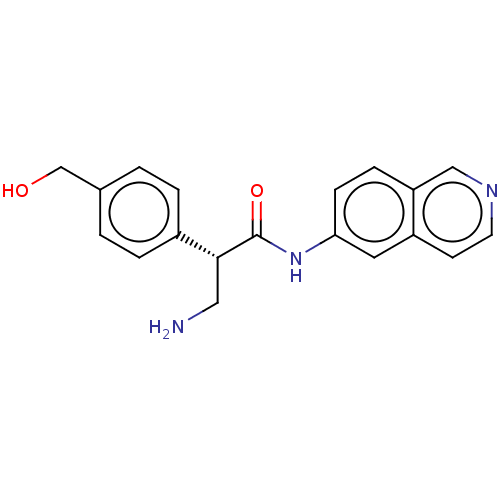 (CHEMBL4753043 | US11608319, Compound AR-13503) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546246 (CHEMBL4753043 | US11608319, Compound AR-13503) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 2 (Cricetulus griseus (Chinese hamster)) | BDBM121106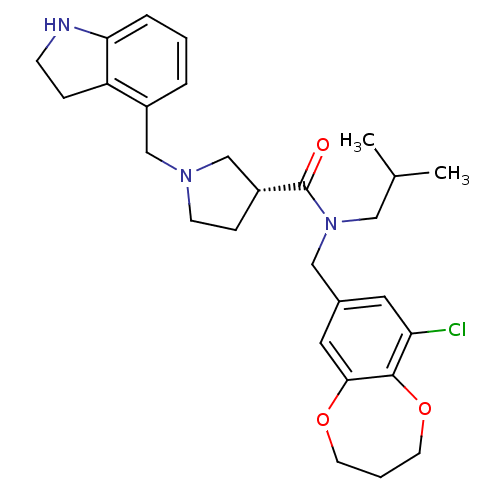 (US8722896, (-)-(3R)-1-(Indolin-4-ylmethyl)- N-(9-c...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.206 | -57.5 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
The Regents of the University of California US Patent | Assay Description An aequorin-based luminescent assay for calcium mobilization was used to measure mobilization of intracellular Ca2+ (Bullock et al., Mol Pharmacol 65... | US Patent US8722896 (2014) BindingDB Entry DOI: 10.7270/Q29G5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha3beta4 nAChR (unknown origin) expressed in HEK cell membranes after 4 hrs by liquid scintillation counting ... | J Nat Prod 81: 1029-1035 (2018) Article DOI: 10.1021/acs.jnatprod.8b00062 BindingDB Entry DOI: 10.7270/Q2R49TF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50465459 (CHEMBL4290888) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR (unknown origin) expressed in HEK cell membranes after 4 hrs by liquid scintillation counting ... | J Nat Prod 81: 1029-1035 (2018) Article DOI: 10.1021/acs.jnatprod.8b00062 BindingDB Entry DOI: 10.7270/Q2R49TF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 2 (Cricetulus griseus (Chinese hamster)) | BDBM121324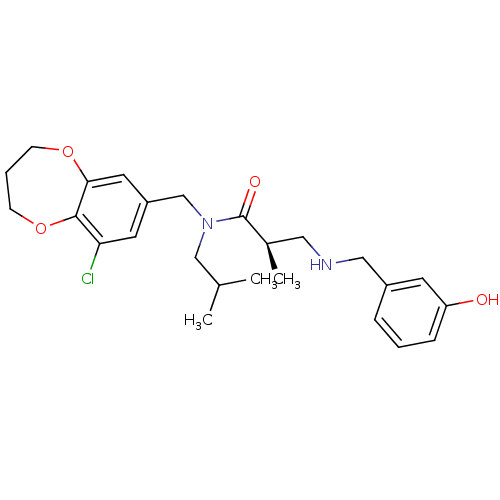 (US8722896, (-)-(2R)-2-Methyl-3-((3- hydroxy)benzyl...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | -56.7 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
The Regents of the University of California US Patent | Assay Description An aequorin-based luminescent assay for calcium mobilization was used to measure mobilization of intracellular Ca2+ (Bullock et al., Mol Pharmacol 65... | US Patent US8722896 (2014) BindingDB Entry DOI: 10.7270/Q29G5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123995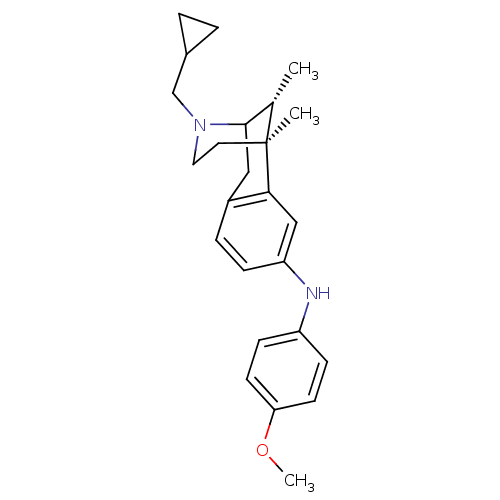 ((3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4,5,6-hex...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 from guinea pig brain using [3H]U69,593 as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8143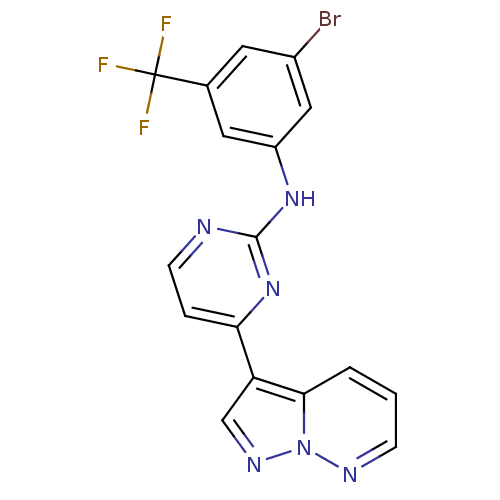 (N-[3-bromo-5-(trifluoromethyl)phenyl]-4-{pyrazolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123994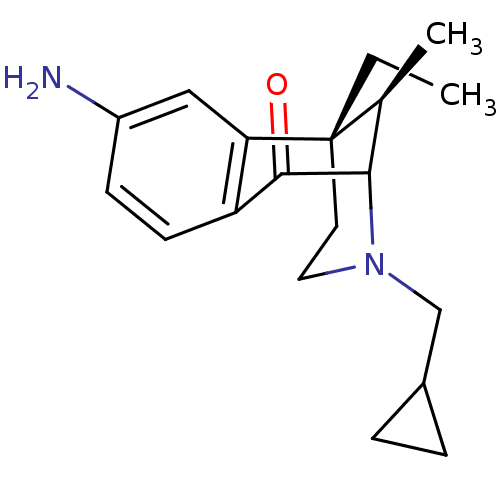 (8-Amino-3-cyclopropylmethyl-6-ethyl-11-methyl-3,4,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 from guinea pig brain using [3H]U69,593 as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50465456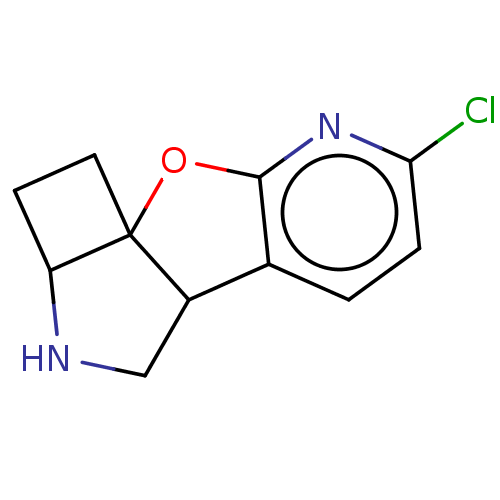 (CHEMBL4283703) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nAChR (unknown origin) expressed in HEK cell membranes after 4 hrs by liquid scintillation counting ... | J Nat Prod 81: 1029-1035 (2018) Article DOI: 10.1021/acs.jnatprod.8b00062 BindingDB Entry DOI: 10.7270/Q2R49TF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50123995 ((3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4,5,6-hex...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 from guinea pig brain using [3H]DAMGO as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123997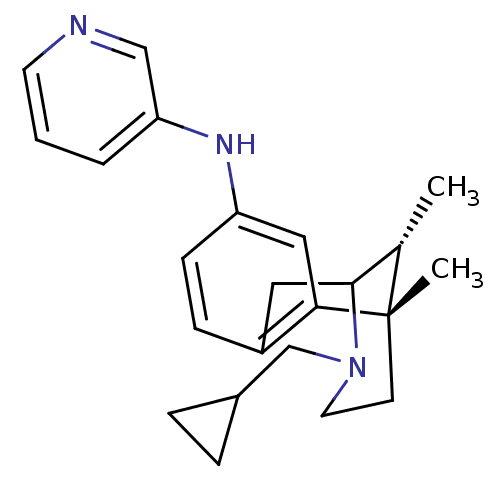 ((3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4,5,6-hex...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 from guinea pig brain using [3H]U69,593 as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 2 (Cricetulus griseus (Chinese hamster)) | BDBM121105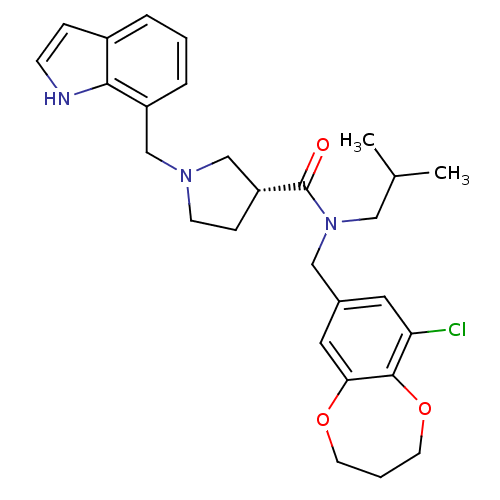 (US8722896, (-)-(3R)-1-(Indol-7-ylmethyl)-N- (9-chl...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.514 | -55.2 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
The Regents of the University of California US Patent | Assay Description An aequorin-based luminescent assay for calcium mobilization was used to measure mobilization of intracellular Ca2+ (Bullock et al., Mol Pharmacol 65... | US Patent US8722896 (2014) BindingDB Entry DOI: 10.7270/Q29G5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50297321 (CHEMBL551613 | Isoindolin-2-yl(4-(spiro[chromene-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells | J Med Chem 52: 5685-702 (2009) Article DOI: 10.1021/jm900773n BindingDB Entry DOI: 10.7270/Q2VD6ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50085020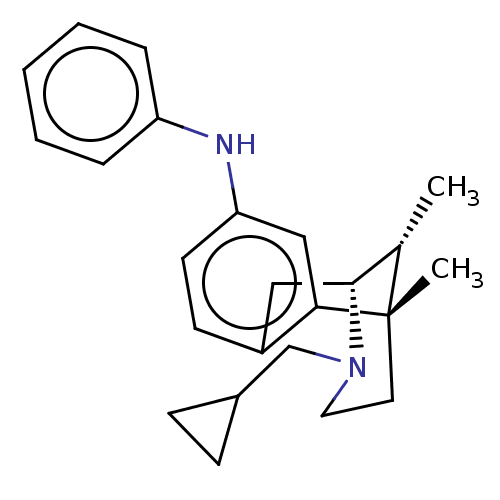 (((6S,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 from guinea pig brain using [3H]U69,593 as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 2 (Cricetulus griseus (Chinese hamster)) | BDBM121086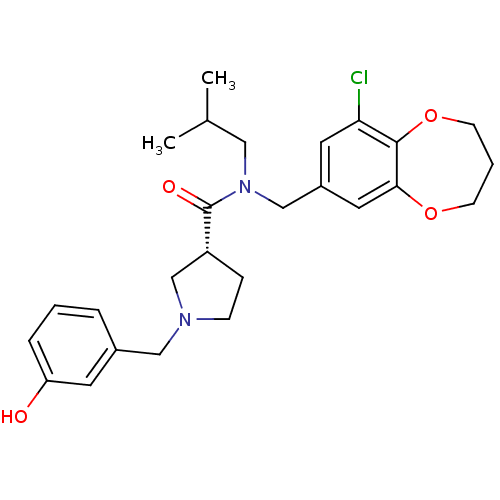 (US8722896, (-)-(3R)-1-(3-Hydroxybenzyl)- N-(9-chlo...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.550 | -55.0 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
The Regents of the University of California US Patent | Assay Description An aequorin-based luminescent assay for calcium mobilization was used to measure mobilization of intracellular Ca2+ (Bullock et al., Mol Pharmacol 65... | US Patent US8722896 (2014) BindingDB Entry DOI: 10.7270/Q29G5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 1 (Cricetulus griseus (Chinese hamster)) | BDBM121307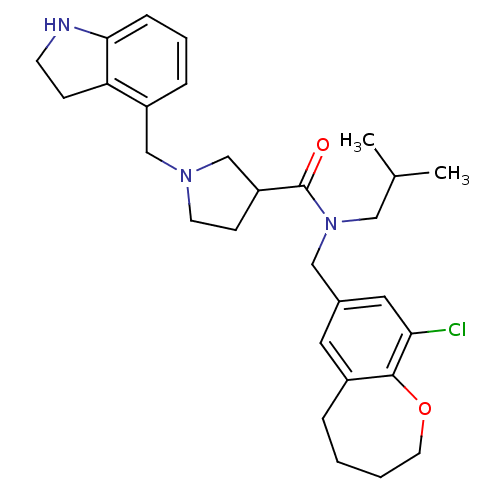 (US8722896, (-)-(3R)-1-(Indolin-4-ylmethyl)- N-(9-c...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.560 | -54.9 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
The Regents of the University of California US Patent | Assay Description An aequorin-based luminescent assay for calcium mobilization was used to measure mobilization of intracellular Ca2+ (Bullock et al., Mol Pharmacol 65... | US Patent US8722896 (2014) BindingDB Entry DOI: 10.7270/Q29G5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123988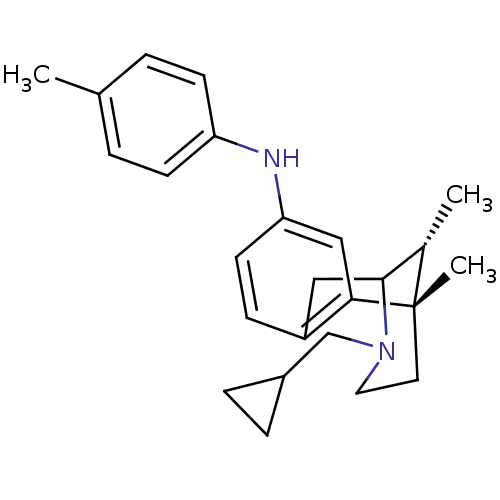 ((3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4,5,6-hex...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 from guinea pig brain using [3H]U69,593 as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 from guinea pig brain using [3H]naltrindole as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50000091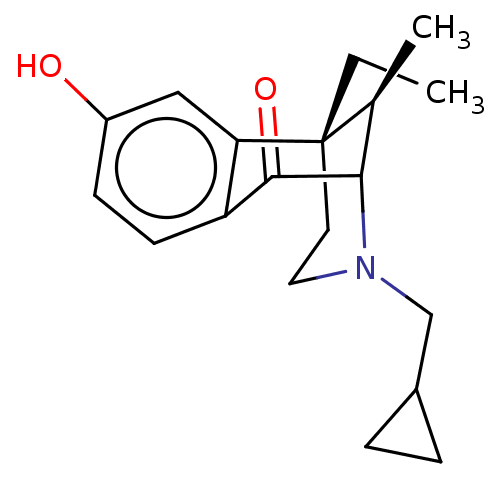 ((6S,11R)-3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 from guinea pig brain using [3H]U69,593 as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50369890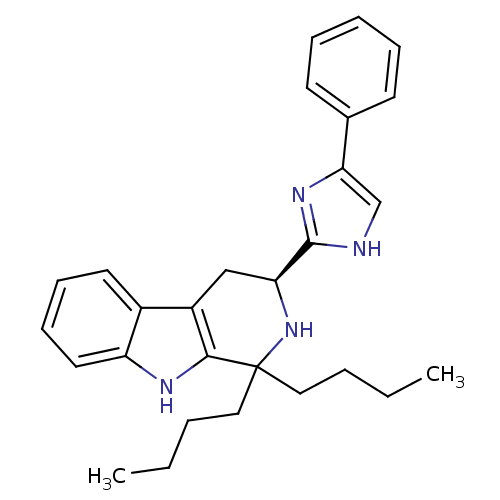 (CHEMBL1237140 | CHEMBL1788167) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50123997 ((3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4,5,6-hex...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 from guinea pig brain using [3H]DAMGO as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50085021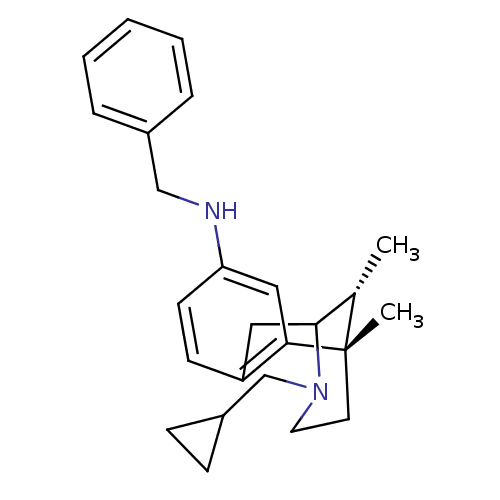 (Benzyl-((6S,11R)-3-cyclopropylmethyl-6,11-dimethyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 from guinea pig brain using [3H]DAMGO as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 2 (Cricetulus griseus (Chinese hamster)) | BDBM121068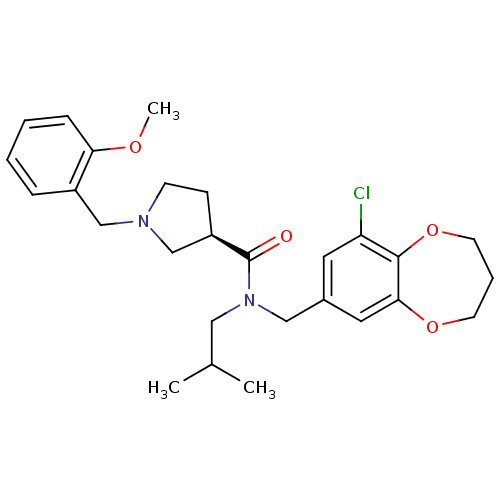 (US8722896, (-)-(3R)-1-(2-Methoxybenzyl)- N-(9-chlo...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.680 | -54.4 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
The Regents of the University of California US Patent | Assay Description An aequorin-based luminescent assay for calcium mobilization was used to measure mobilization of intracellular Ca2+ (Bullock et al., Mol Pharmacol 65... | US Patent US8722896 (2014) BindingDB Entry DOI: 10.7270/Q29G5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50297339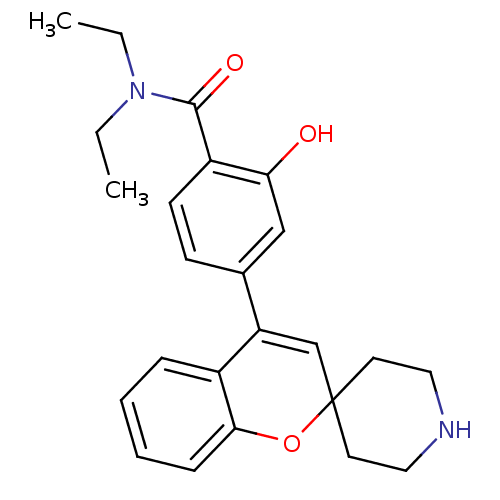 (CHEMBL557458 | N,N-Diethyl-2-hydroxy-4-(spiro[chro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells | J Med Chem 52: 5685-702 (2009) Article DOI: 10.1021/jm900773n BindingDB Entry DOI: 10.7270/Q2VD6ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 2 (Cricetulus griseus (Chinese hamster)) | BDBM121116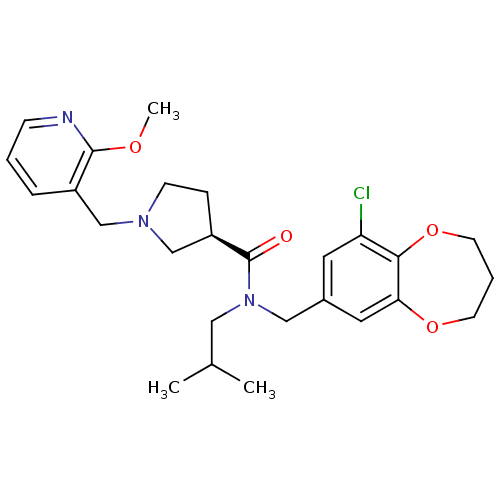 (US8722896, (-)-(3R)-N-(9-chloro-3,4- dihydro-2H-1,...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.710 | -54.3 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
The Regents of the University of California US Patent | Assay Description An aequorin-based luminescent assay for calcium mobilization was used to measure mobilization of intracellular Ca2+ (Bullock et al., Mol Pharmacol 65... | US Patent US8722896 (2014) BindingDB Entry DOI: 10.7270/Q29G5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 2 (Cricetulus griseus (Chinese hamster)) | BDBM121040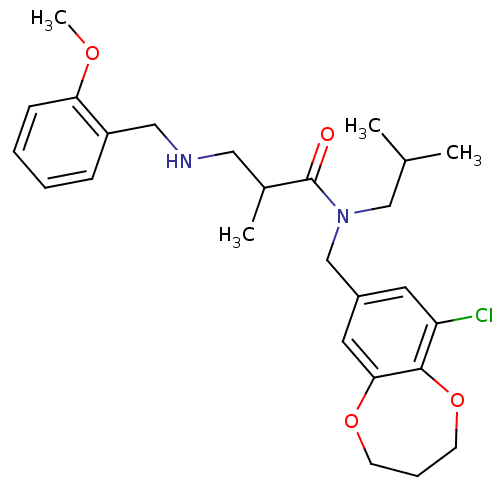 (US8722896, (+/-)-2-Methyl-3-((2- methoxy)benzylami...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.720 | -54.3 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
The Regents of the University of California US Patent | Assay Description An aequorin-based luminescent assay for calcium mobilization was used to measure mobilization of intracellular Ca2+ (Bullock et al., Mol Pharmacol 65... | US Patent US8722896 (2014) BindingDB Entry DOI: 10.7270/Q29G5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50000091 ((6S,11R)-3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 from guinea pig brain using [3H]DAMGO as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50124002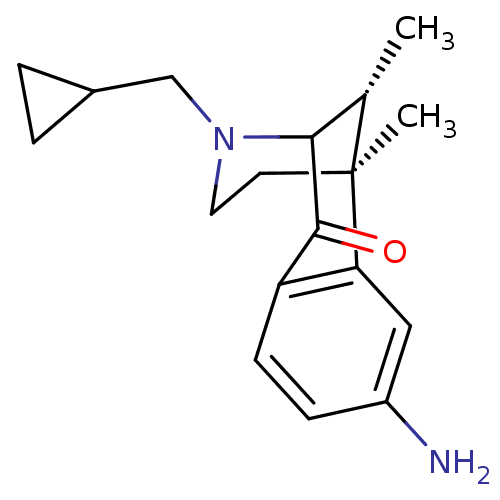 (8-Amino-3-cyclopropylmethyl-6,11-dimethyl-3,4,5,6-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 from guinea pig brain using [3H]U69,593 as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50213144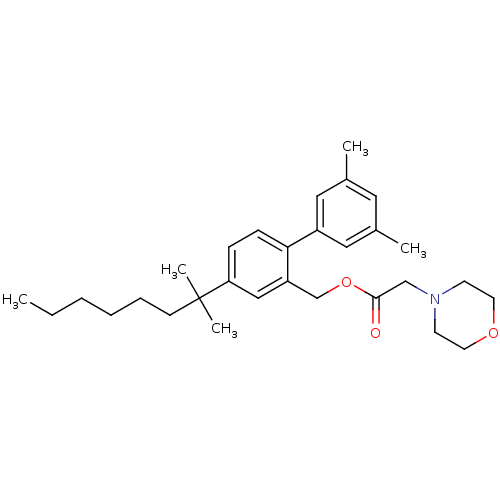 (CHEMBL233184 | morpholin-4-yl-acetic acid 4-(1,1-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from cloned human CB2 receptor | Bioorg Med Chem Lett 17: 3652-6 (2007) Article DOI: 10.1016/j.bmcl.2007.04.059 BindingDB Entry DOI: 10.7270/Q2GM8703 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 2 (Cricetulus griseus (Chinese hamster)) | BDBM121070 (US8722896, (-)-(3R)-1-(2-Methoxybenzyl)- N-(9-fluo...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.810 | -54.0 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
The Regents of the University of California US Patent | Assay Description An aequorin-based luminescent assay for calcium mobilization was used to measure mobilization of intracellular Ca2+ (Bullock et al., Mol Pharmacol 65... | US Patent US8722896 (2014) BindingDB Entry DOI: 10.7270/Q29G5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021074 (CHEMBL3287628) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.815 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 2 (Cricetulus griseus (Chinese hamster)) | BDBM121319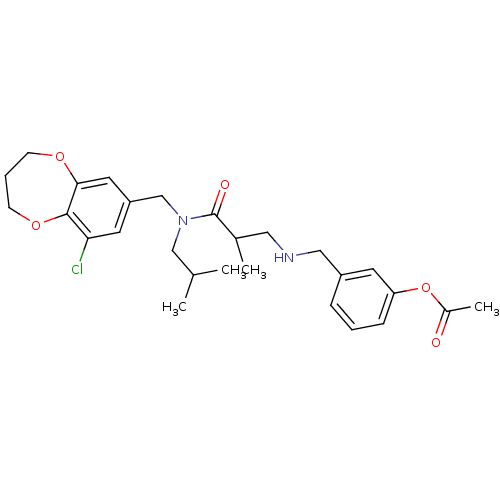 (US8722896, (+/-)-2-Methyl-3-((3- acetoxy)benzylami...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.890 | -53.7 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
The Regents of the University of California US Patent | Assay Description An aequorin-based luminescent assay for calcium mobilization was used to measure mobilization of intracellular Ca2+ (Bullock et al., Mol Pharmacol 65... | US Patent US8722896 (2014) BindingDB Entry DOI: 10.7270/Q29G5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50123988 ((3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4,5,6-hex...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 from guinea pig brain using [3H]DAMGO as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50297329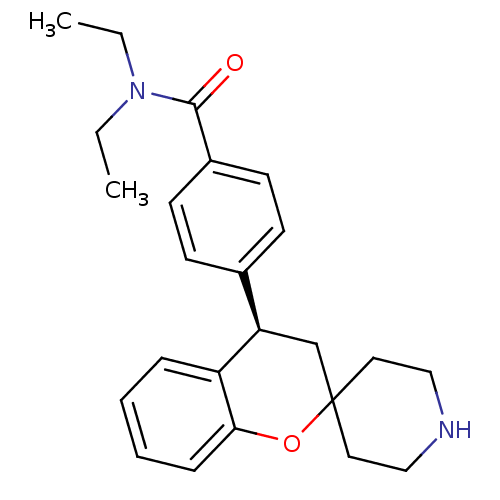 ((R)-N,N-diethyl-4-(spiro[chroman-2,4'-piperidine]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells | J Med Chem 52: 5685-702 (2009) Article DOI: 10.1021/jm900773n BindingDB Entry DOI: 10.7270/Q2VD6ZH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prokineticin receptor 2 (Cricetulus griseus (Chinese hamster)) | BDBM121324 (US8722896, (-)-(2R)-2-Methyl-3-((3- hydroxy)benzyl...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.930 | -53.6 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
The Regents of the University of California US Patent | Assay Description An aequorin-based luminescent assay for calcium mobilization was used to measure mobilization of intracellular Ca2+ (Bullock et al., Mol Pharmacol 65... | US Patent US8722896 (2014) BindingDB Entry DOI: 10.7270/Q29G5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8138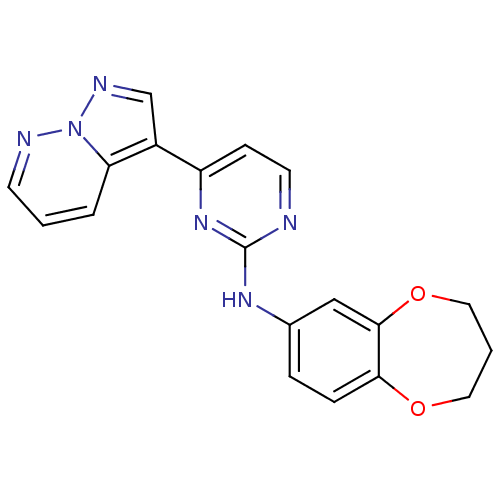 (N-(3,4-dihydro-2H-1,5-benzodioxepin-7-yl)-4-{pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8145 (N-(3,5-dichlorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8136 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8146 (N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123985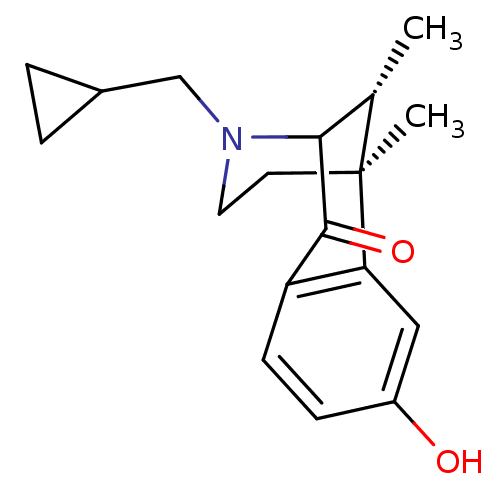 (3-Cyclopropylmethyl-8-hydroxy-6,11-dimethyl-3,4,5,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 from guinea pig brain using [3H]U69,593 as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50546247 (AR-11324 FREE BASE | AR-13324 | Netarsudil | US114...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50546247 (AR-11324 FREE BASE | AR-13324 | Netarsudil | US114...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123993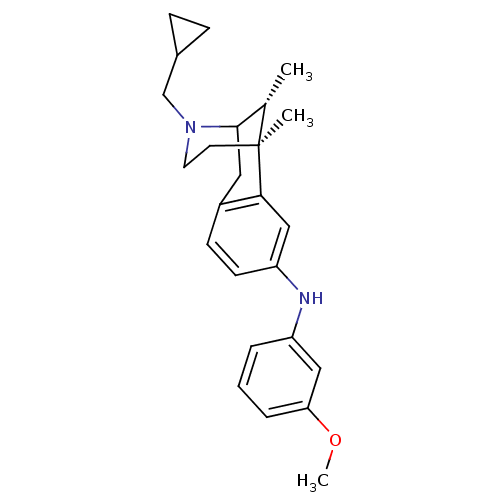 ((3-Cyclopropylmethyl-6,11-dimethyl-1,2,3,4,5,6-hex...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 from guinea pig brain using [3H]U69,593 as radioligand | J Med Chem 46: 838-49 (2003) Article DOI: 10.1021/jm020429w BindingDB Entry DOI: 10.7270/Q2Z31Z09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50213141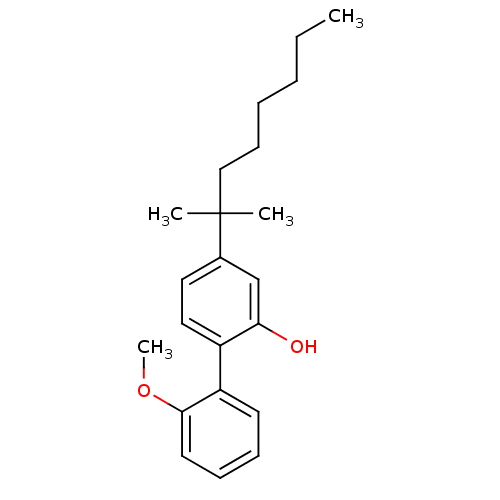 (4-(1,1-dimethyl-heptyl)-2'-methoxy-biphenyl-2-ol |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from cloned human CB2 receptor | Bioorg Med Chem Lett 17: 3652-6 (2007) Article DOI: 10.1016/j.bmcl.2007.04.059 BindingDB Entry DOI: 10.7270/Q2GM8703 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7800 total ) | Next | Last >> |