 Found 208 hits with Last Name = 'hoerer' and Initial = 's'
Found 208 hits with Last Name = 'hoerer' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329791
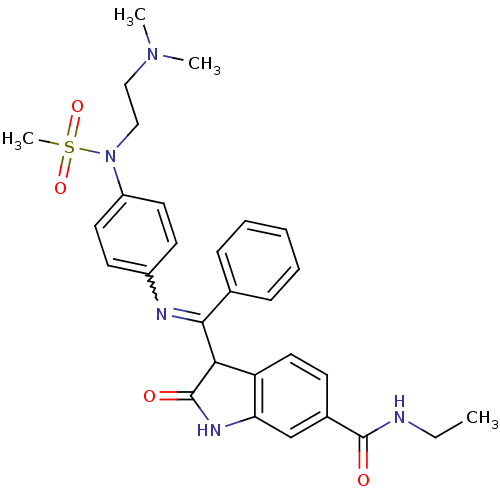
((Z)-3-((4-(N-(2-(dimethylamino)ethyl)methylsulfona...)Show SMILES CCNC(=O)c1ccc2C(C(=Nc3ccc(cc3)N(CCN(C)C)S(C)(=O)=O)c3ccccc3)C(=O)Nc2c1 |w:11.11| Show InChI InChI=1S/C29H33N5O4S/c1-5-30-28(35)21-11-16-24-25(19-21)32-29(36)26(24)27(20-9-7-6-8-10-20)31-22-12-14-23(15-13-22)34(39(4,37)38)18-17-33(2)3/h6-16,19,26H,5,17-18H2,1-4H3,(H,30,35)(H,32,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285776
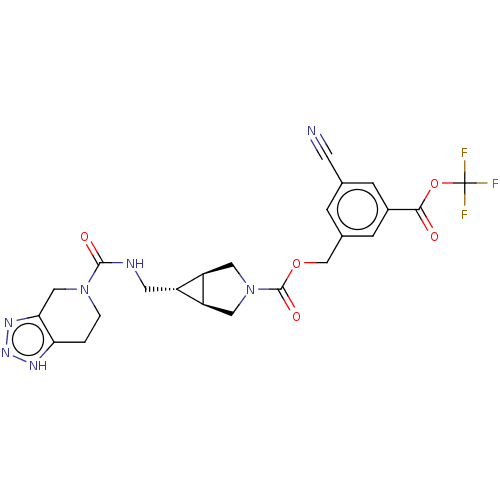
(CHEMBL4172309)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)N1CCc2[nH]nnc2C1)C(=O)OCc1cc(cc(c1)C(=O)OC(F)(F)F)C#N |r| Show InChI InChI=1S/C23H22F3N7O5/c24-23(25,26)38-20(34)14-4-12(6-27)3-13(5-14)11-37-22(36)33-8-16-15(17(16)9-33)7-28-21(35)32-2-1-18-19(10-32)30-31-29-18/h3-5,15-17H,1-2,7-11H2,(H,28,35)(H,29,30,31)/t15-,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285750
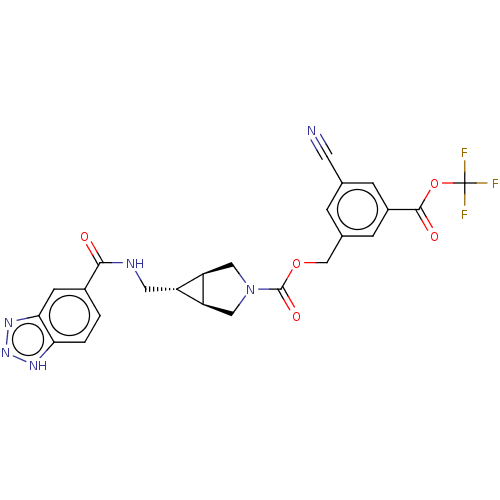
(CHEMBL4173049)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]nnc2c1)C(=O)OCc1cc(cc(c1)C(=O)OC(F)(F)F)C#N |r| Show InChI InChI=1S/C24H19F3N6O5/c25-24(26,27)38-22(35)15-4-12(7-28)3-13(5-15)11-37-23(36)33-9-17-16(18(17)10-33)8-29-21(34)14-1-2-19-20(6-14)31-32-30-19/h1-6,16-18H,8-11H2,(H,29,34)(H,30,31,32)/t16-,17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285745
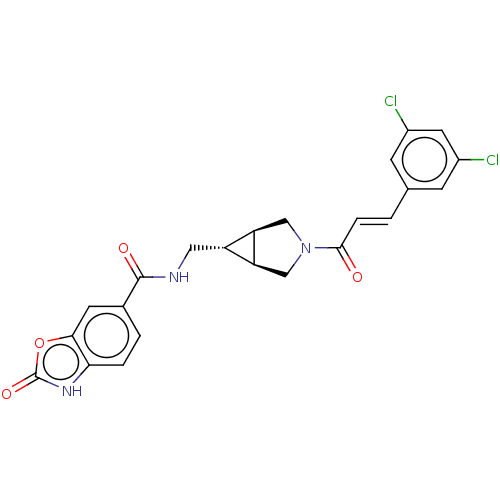
(CHEMBL4162641)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)\C=C\c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C23H19Cl2N3O4/c24-14-5-12(6-15(25)8-14)1-4-21(29)28-10-17-16(18(17)11-28)9-26-22(30)13-2-3-19-20(7-13)32-23(31)27-19/h1-8,16-18H,9-11H2,(H,26,30)(H,27,31)/b4-1+/t16-,17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285744
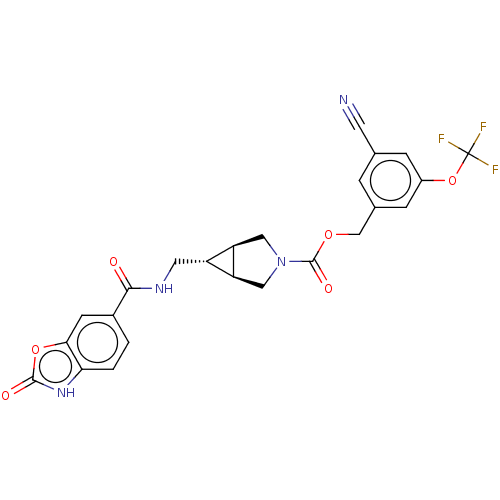
(CHEMBL4168498)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)OCc1cc(OC(F)(F)F)cc(c1)C#N |r| Show InChI InChI=1S/C24H19F3N4O6/c25-24(26,27)37-15-4-12(7-28)3-13(5-15)11-35-23(34)31-9-17-16(18(17)10-31)8-29-21(32)14-1-2-19-20(6-14)36-22(33)30-19/h1-6,16-18H,8-11H2,(H,29,32)(H,30,33)/t16-,17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50285777
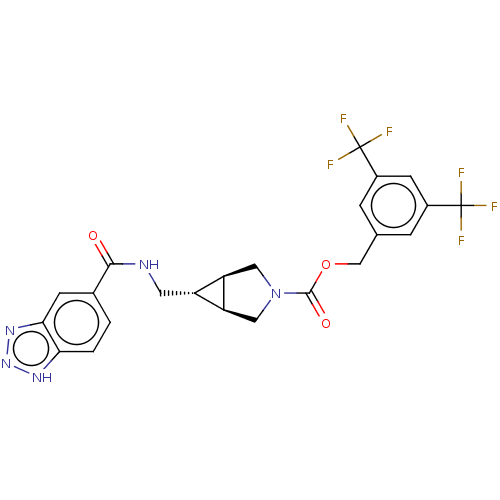
(CHEMBL4165749)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]nnc2c1)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H19F6N5O3/c24-22(25,26)13-3-11(4-14(6-13)23(27,28)29)10-37-21(36)34-8-16-15(17(16)9-34)7-30-20(35)12-1-2-18-19(5-12)32-33-31-18/h1-6,15-17H,7-10H2,(H,30,35)(H,31,32,33)/t15-,16-,17+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human ATX |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285748
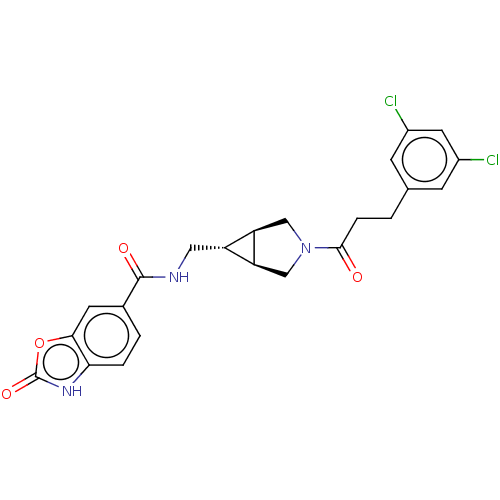
(CHEMBL4173341)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)CCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C23H21Cl2N3O4/c24-14-5-12(6-15(25)8-14)1-4-21(29)28-10-17-16(18(17)11-28)9-26-22(30)13-2-3-19-20(7-13)32-23(31)27-19/h2-3,5-8,16-18H,1,4,9-11H2,(H,26,30)(H,27,31)/t16-,17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285774
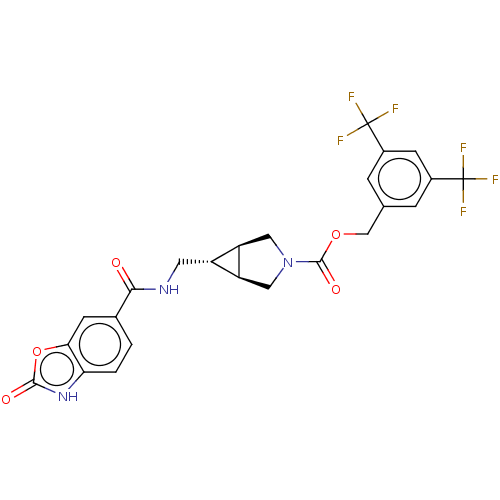
(CHEMBL4169550)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H19F6N3O5/c25-23(26,27)13-3-11(4-14(6-13)24(28,29)30)10-37-22(36)33-8-16-15(17(16)9-33)7-31-20(34)12-1-2-18-19(5-12)38-21(35)32-18/h1-6,15-17H,7-10H2,(H,31,34)(H,32,35)/t15-,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329795
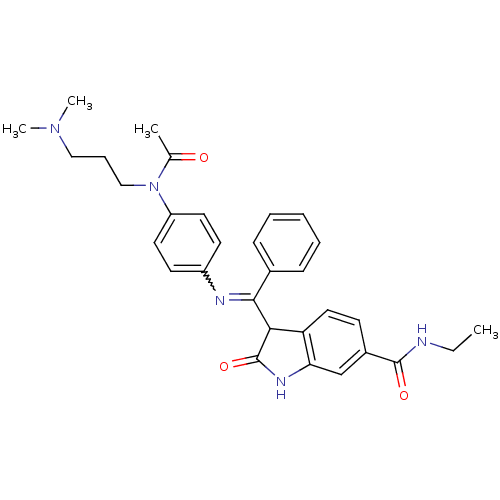
((Z)-3-((4-(N-(3-(dimethylamino)propyl)acetamido)ph...)Show SMILES CCNC(=O)c1ccc2C(C(=Nc3ccc(cc3)N(CCCN(C)C)C(C)=O)c3ccccc3)C(=O)Nc2c1 |w:11.11| Show InChI InChI=1S/C31H35N5O3/c1-5-32-30(38)23-12-17-26-27(20-23)34-31(39)28(26)29(22-10-7-6-8-11-22)33-24-13-15-25(16-14-24)36(21(2)37)19-9-18-35(3)4/h6-8,10-17,20,28H,5,9,18-19H2,1-4H3,(H,32,38)(H,34,39) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329793

((Z)-3-((4-(N-(2-(dimethylamino)ethyl)acetamido)phe...)Show SMILES CCNC(=O)c1ccc2C(C(=Nc3ccc(cc3)N(CCN(C)C)C(C)=O)c3ccccc3)C(=O)Nc2c1 |w:11.11| Show InChI InChI=1S/C30H33N5O3/c1-5-31-29(37)22-11-16-25-26(19-22)33-30(38)27(25)28(21-9-7-6-8-10-21)32-23-12-14-24(15-13-23)35(20(2)36)18-17-34(3)4/h6-16,19,27H,5,17-18H2,1-4H3,(H,31,37)(H,33,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50187693

(CHEMBL3186509)Show SMILES Clc1cc(Cl)cc(COC(=O)N2CCN(CCC(=O)c3ccc4[nH]c(=O)oc4c3)CC2)c1 Show InChI InChI=1S/C22H21Cl2N3O5/c23-16-9-14(10-17(24)12-16)13-31-22(30)27-7-5-26(6-8-27)4-3-19(28)15-1-2-18-20(11-15)32-21(29)25-18/h1-2,9-12H,3-8,13H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human full length ATX expressed in HEK cells using FS-3 as substrate incubated for 15 mins followed by substrate addition measured afte... |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285751
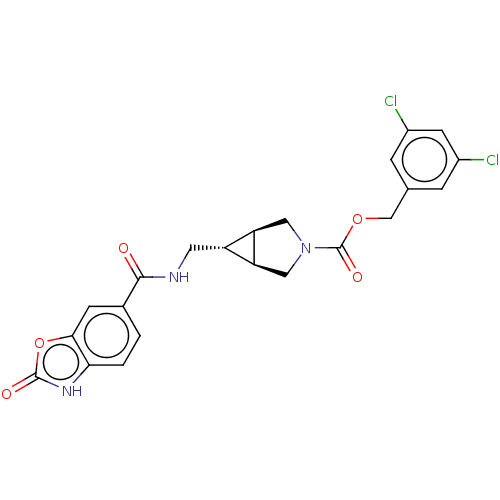
(CHEMBL4169912)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C22H19Cl2N3O5/c23-13-3-11(4-14(24)6-13)10-31-22(30)27-8-16-15(17(16)9-27)7-25-20(28)12-1-2-18-19(5-12)32-21(29)26-18/h1-6,15-17H,7-10H2,(H,25,28)(H,26,29)/t15-,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285777

(CHEMBL4165749)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]nnc2c1)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H19F6N5O3/c24-22(25,26)13-3-11(4-14(6-13)23(27,28)29)10-37-21(36)34-8-16-15(17(16)9-34)7-30-20(35)12-1-2-18-19(5-12)32-33-31-18/h1-6,15-17H,7-10H2,(H,30,35)(H,31,32,33)/t15-,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285774

(CHEMBL4169550)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H19F6N3O5/c25-23(26,27)13-3-11(4-14(6-13)24(28,29)30)10-37-22(36)33-8-16-15(17(16)9-33)7-31-20(34)12-1-2-18-19(5-12)38-21(35)32-18/h1-6,15-17H,7-10H2,(H,31,34)(H,32,35)/t15-,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of ATX in rat whole blood using LPA 17:0 as substrate after 1 hr by LC-MS/MS analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285773
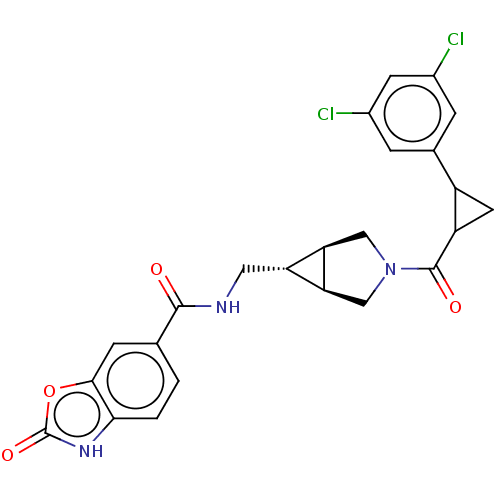
(CHEMBL4170966)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)C1CC1c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C24H21Cl2N3O4/c25-13-3-12(4-14(26)6-13)15-7-16(15)23(31)29-9-18-17(19(18)10-29)8-27-22(30)11-1-2-20-21(5-11)33-24(32)28-20/h1-6,15-19H,7-10H2,(H,27,30)(H,28,32)/t15?,16?,17-,18-,19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285743
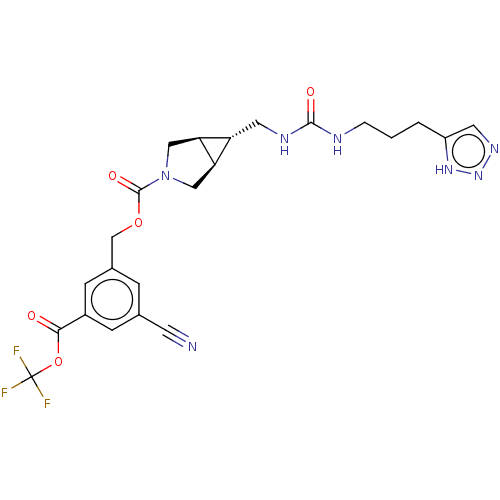
(CHEMBL4169136)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)NCCCc1cnn[nH]1)C(=O)OCc1cc(cc(c1)C(=O)OC(F)(F)F)C#N |r| Show InChI InChI=1S/C23H24F3N7O5/c24-23(25,26)38-20(34)15-5-13(7-27)4-14(6-15)12-37-22(36)33-10-18-17(19(18)11-33)9-29-21(35)28-3-1-2-16-8-30-32-31-16/h4-6,8,17-19H,1-3,9-12H2,(H2,28,29,35)(H,30,31,32)/t17-,18-,19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285775
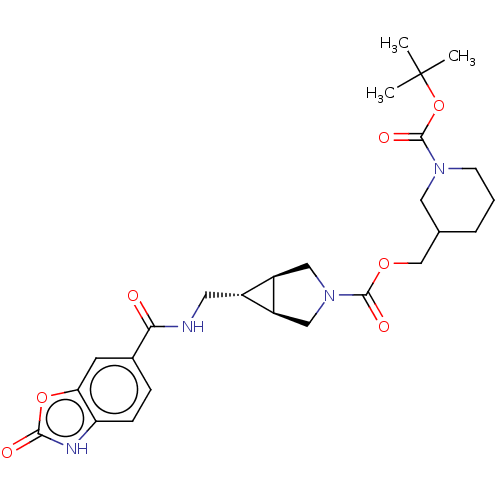
(CHEMBL4159308)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)OCC1CCCN(C1)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C26H34N4O7/c1-26(2,3)37-25(34)29-8-4-5-15(11-29)14-35-24(33)30-12-18-17(19(18)13-30)10-27-22(31)16-6-7-20-21(9-16)36-23(32)28-20/h6-7,9,15,17-19H,4-5,8,10-14H2,1-3H3,(H,27,31)(H,28,32)/t15?,17-,18-,19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of ATX in rat whole blood using LPA 17:0 as substrate after 1 hr by LC-MS/MS analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329792
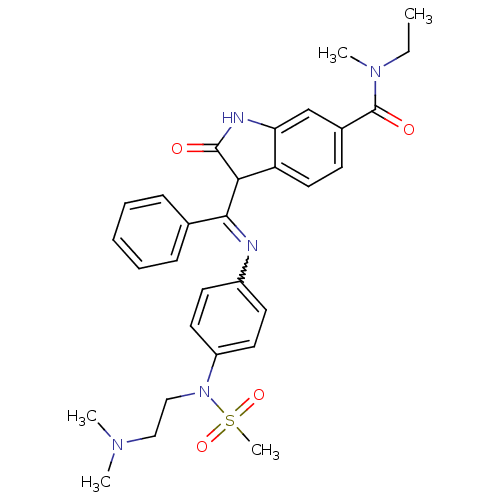
((Z)-3-((4-(N-(2-(dimethylamino)ethyl)methylsulfona...)Show SMILES CCN(C)C(=O)c1ccc2C(C(=Nc3ccc(cc3)N(CCN(C)C)S(C)(=O)=O)c3ccccc3)C(=O)Nc2c1 |w:12.12| Show InChI InChI=1S/C30H35N5O4S/c1-6-34(4)30(37)22-12-17-25-26(20-22)32-29(36)27(25)28(21-10-8-7-9-11-21)31-23-13-15-24(16-14-23)35(40(5,38)39)19-18-33(2)3/h7-17,20,27H,6,18-19H2,1-5H3,(H,32,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329798
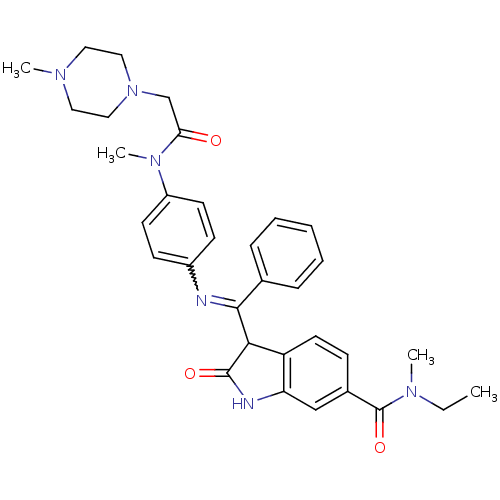
((Z)-N-ethyl-N-methyl-3-((4-(N-methyl-2-(4-methylpi...)Show SMILES CCN(C)C(=O)c1ccc2C(C(=Nc3ccc(cc3)N(C)C(=O)CN3CCN(C)CC3)c3ccccc3)C(=O)Nc2c1 |w:12.12| Show InChI InChI=1S/C33H38N6O3/c1-5-37(3)33(42)24-11-16-27-28(21-24)35-32(41)30(27)31(23-9-7-6-8-10-23)34-25-12-14-26(15-13-25)38(4)29(40)22-39-19-17-36(2)18-20-39/h6-16,21,30H,5,17-20,22H2,1-4H3,(H,35,41) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285748

(CHEMBL4173341)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)CCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C23H21Cl2N3O4/c24-14-5-12(6-15(25)8-14)1-4-21(29)28-10-17-16(18(17)11-28)9-26-22(30)13-2-3-19-20(7-13)32-23(31)27-19/h2-3,5-8,16-18H,1,4,9-11H2,(H,26,30)(H,27,31)/t16-,17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of ATX in rat whole blood using LPA 17:0 as substrate after 1 hr by LC-MS/MS analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329794
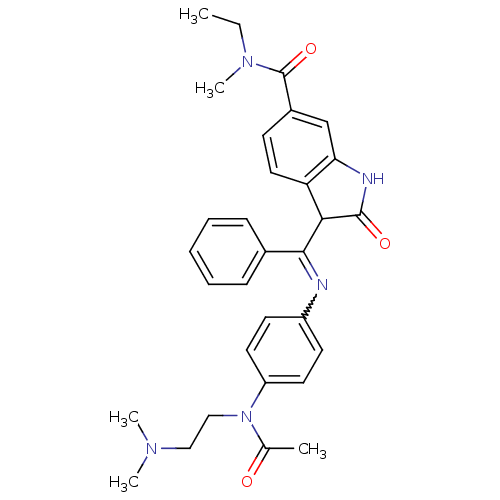
((Z)-3-((4-(N-(2-(dimethylamino)ethyl)acetamido)phe...)Show SMILES CCN(C)C(=O)c1ccc2C(C(=Nc3ccc(cc3)N(CCN(C)C)C(C)=O)c3ccccc3)C(=O)Nc2c1 |w:12.12| Show InChI InChI=1S/C31H35N5O3/c1-6-35(5)31(39)23-12-17-26-27(20-23)33-30(38)28(26)29(22-10-8-7-9-11-22)32-24-13-15-25(16-14-24)36(21(2)37)19-18-34(3)4/h7-17,20,28H,6,18-19H2,1-5H3,(H,33,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50272797
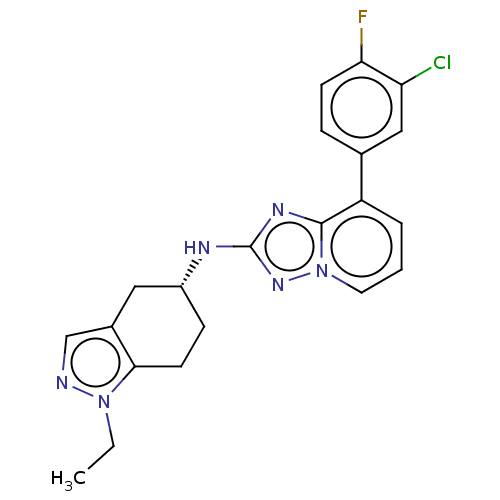
(CHEMBL4126886)Show SMILES CCn1ncc2C[C@@H](CCc12)Nc1nc2c(cccn2n1)-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C21H20ClFN6/c1-2-28-19-8-6-15(10-14(19)12-24-28)25-21-26-20-16(4-3-9-29(20)27-21)13-5-7-18(23)17(22)11-13/h3-5,7,9,11-12,15H,2,6,8,10H2,1H3,(H,25,27)/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells expressing human wild type APP assessed as inhibition of amyloid beta 42 production after 22 hrs by e... |
Bioorg Med Chem 26: 3227-3241 (2018)
Article DOI: 10.1016/j.bmc.2018.04.053
BindingDB Entry DOI: 10.7270/Q24X5B97 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329797
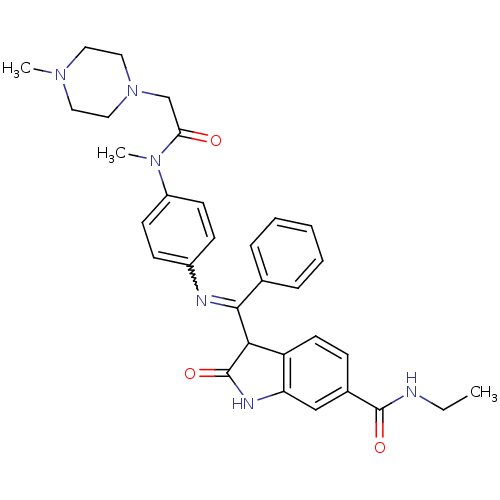
((Z)-N-ethyl-3-((4-(N-methyl-2-(4-methylpiperazin-1...)Show SMILES CCNC(=O)c1ccc2C(C(=Nc3ccc(cc3)N(C)C(=O)CN3CCN(C)CC3)c3ccccc3)C(=O)Nc2c1 |w:11.11| Show InChI InChI=1S/C32H36N6O3/c1-4-33-31(40)23-10-15-26-27(20-23)35-32(41)29(26)30(22-8-6-5-7-9-22)34-24-11-13-25(14-12-24)37(3)28(39)21-38-18-16-36(2)17-19-38/h5-15,20,29H,4,16-19,21H2,1-3H3,(H,33,40)(H,35,41) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329796
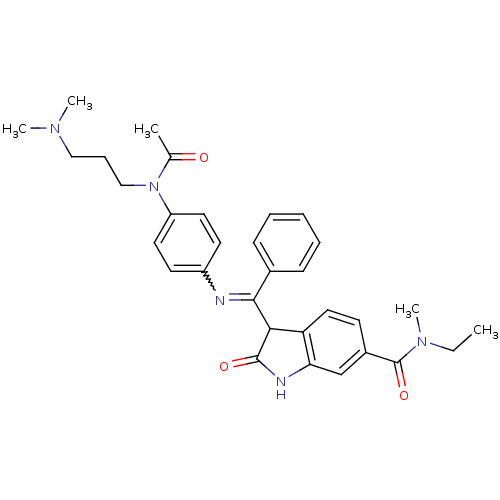
((Z)-3-((4-(N-(3-(dimethylamino)propyl)acetamido)ph...)Show SMILES CCN(C)C(=O)c1ccc2C(C(=Nc3ccc(cc3)N(CCCN(C)C)C(C)=O)c3ccccc3)C(=O)Nc2c1 |w:12.12| Show InChI InChI=1S/C32H37N5O3/c1-6-36(5)32(40)24-13-18-27-28(21-24)34-31(39)29(27)30(23-11-8-7-9-12-23)33-25-14-16-26(17-15-25)37(22(2)38)20-10-19-35(3)4/h7-9,11-18,21,29H,6,10,19-20H2,1-5H3,(H,34,39) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285778
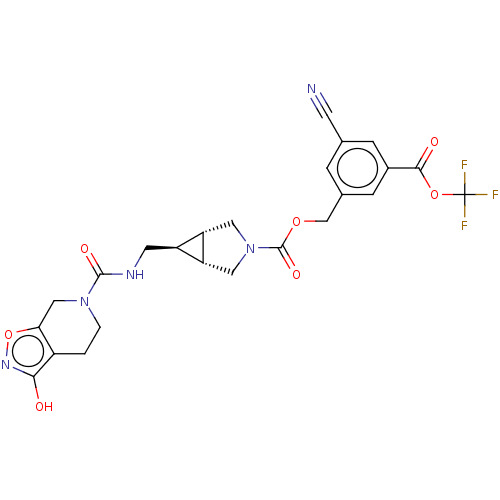
(CHEMBL4164935)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)N1CCc2c(O)noc2C1)C(=O)OCc1cc(cc(c1)C(=O)OC(F)(F)F)C#N |r| Show InChI InChI=1S/C24H22F3N5O7/c25-24(26,27)38-21(34)14-4-12(6-28)3-13(5-14)11-37-23(36)32-8-17-16(18(17)9-32)7-29-22(35)31-2-1-15-19(10-31)39-30-20(15)33/h3-5,16-18H,1-2,7-11H2,(H,29,35)(H,30,33)/t16-,17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285742

(CHEMBL4163050)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)NCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C22H20Cl2N4O4/c23-13-3-11(4-14(24)6-13)7-26-21(30)28-9-16-15(17(16)10-28)8-25-20(29)12-1-2-18-19(5-12)32-22(31)27-18/h1-6,15-17H,7-10H2,(H,25,29)(H,26,30)(H,27,31)/t15-,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329814
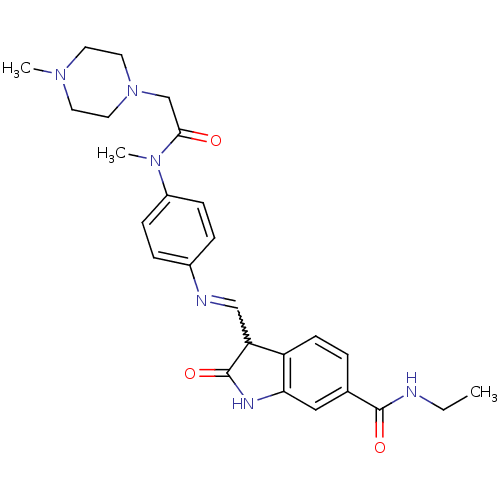
((Z)-N-ethyl-3-((4-(N-methyl-2-(4-methylpiperazin-1...)Show SMILES CCNC(=O)c1ccc2C(C=Nc3ccc(cc3)N(C)C(=O)CN3CCN(C)CC3)C(=O)Nc2c1 |w:10.9| Show InChI InChI=1S/C26H32N6O3/c1-4-27-25(34)18-5-10-21-22(26(35)29-23(21)15-18)16-28-19-6-8-20(9-7-19)31(3)24(33)17-32-13-11-30(2)12-14-32/h5-10,15-16,22H,4,11-14,17H2,1-3H3,(H,27,34)(H,29,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329813
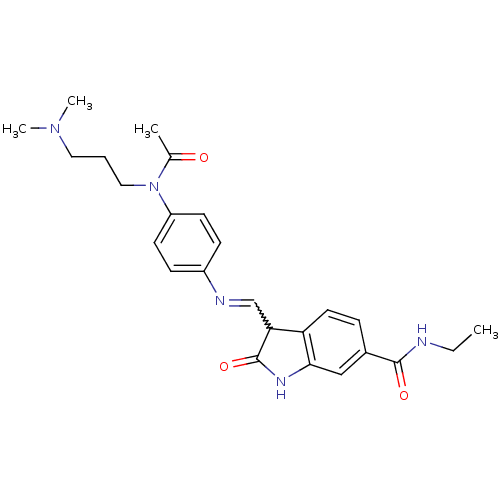
((Z)-3-((4-(N-(3-(dimethylamino)propyl)acetamido)ph...)Show SMILES CCNC(=O)c1ccc2C(C=Nc3ccc(cc3)N(CCCN(C)C)C(C)=O)C(=O)Nc2c1 |w:10.9| Show InChI InChI=1S/C25H31N5O3/c1-5-26-24(32)18-7-12-21-22(25(33)28-23(21)15-18)16-27-19-8-10-20(11-9-19)30(17(2)31)14-6-13-29(3)4/h7-12,15-16,22H,5-6,13-14H2,1-4H3,(H,26,32)(H,28,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285750

(CHEMBL4173049)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]nnc2c1)C(=O)OCc1cc(cc(c1)C(=O)OC(F)(F)F)C#N |r| Show InChI InChI=1S/C24H19F3N6O5/c25-24(26,27)38-22(35)15-4-12(7-28)3-13(5-15)11-37-23(36)33-9-17-16(18(17)10-33)8-29-21(34)14-1-2-19-20(6-14)31-32-30-19/h1-6,16-18H,8-11H2,(H,29,34)(H,30,31,32)/t16-,17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of ATX in rat whole blood using LPA 17:0 as substrate after 1 hr by LC-MS/MS analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329799
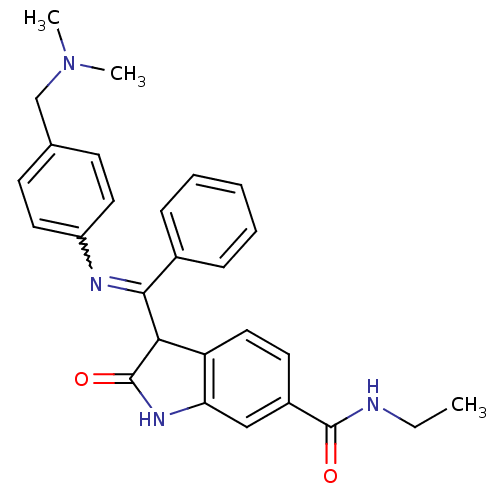
((Z)-3-((4-((dimethylamino)methyl)phenylamino)(phen...)Show SMILES CCNC(=O)c1ccc2C(C(=Nc3ccc(CN(C)C)cc3)c3ccccc3)C(=O)Nc2c1 |w:11.11| Show InChI InChI=1S/C27H28N4O2/c1-4-28-26(32)20-12-15-22-23(16-20)30-27(33)24(22)25(19-8-6-5-7-9-19)29-21-13-10-18(11-14-21)17-31(2)3/h5-16,24H,4,17H2,1-3H3,(H,28,32)(H,30,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50272769
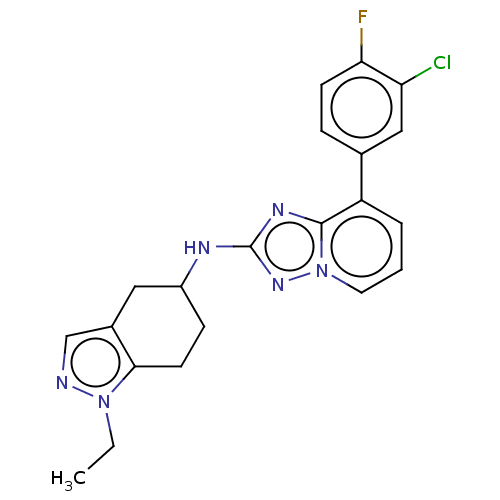
(CHEMBL4127386)Show SMILES CCn1ncc2CC(CCc12)Nc1nc2c(cccn2n1)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C21H20ClFN6/c1-2-28-19-8-6-15(10-14(19)12-24-28)25-21-26-20-16(4-3-9-29(20)27-21)13-5-7-18(23)17(22)11-13/h3-5,7,9,11-12,15H,2,6,8,10H2,1H3,(H,25,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells expressing human wild type APP assessed as inhibition of amyloid beta 42 production after 22 hrs by e... |
Bioorg Med Chem 26: 3227-3241 (2018)
Article DOI: 10.1016/j.bmc.2018.04.053
BindingDB Entry DOI: 10.7270/Q24X5B97 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329784
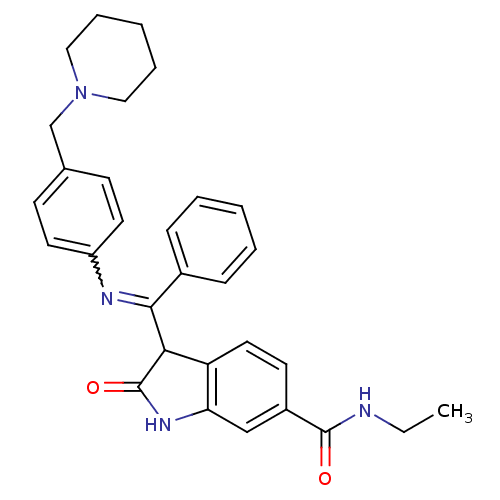
((Z)-3-[Phenyl-(4-piperidin-1-ylmethyl-phenylamino)...)Show SMILES CCNC(=O)c1ccc2C(C(=Nc3ccc(CN4CCCCC4)cc3)c3ccccc3)C(=O)Nc2c1 |w:11.11| Show InChI InChI=1S/C30H32N4O2/c1-2-31-29(35)23-13-16-25-26(19-23)33-30(36)27(25)28(22-9-5-3-6-10-22)32-24-14-11-21(12-15-24)20-34-17-7-4-8-18-34/h3,5-6,9-16,19,27H,2,4,7-8,17-18,20H2,1H3,(H,31,35)(H,33,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329801
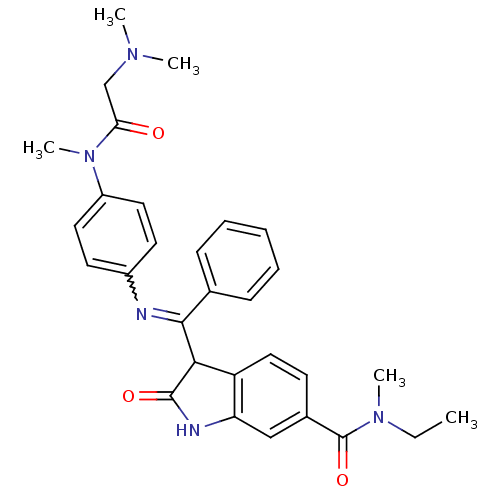
((Z)-3-((4-(2-(dimethylamino)-N-methylacetamido)phe...)Show SMILES CCN(C)C(=O)c1ccc2C(C(=Nc3ccc(cc3)N(C)C(=O)CN(C)C)c3ccccc3)C(=O)Nc2c1 |w:12.12| Show InChI InChI=1S/C30H33N5O3/c1-6-34(4)30(38)21-12-17-24-25(18-21)32-29(37)27(24)28(20-10-8-7-9-11-20)31-22-13-15-23(16-14-22)35(5)26(36)19-33(2)3/h7-18,27H,6,19H2,1-5H3,(H,32,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50285777

(CHEMBL4165749)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]nnc2c1)C(=O)OCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H19F6N5O3/c24-22(25,26)13-3-11(4-14(6-13)23(27,28)29)10-37-21(36)34-8-16-15(17(16)9-34)7-30-20(35)12-1-2-18-19(5-12)32-33-31-18/h1-6,15-17H,7-10H2,(H,30,35)(H,31,32,33)/t15-,16-,17+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of ATX in human whole blood |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50272768

(CHEMBL4129994)Show SMILES CCn1ncc2CC(CCc12)Nc1nc2c(cccn2n1)-c1cc(F)c(F)c(F)c1 Show InChI InChI=1S/C21H19F3N6/c1-2-29-18-6-5-14(8-13(18)11-25-29)26-21-27-20-15(4-3-7-30(20)28-21)12-9-16(22)19(24)17(23)10-12/h3-4,7,9-11,14H,2,5-6,8H2,1H3,(H,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells expressing human wild type APP assessed as inhibition of amyloid beta 42 production after 22 hrs by e... |
Bioorg Med Chem 26: 3227-3241 (2018)
Article DOI: 10.1016/j.bmc.2018.04.053
BindingDB Entry DOI: 10.7270/Q24X5B97 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329802
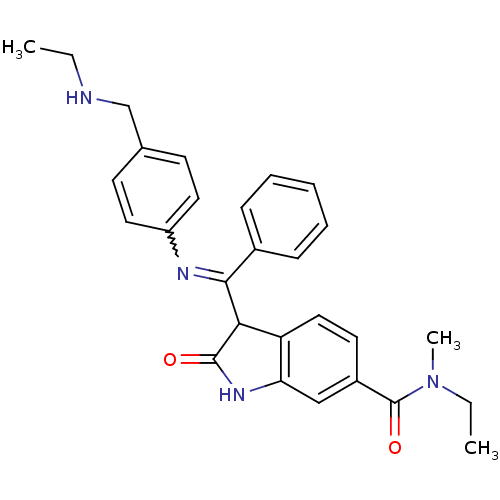
((Z)-N-ethyl-3-((4-((ethylamino)methyl)phenylamino)...)Show SMILES CCNCc1ccc(cc1)N=C(C1C(=O)Nc2cc(ccc12)C(=O)N(C)CC)c1ccccc1 |w:10.10| Show InChI InChI=1S/C28H30N4O2/c1-4-29-18-19-11-14-22(15-12-19)30-26(20-9-7-6-8-10-20)25-23-16-13-21(28(34)32(3)5-2)17-24(23)31-27(25)33/h6-17,25,29H,4-5,18H2,1-3H3,(H,31,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50279165

(CHEMBL1203982 | CHEMBL497724)Show SMILES CNC(=O)c1ccc2C(C(=Nc3ccc(CN4CCCCC4)cc3)c3ccccc3)C(=O)Nc2c1 |w:10.10| Show InChI InChI=1S/C29H30N4O2/c1-30-28(34)22-12-15-24-25(18-22)32-29(35)26(24)27(21-8-4-2-5-9-21)31-23-13-10-20(11-14-23)19-33-16-6-3-7-17-33/h2,4-5,8-15,18,26H,3,6-7,16-17,19H2,1H3,(H,30,34)(H,32,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285776

(CHEMBL4172309)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)N1CCc2[nH]nnc2C1)C(=O)OCc1cc(cc(c1)C(=O)OC(F)(F)F)C#N |r| Show InChI InChI=1S/C23H22F3N7O5/c24-23(25,26)38-20(34)14-4-12(6-27)3-13(5-14)11-37-22(36)33-8-16-15(17(16)9-33)7-28-21(35)32-2-1-18-19(10-32)30-31-29-18/h3-5,15-17H,1-2,7-11H2,(H,28,35)(H,29,30,31)/t15-,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of ATX in rat whole blood using LPA 17:0 as substrate after 1 hr by LC-MS/MS analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329803
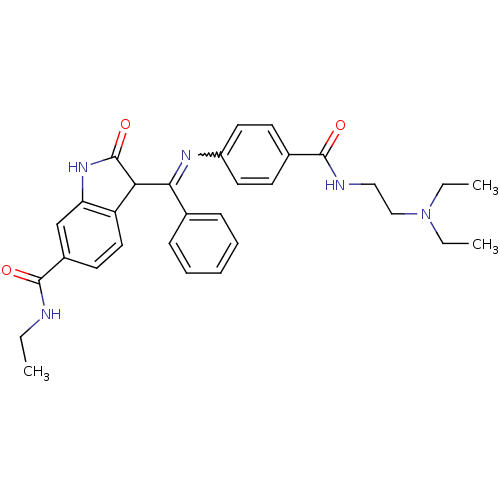
((Z)-3-((4-(2-(diethylamino)ethylcarbamoyl)phenylam...)Show SMILES CCNC(=O)c1ccc2C(C(=Nc3ccc(cc3)C(=O)NCCN(CC)CC)c3ccccc3)C(=O)Nc2c1 |w:11.11| Show InChI InChI=1S/C31H35N5O3/c1-4-32-30(38)23-14-17-25-26(20-23)35-31(39)27(25)28(21-10-8-7-9-11-21)34-24-15-12-22(13-16-24)29(37)33-18-19-36(5-2)6-3/h7-17,20,27H,4-6,18-19H2,1-3H3,(H,32,38)(H,33,37)(H,35,39) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50279164

((3Z)-N-ETHYL-N-METHYL-2-OXO-3-(PHENYL{[4-(PIPERIDI...)Show SMILES CCN(C)C(=O)c1ccc2C(C(=Nc3ccc(CN4CCCCC4)cc3)c3ccccc3)C(=O)Nc2c1 |w:12.12| Show InChI InChI=1S/C31H34N4O2/c1-3-34(2)31(37)24-14-17-26-27(20-24)33-30(36)28(26)29(23-10-6-4-7-11-23)32-25-15-12-22(13-16-25)21-35-18-8-5-9-19-35/h4,6-7,10-17,20,28H,3,5,8-9,18-19,21H2,1-2H3,(H,33,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50279163
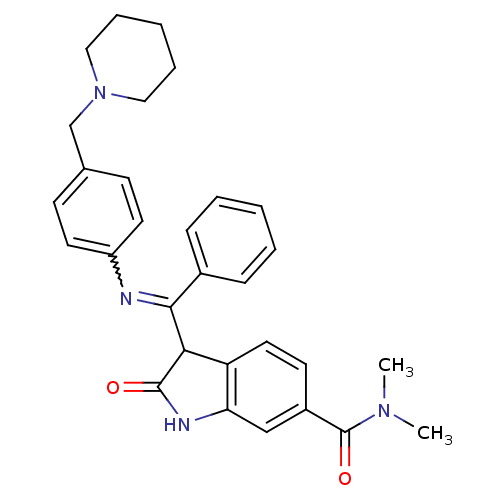
(CHEMBL1203981 | CHEMBL496861)Show SMILES CN(C)C(=O)c1ccc2C(C(=Nc3ccc(CN4CCCCC4)cc3)c3ccccc3)C(=O)Nc2c1 |w:11.11| Show InChI InChI=1S/C30H32N4O2/c1-33(2)30(36)23-13-16-25-26(19-23)32-29(35)27(25)28(22-9-5-3-6-10-22)31-24-14-11-21(12-15-24)20-34-17-7-4-8-18-34/h3,5-6,9-16,19,27H,4,7-8,17-18,20H2,1-2H3,(H,32,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329800
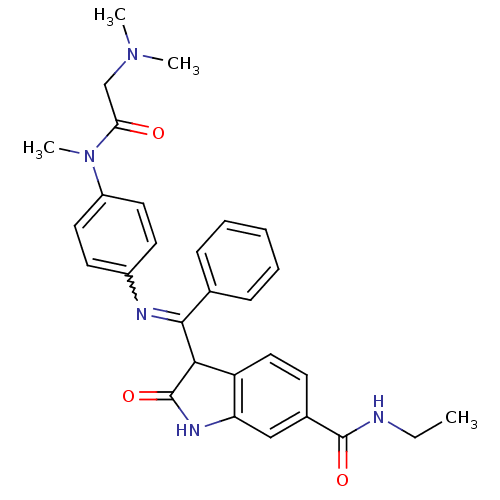
((Z)-3-((4-(2-(dimethylamino)-N-methylacetamido)phe...)Show SMILES CCNC(=O)c1ccc2C(C(=Nc3ccc(cc3)N(C)C(=O)CN(C)C)c3ccccc3)C(=O)Nc2c1 |w:11.11| Show InChI InChI=1S/C29H31N5O3/c1-5-30-28(36)20-11-16-23-24(17-20)32-29(37)26(23)27(19-9-7-6-8-10-19)31-21-12-14-22(15-13-21)34(4)25(35)18-33(2)3/h6-17,26H,5,18H2,1-4H3,(H,30,36)(H,32,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50272886
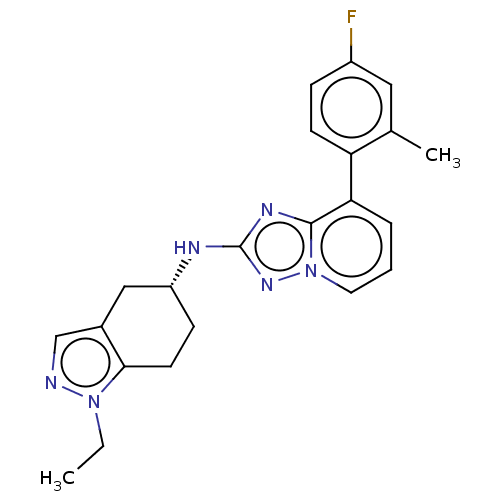
(CHEMBL4127978)Show SMILES CCn1ncc2C[C@@H](CCc12)Nc1nc2c(cccn2n1)-c1ccc(F)cc1C |r| Show InChI InChI=1S/C22H23FN6/c1-3-28-20-9-7-17(12-15(20)13-24-28)25-22-26-21-19(5-4-10-29(21)27-22)18-8-6-16(23)11-14(18)2/h4-6,8,10-11,13,17H,3,7,9,12H2,1-2H3,(H,25,27)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells expressing human wild type APP assessed as inhibition of amyloid beta 42 production after 22 hrs by e... |
Bioorg Med Chem 26: 3227-3241 (2018)
Article DOI: 10.1016/j.bmc.2018.04.053
BindingDB Entry DOI: 10.7270/Q24X5B97 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50272887
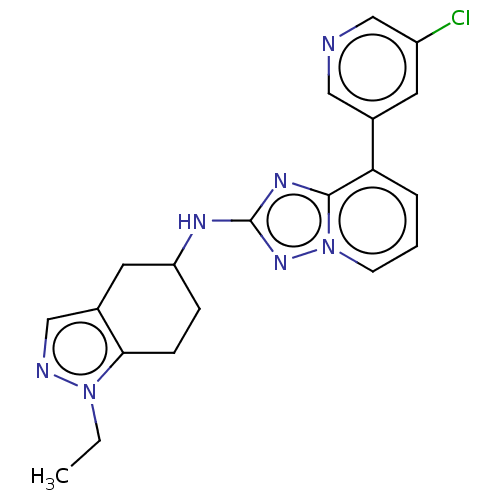
(CHEMBL4126888)Show SMILES CCn1ncc2CC(CCc12)Nc1nc2c(cccn2n1)-c1cncc(Cl)c1 Show InChI InChI=1S/C20H20ClN7/c1-2-27-18-6-5-16(9-14(18)11-23-27)24-20-25-19-17(4-3-7-28(19)26-20)13-8-15(21)12-22-10-13/h3-4,7-8,10-12,16H,2,5-6,9H2,1H3,(H,24,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells expressing human wild type APP assessed as inhibition of amyloid beta 42 production after 22 hrs by e... |
Bioorg Med Chem 26: 3227-3241 (2018)
Article DOI: 10.1016/j.bmc.2018.04.053
BindingDB Entry DOI: 10.7270/Q24X5B97 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50272771
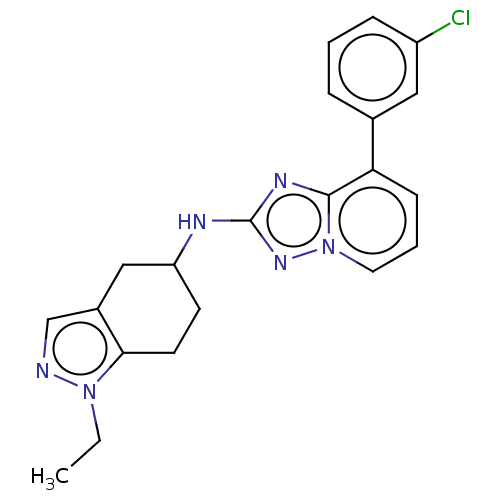
(CHEMBL4128038)Show SMILES CCn1ncc2CC(CCc12)Nc1nc2c(cccn2n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C21H21ClN6/c1-2-27-19-9-8-17(12-15(19)13-23-27)24-21-25-20-18(7-4-10-28(20)26-21)14-5-3-6-16(22)11-14/h3-7,10-11,13,17H,2,8-9,12H2,1H3,(H,24,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells expressing human wild type APP assessed as inhibition of amyloid beta 42 production after 22 hrs by e... |
Bioorg Med Chem 26: 3227-3241 (2018)
Article DOI: 10.1016/j.bmc.2018.04.053
BindingDB Entry DOI: 10.7270/Q24X5B97 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329805
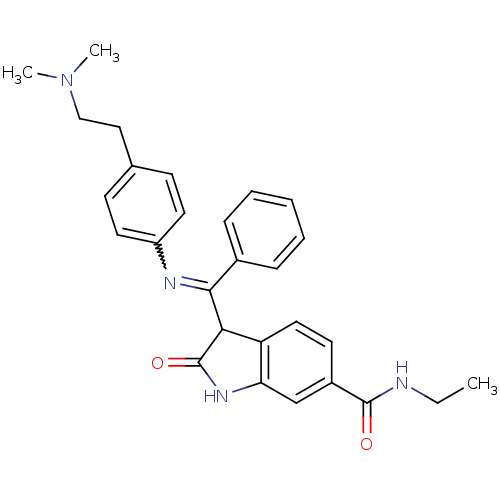
((Z)-3-((4-(2-(dimethylamino)ethyl)phenylamino)(phe...)Show SMILES CCNC(=O)c1ccc2C(C(=Nc3ccc(CCN(C)C)cc3)c3ccccc3)C(=O)Nc2c1 |w:11.11| Show InChI InChI=1S/C28H30N4O2/c1-4-29-27(33)21-12-15-23-24(18-21)31-28(34)25(23)26(20-8-6-5-7-9-20)30-22-13-10-19(11-14-22)16-17-32(2)3/h5-15,18,25H,4,16-17H2,1-3H3,(H,29,33)(H,31,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285775

(CHEMBL4159308)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)OCC1CCCN(C1)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C26H34N4O7/c1-26(2,3)37-25(34)29-8-4-5-15(11-29)14-35-24(33)30-12-18-17(19(18)13-30)10-27-22(31)16-6-7-20-21(9-16)36-23(32)28-20/h6-7,9,15,17-19H,4-5,8,10-14H2,1-3H3,(H,27,31)(H,28,32)/t15?,17-,18-,19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat ATX using LPC 18:1 as substrate after 2 hrs by rapidfire/MS-based analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50272888
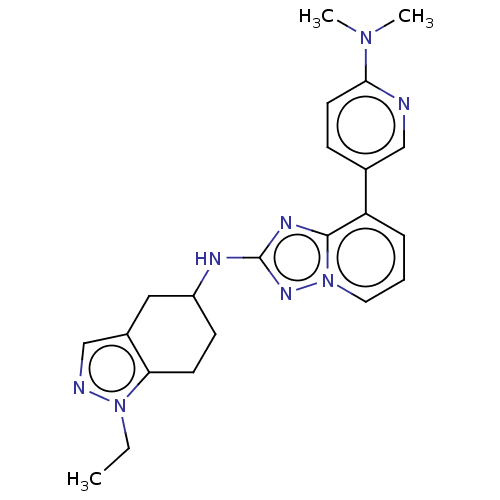
(CHEMBL4130152)Show SMILES CCn1ncc2CC(CCc12)Nc1nc2c(cccn2n1)-c1ccc(nc1)N(C)C Show InChI InChI=1S/C22H26N8/c1-4-29-19-9-8-17(12-16(19)14-24-29)25-22-26-21-18(6-5-11-30(21)27-22)15-7-10-20(23-13-15)28(2)3/h5-7,10-11,13-14,17H,4,8-9,12H2,1-3H3,(H,25,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Modulation of gamma secretase in human H4 cells expressing human wild type APP assessed as inhibition of amyloid beta 42 production after 22 hrs by e... |
Bioorg Med Chem 26: 3227-3241 (2018)
Article DOI: 10.1016/j.bmc.2018.04.053
BindingDB Entry DOI: 10.7270/Q24X5B97 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50329816
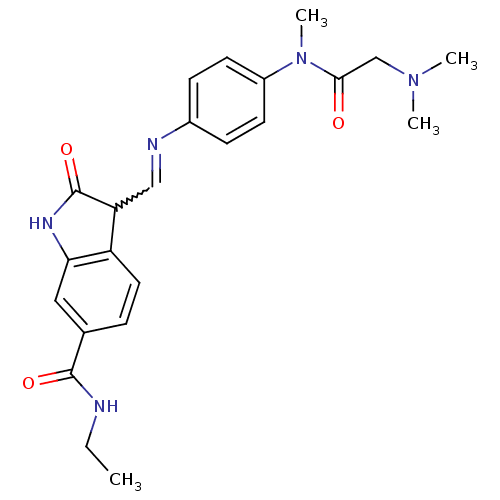
((Z)-3-((4-(2-(dimethylamino)-N-methylacetamido)phe...)Show SMILES CCNC(=O)c1ccc2C(C=Nc3ccc(cc3)N(C)C(=O)CN(C)C)C(=O)Nc2c1 |w:10.9| Show InChI InChI=1S/C23H27N5O3/c1-5-24-22(30)15-6-11-18-19(23(31)26-20(18)12-15)13-25-16-7-9-17(10-8-16)28(4)21(29)14-27(2)3/h6-13,19H,5,14H2,1-4H3,(H,24,30)(H,26,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co KG
Curated by ChEMBL
| Assay Description
Inhibition of TGFbeta receptor |
J Med Chem 53: 7287-95 (2010)
Article DOI: 10.1021/jm100812a
BindingDB Entry DOI: 10.7270/Q2F47PCW |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Rattus norvegicus) | BDBM50285751

(CHEMBL4169912)Show SMILES [H][C@@]12CN(C[C@]1([H])[C@H]2CNC(=O)c1ccc2[nH]c(=O)oc2c1)C(=O)OCc1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C22H19Cl2N3O5/c23-13-3-11(4-14(24)6-13)10-31-22(30)27-8-16-15(17(16)9-27)7-25-20(28)12-1-2-18-19(5-12)32-21(29)26-18/h1-6,15-17H,7-10H2,(H,25,28)(H,26,29)/t15-,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of ATX in rat whole blood using LPA 17:0 as substrate after 1 hr by LC-MS/MS analysis |
ACS Med Chem Lett 8: 1252-1257 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00312
BindingDB Entry DOI: 10.7270/Q2T43WNV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data