

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016326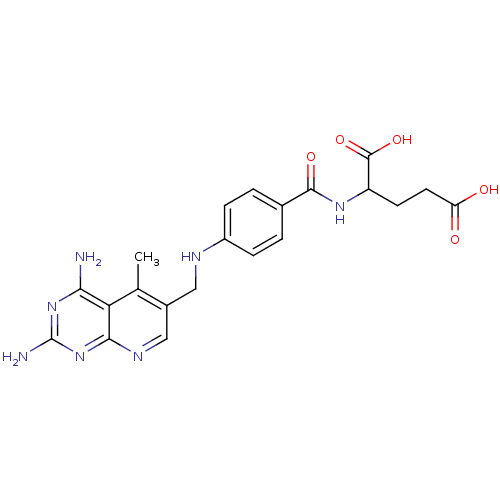 (2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM66082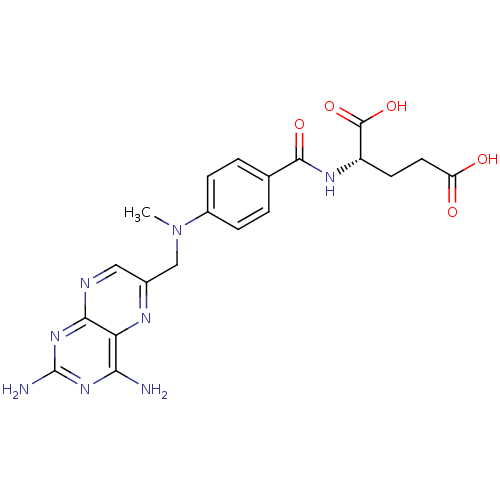 ((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50023681 (2-{4-[(2,4-Diamino-5,7-dimethyl-pyrido[2,3-d]pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50023680 (2-{4-[(2,4-Diamino-7-phenyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50023682 (2-{4-[(2,4-Diamino-5-methyl-7-phenyl-pyrido[2,3-d]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50023683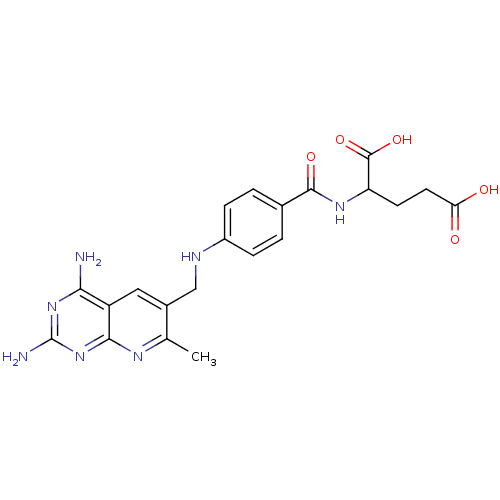 (2-{4-[(2,4-Diamino-7-methyl-pyrido[2,3-d]pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50023684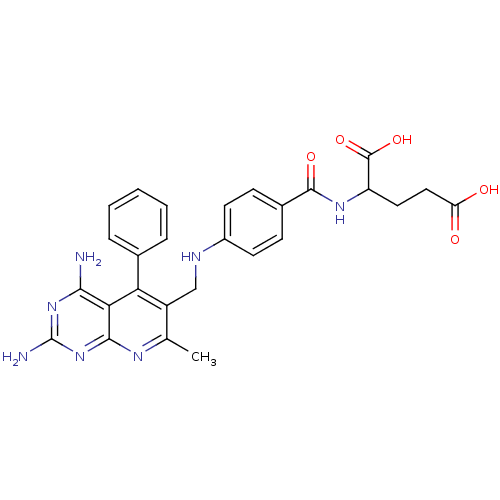 (2-{4-[(2,4-Diamino-7-methyl-5-phenyl-pyrido[2,3-d]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University Curated by ChEMBL | Assay Description Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells | J Med Chem 31: 1209-15 (1988) BindingDB Entry DOI: 10.7270/Q2930S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073270 (CHEMBL333781 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073266 (CHEMBL119898 | {2-[(1S,3S,4S)-1-Benzyl-4-((2S,3S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50519598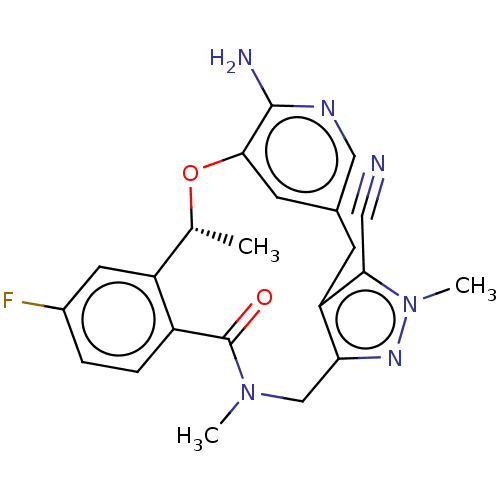 (CHEMBL4436406) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of ALK (unknown origin) | J Med Chem 62: 10927-10954 (2019) Article DOI: 10.1021/acs.jmedchem.9b00446 BindingDB Entry DOI: 10.7270/Q2S185WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073250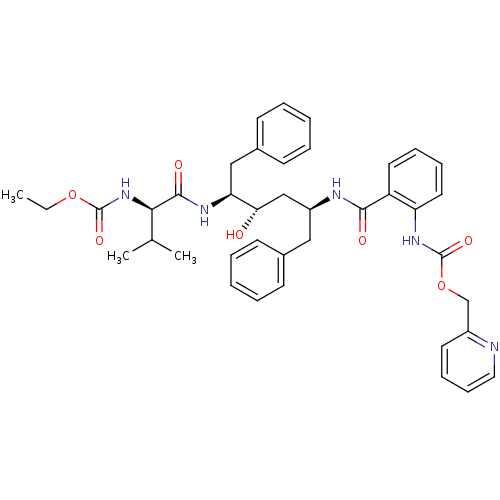 (CHEMBL333420 | {2-[(1S,3S,4S)-1-Benzyl-4-((R)-2-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073253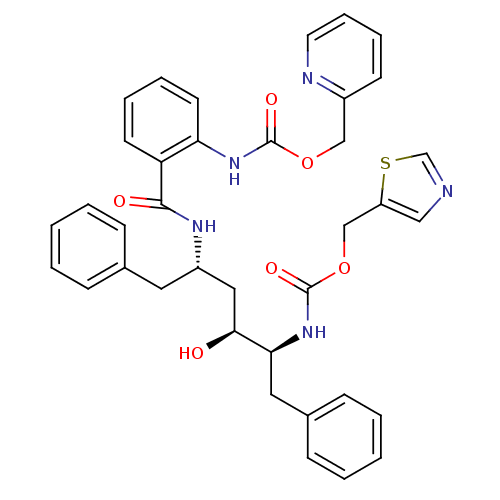 (CHEMBL278935 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073269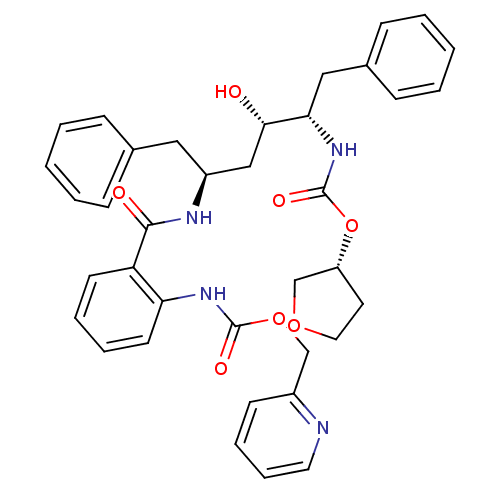 ((2-{(1S,3S,4S)-1-Benzyl-3-hydroxy-5-phenyl-4-[(R)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090731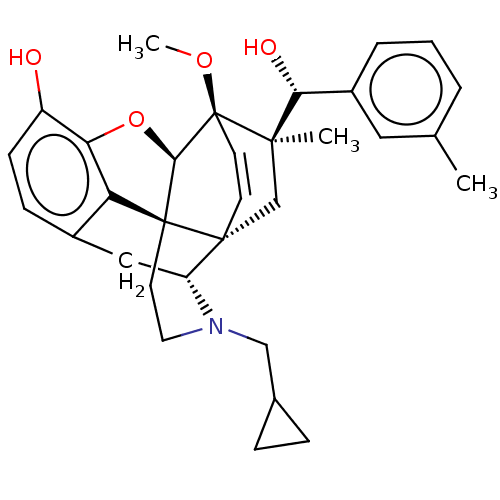 (CHEMBL3581741 | US9259422, 22, R = 3-MePh- BU10112...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090760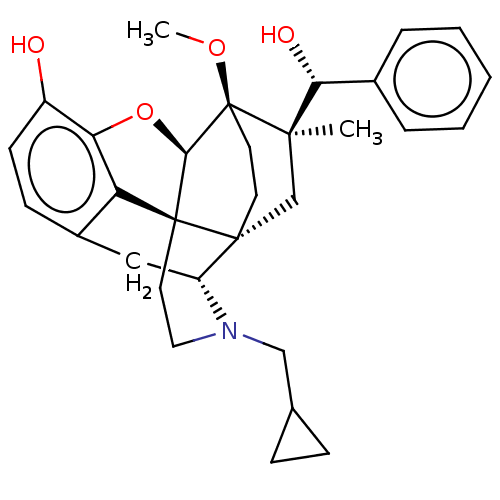 (CHEMBL3581750 | US9259422, 30, R = Ph-BU10119 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50015003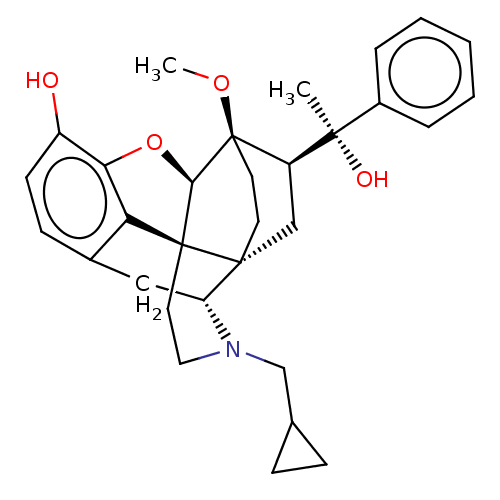 (CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM153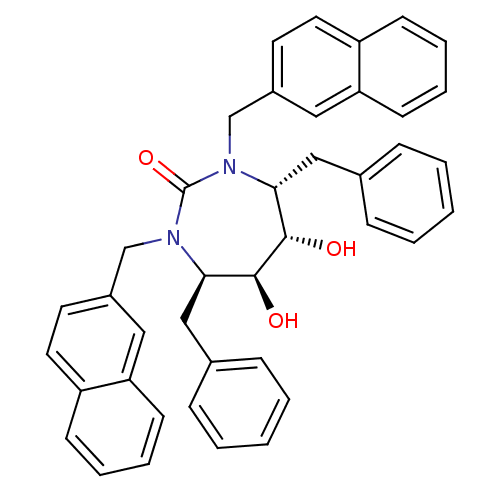 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(n...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 51: 4280-8 (2008) Article DOI: 10.1021/jm800242q BindingDB Entry DOI: 10.7270/Q2S1858S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090755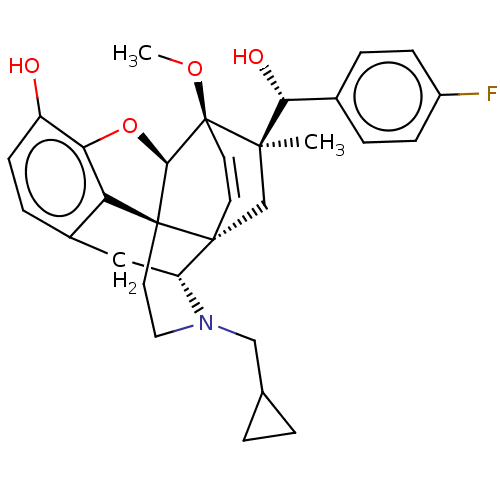 (CHEMBL3581743 | US9259422, 22, R = 4-FPh- BU10120 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090766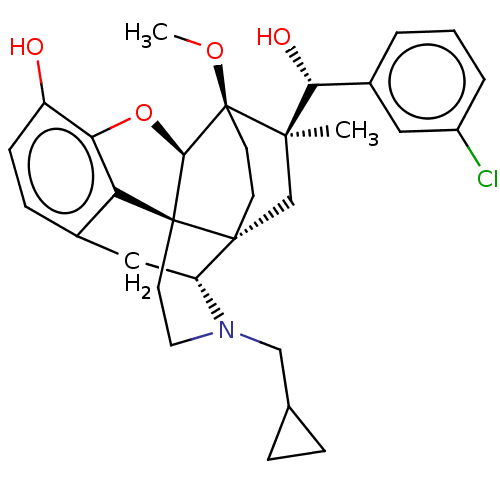 (CHEMBL3581756) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073252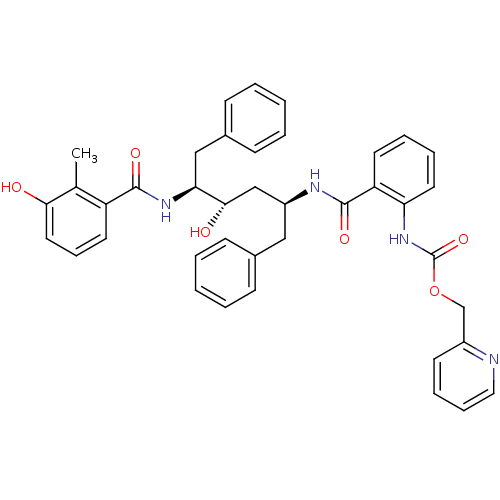 (CHEMBL324157 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073254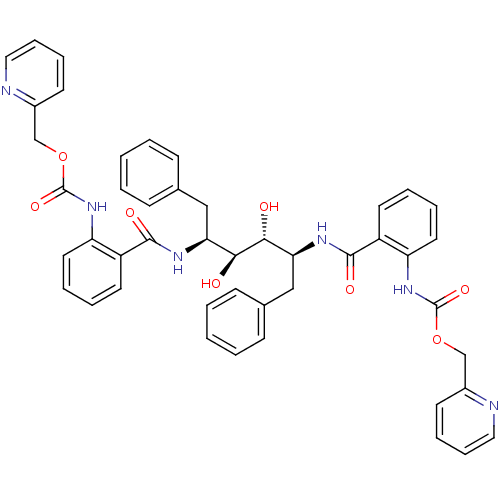 (Anthranilamide derivative | CHEMBL408110) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50519598 (CHEMBL4436406) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Inhibition of ROS1 (unknown origin) | J Med Chem 62: 10927-10954 (2019) Article DOI: 10.1021/acs.jmedchem.9b00446 BindingDB Entry DOI: 10.7270/Q2S185WV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073265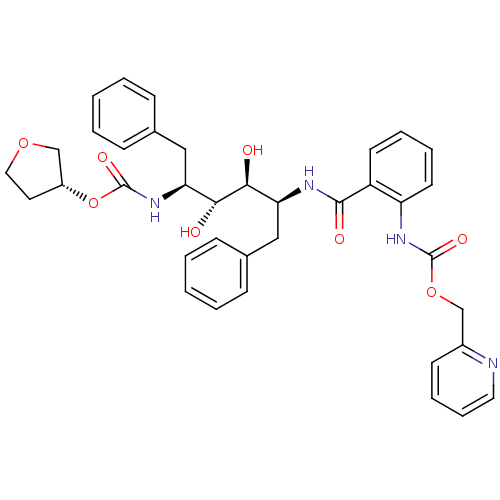 ((2-{(1S,2S,3R,4S)-1-Benzyl-2,3-dihydroxy-5-phenyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090782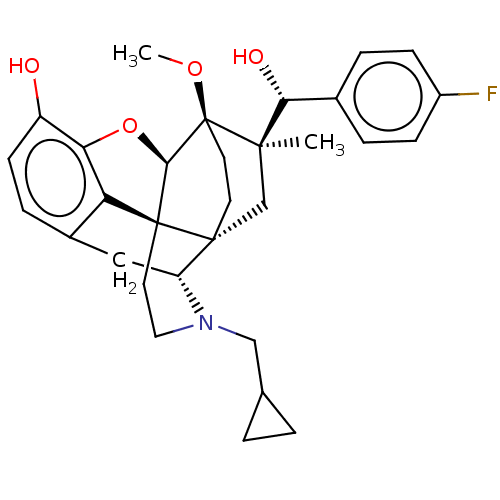 (CHEMBL3581754) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090694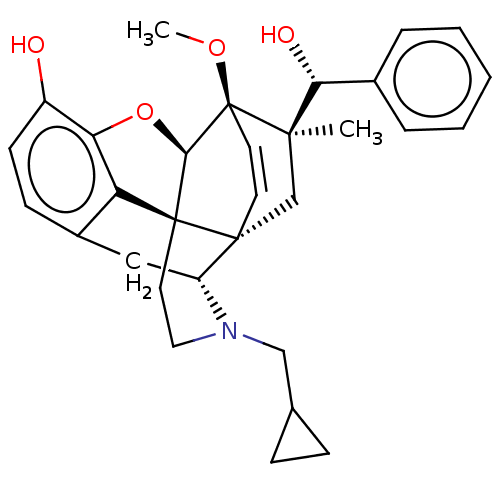 (CHEMBL3581740 | US9259422, 22, R = Ph-BU128 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090694 (CHEMBL3581740 | US9259422, 22, R = Ph-BU128 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090767 (CHEMBL3581757) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073268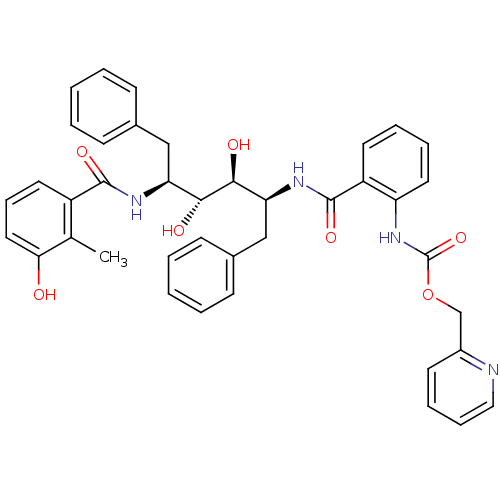 (CHEMBL331294 | {2-[(1S,2S,3R,4S)-1-Benzyl-2,3-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090766 (CHEMBL3581756) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090765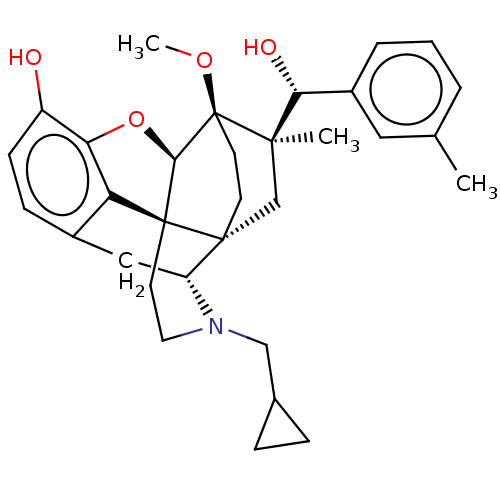 (CHEMBL3581752) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090782 (CHEMBL3581754) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090760 (CHEMBL3581750 | US9259422, 30, R = Ph-BU10119 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090765 (CHEMBL3581752) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090768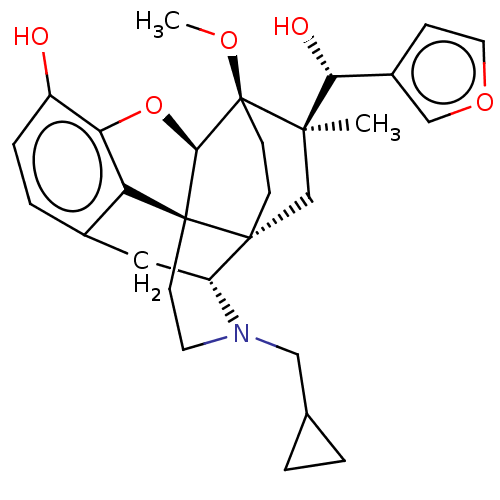 (CHEMBL3581762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090769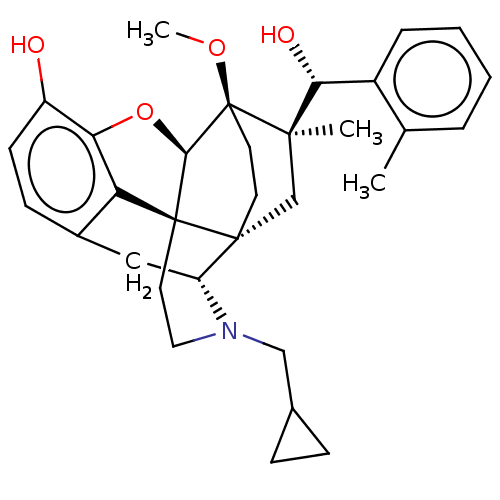 (CHEMBL3581751) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073263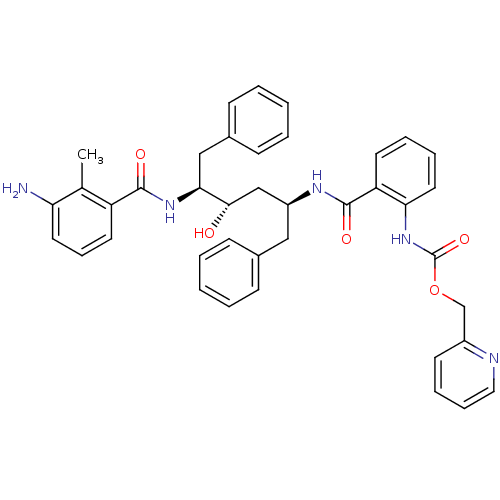 (CHEMBL117629 | {2-[(1S,3S,4S)-4-(3-Amino-2-methyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090767 (CHEMBL3581757) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073262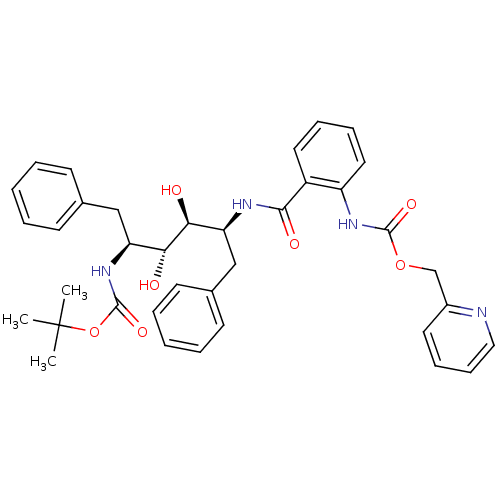 (CHEMBL333290 | [2-((1S,2S,3R,4S)-1-Benzyl-4-tert-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073244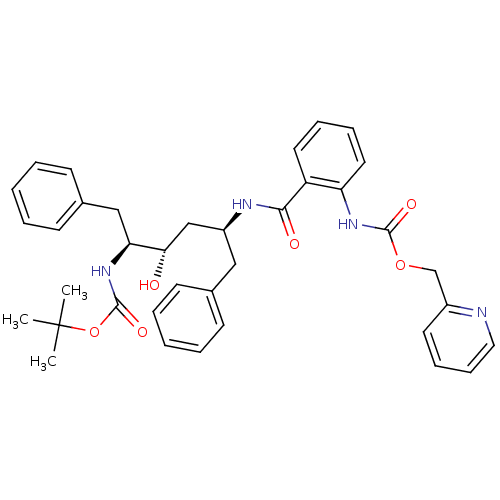 (CHEMBL332825 | [2-((1S,3S,4S)-1-Benzyl-4-tert-buto...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090768 (CHEMBL3581762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090773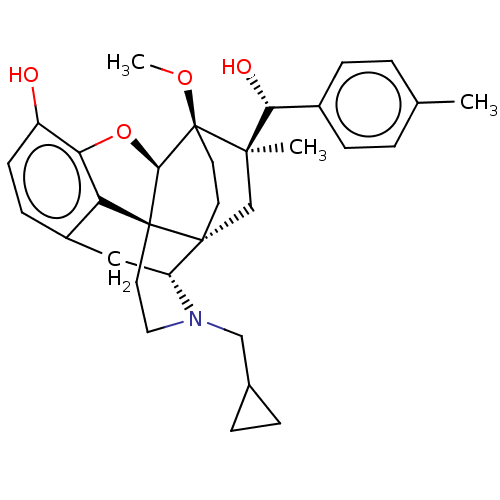 (CHEMBL3581753) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090773 (CHEMBL3581753) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090755 (CHEMBL3581743 | US9259422, 22, R = 4-FPh- BU10120 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090731 (CHEMBL3581741 | US9259422, 22, R = 3-MePh- BU10112...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50015003 (CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090769 (CHEMBL3581751) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090760 (CHEMBL3581750 | US9259422, 30, R = Ph-BU10119 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat delta opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073248 (CHEMBL119745 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3721 total ) | Next | Last >> |