

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103072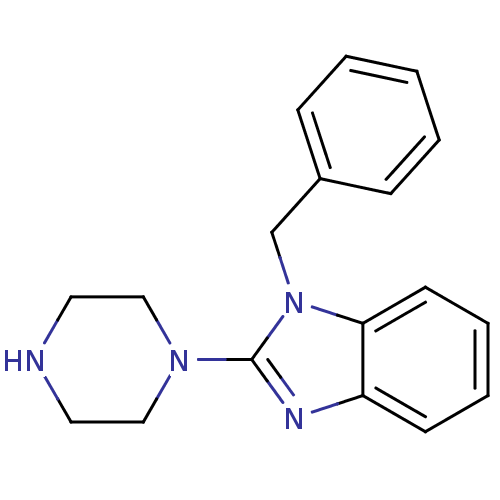 (1-Benzyl-2-piperazin-1-yl-1H-benzoimidazole | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103070 (1-(4-Methoxy-benzyl)-2-piperazin-1-yl-1H-benzoimid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103076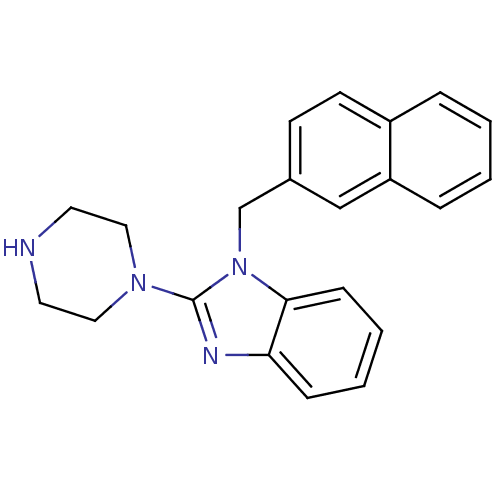 (1-Naphthalen-2-ylmethyl-2-piperazin-1-yl-1H-benzoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103075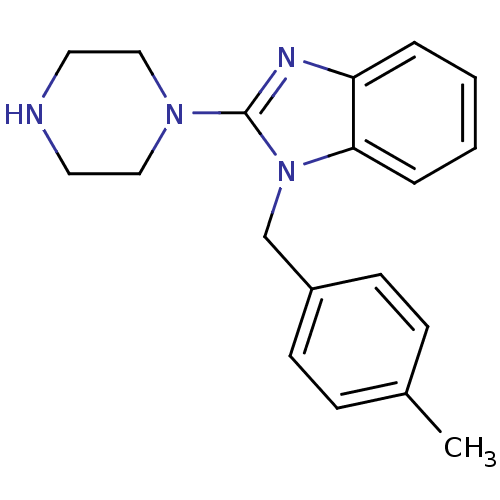 (1-(4-Methyl-benzyl)-2-piperazin-1-yl-1H-benzoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1B (Homo sapiens (Human)) | BDBM50029191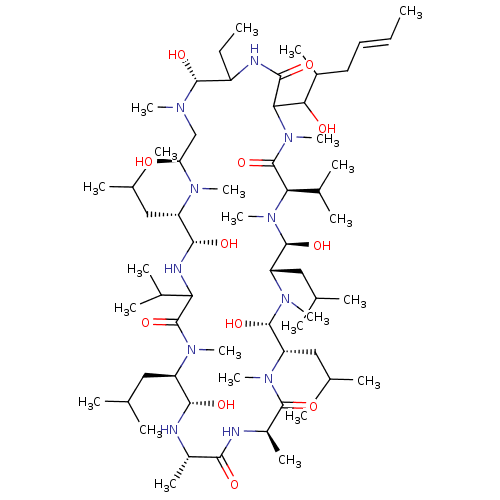 (15-Benzyl-30-ethyl-12-hydroxymethyl-33-(1-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description The compound was tested for inhibition of Peptidyl-propyl isomerase (PPIase). | J Med Chem 38: 4164-70 (1995) BindingDB Entry DOI: 10.7270/Q2X34WGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103069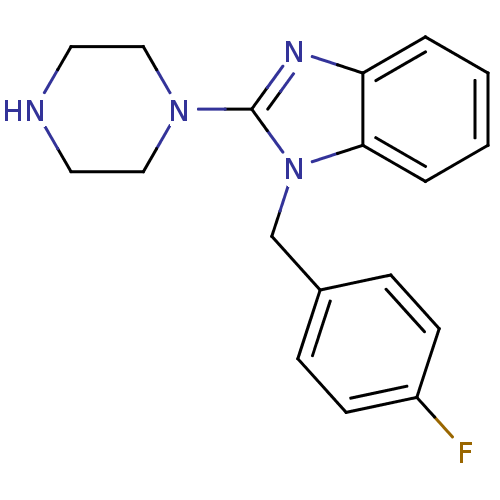 (1-(4-Fluoro-benzyl)-2-piperazin-1-yl-1H-benzoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1B (Homo sapiens (Human)) | BDBM50029190 (15-Benzyl-30-ethyl-33-(1-hydroxy-2-methyl-hex-4-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description The compound was tested for inhibition of Peptidyl-propyl isomerase (PPIase). | J Med Chem 38: 4164-70 (1995) BindingDB Entry DOI: 10.7270/Q2X34WGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1B (Homo sapiens (Human)) | BDBM50029192 (15-Benzyl-30-ethyl-12-hydroxymethyl-33-(1-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description The compound was tested for inhibition of Peptidyl-propyl isomerase (PPIase). | J Med Chem 38: 4164-70 (1995) BindingDB Entry DOI: 10.7270/Q2X34WGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1B (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description The compound was tested for inhibition of Peptidyl-propyl isomerase (PPIase). | J Med Chem 38: 4164-70 (1995) BindingDB Entry DOI: 10.7270/Q2X34WGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103073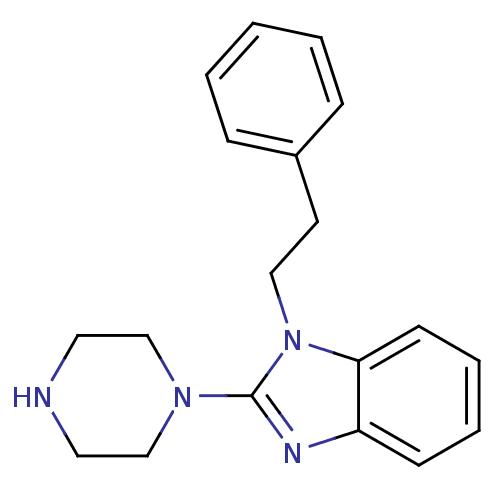 (1-Phenethyl-2-piperazin-1-yl-1H-benzoimidazole | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103071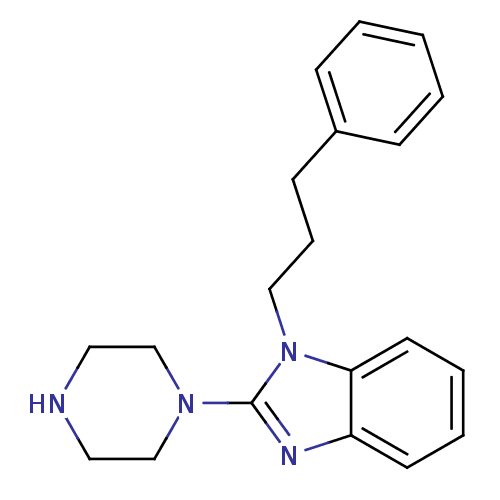 (1-(3-Phenyl-propyl)-2-piperazin-1-yl-1H-benzoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103077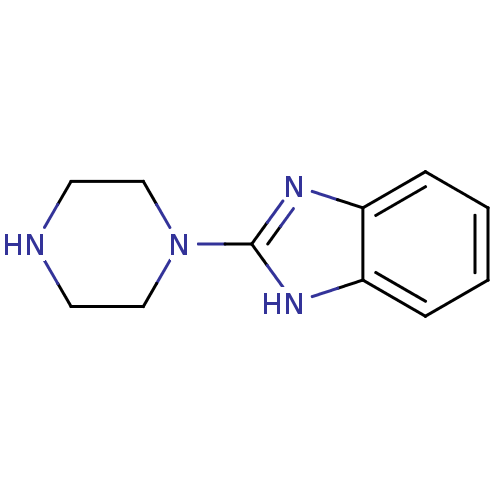 (2-Piperazin-1-yl-1H-benzoimidazole | CHEMBL292066) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50053608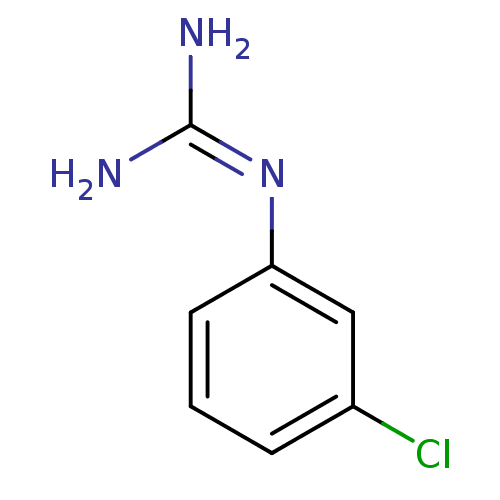 (1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from 5-HT3 receptor (unknown origin) expressed in mouse/rat NG108-15 cells after 30 mins by by liquid scintillation count... | Bioorg Med Chem Lett 23: 5945-8 (2013) Article DOI: 10.1016/j.bmcl.2013.08.072 BindingDB Entry DOI: 10.7270/Q2ZK5J31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1B (Homo sapiens (Human)) | BDBM50029194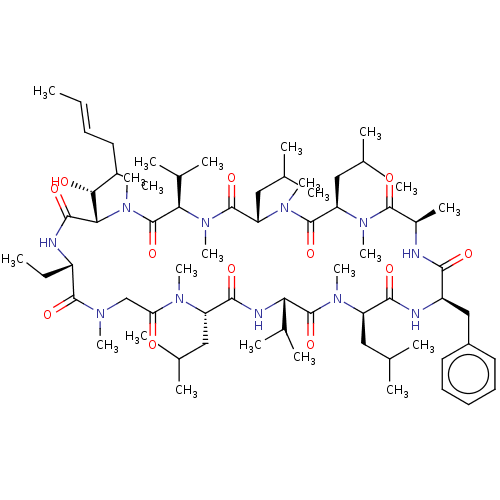 (15-Benzyl-30-ethyl-33-(1-hydroxy-2-methyl-hex-4-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description The compound was tested for inhibition of Peptidyl-propyl isomerase (PPIase). | J Med Chem 38: 4164-70 (1995) BindingDB Entry DOI: 10.7270/Q2X34WGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50441458 (CHEMBL2436555) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from 5-HT3 receptor (unknown origin) expressed in mouse/rat NG108-15 cells after 30 mins by by liquid scintillation count... | Bioorg Med Chem Lett 23: 5945-8 (2013) Article DOI: 10.1016/j.bmcl.2013.08.072 BindingDB Entry DOI: 10.7270/Q2ZK5J31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50324701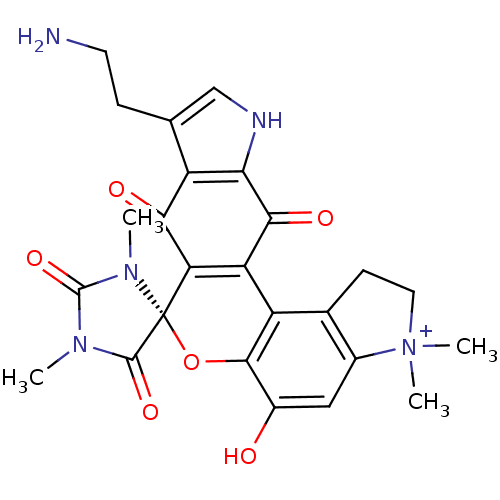 (CHEMBL1221472 | Exiguamine A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase | Nat Chem Biol 4: 535-7 (2008) Article DOI: 10.1038/nchembio.107 BindingDB Entry DOI: 10.7270/Q2P55NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21975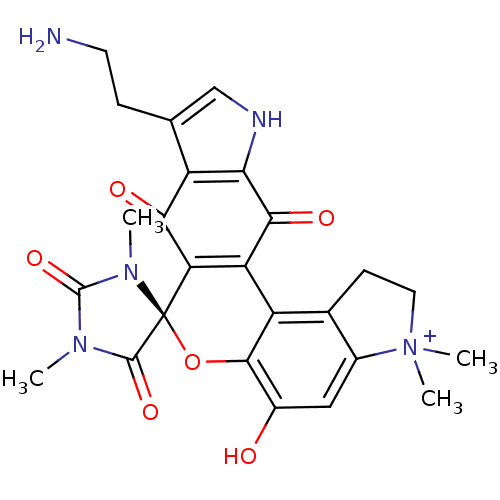 ((4S)-16'-(2-aminoethyl)-9'-hydroxy-1,3,6',6'-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 41 | -43.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50324700 (CHEMBL1221412 | Exiguamine B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase | Nat Chem Biol 4: 535-7 (2008) Article DOI: 10.1038/nchembio.107 BindingDB Entry DOI: 10.7270/Q2P55NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50324699 (CHEMBL1221473 | Seco-exiguamine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant indoleamine-2,3-dioxygenase | Nat Chem Biol 4: 535-7 (2008) Article DOI: 10.1038/nchembio.107 BindingDB Entry DOI: 10.7270/Q2P55NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50441458 (CHEMBL2436555) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from human 5-HT3 receptor expressed in African green monkey COS cells after 90 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 5945-8 (2013) Article DOI: 10.1016/j.bmcl.2013.08.072 BindingDB Entry DOI: 10.7270/Q2ZK5J31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21979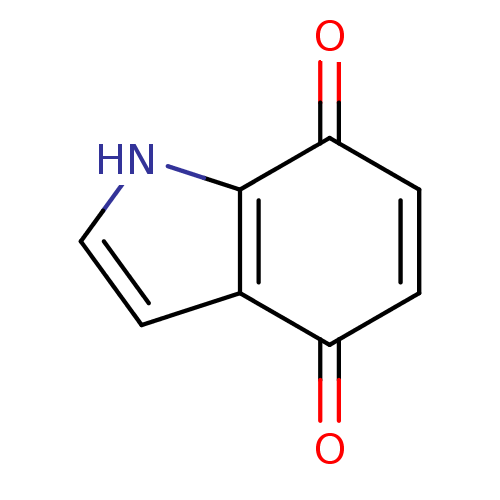 (4,7-dihydro-1H-indole-4,7-dione | Indolequinone, 1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 190 | -39.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21982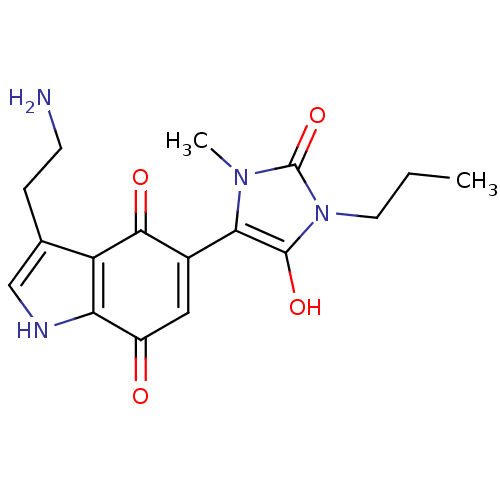 (3-(2-aminoethyl)-5-(3-methyl-2,5-dioxo-1-propylimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | -39.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21981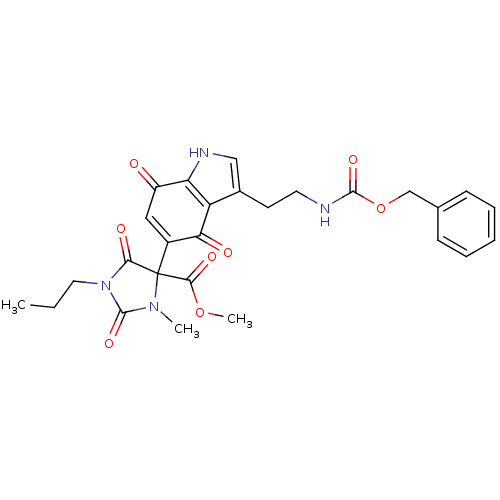 (Tryptamine quinone, 21 | methyl 4-[3-(2-{[(benzylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 260 | -39.1 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21984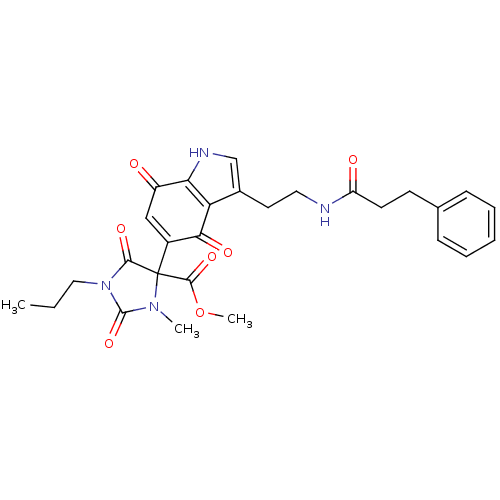 (Tryptamine quinone, 25 | methyl 4-{4,7-dioxo-3-[2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 260 | -39.1 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21980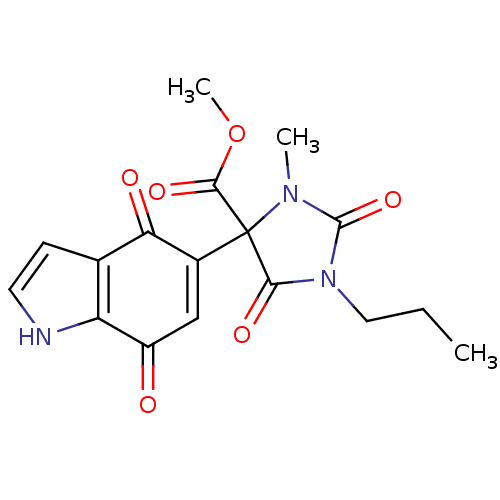 (Indolequinone, 20 | methyl 4-(4,7-dioxo-4,7-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 420 | -37.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21976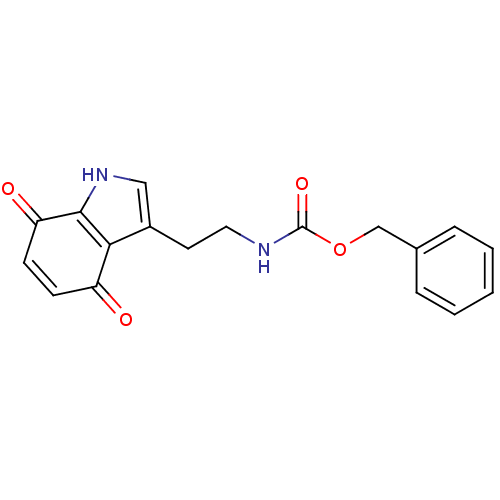 (Tryptamine quinone, 9 | benzyl N-[2-(4,7-dioxo-4,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.49E+3 | -34.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50115644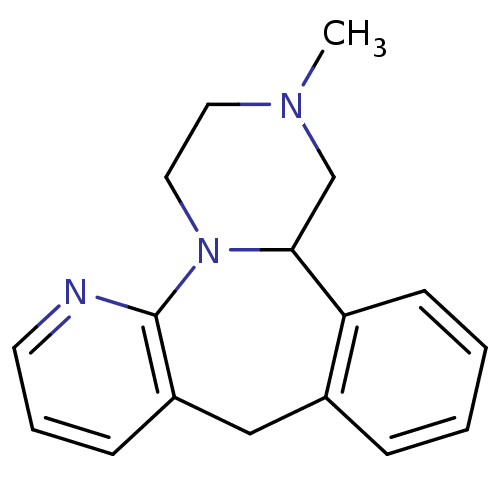 ((+/-)-12-Methyl-1,2,3,4,9,13b-hexahydro-2,4a,5-tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from human 5-HT3A receptor expressed in HEK293 cells by liquid scintillation counting analysis | Bioorg Med Chem Lett 23: 5945-8 (2013) Article DOI: 10.1016/j.bmcl.2013.08.072 BindingDB Entry DOI: 10.7270/Q2ZK5J31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50441458 (CHEMBL2436555) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]Pentazocine from guinea pig sigma1 receptor after 90 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 5945-8 (2013) Article DOI: 10.1016/j.bmcl.2013.08.072 BindingDB Entry DOI: 10.7270/Q2ZK5J31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/Neuronal acetylcholine receptor subunit beta-4 (Homo sapiens (Human)) | BDBM50441458 (CHEMBL2436555) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-epibatidine from human alpha2beta4 nAChR transfected in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 23: 5945-8 (2013) Article DOI: 10.1016/j.bmcl.2013.08.072 BindingDB Entry DOI: 10.7270/Q2ZK5J31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50441458 (CHEMBL2436555) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-epibatidine from human alpha4beta2 nAChR transfected in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 23: 5945-8 (2013) Article DOI: 10.1016/j.bmcl.2013.08.072 BindingDB Entry DOI: 10.7270/Q2ZK5J31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50441458 (CHEMBL2436555) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [[3H]N-methylspiperone from human recombinant dopamine D2 receptor expressed in human fibroblasts after 90 mins by scintillation coun... | Bioorg Med Chem Lett 23: 5945-8 (2013) Article DOI: 10.1016/j.bmcl.2013.08.072 BindingDB Entry DOI: 10.7270/Q2ZK5J31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50441458 (CHEMBL2436555) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]Pyrilamine from human recombinant histamine H1 receptor expressed in HEK cells after 90 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 5945-8 (2013) Article DOI: 10.1016/j.bmcl.2013.08.072 BindingDB Entry DOI: 10.7270/Q2ZK5J31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/Neuronal acetylcholine receptor subunit beta-2 (Homo sapiens (Human)) | BDBM50441458 (CHEMBL2436555) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-epibatidine from human alpha2beta2 nAChR transfected in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 23: 5945-8 (2013) Article DOI: 10.1016/j.bmcl.2013.08.072 BindingDB Entry DOI: 10.7270/Q2ZK5J31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21977 (Tryptamine quinone, 13 | benzyl N-{2-[5-(1,3-dioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.09E+4 | -29.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21973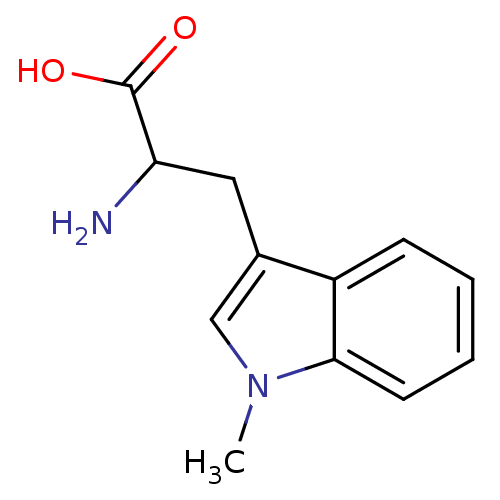 (1-Methyltryptophan, 1 | 2-amino-3-(1-methyl-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.20E+4 | -25.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of British Columbia | Assay Description The ability of the compounds prepared in this study to inhibit purified recombinant human IDO was evaluated with a steady state spectrophotometric as... | J Med Chem 51: 2634-7 (2008) Article DOI: 10.1021/jm800143h BindingDB Entry DOI: 10.7270/Q2MK6B65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9054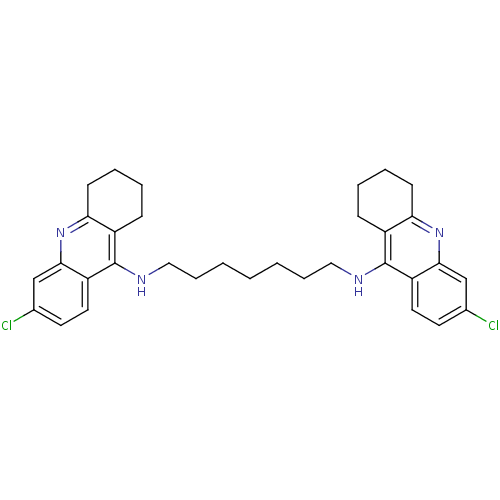 (6-chloro-N-{7-[(6-chloro-1,2,3,4-tetrahydroacridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Mus musculus) | BDBM50354719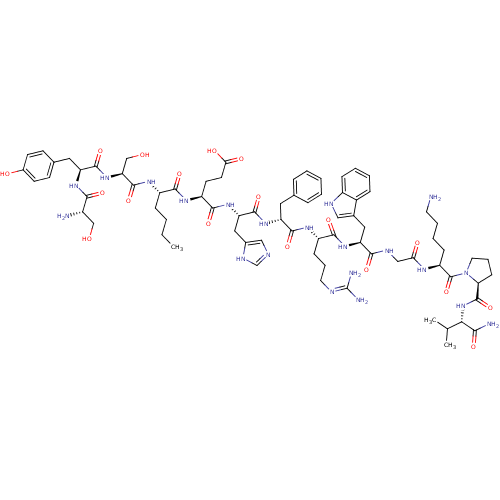 (CHEMBL1834391) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle4-D-Phe7]-alpha-MSH from MC1R in mouse B16-F10 cells after 1.5 hrs by gamma counting | Bioorg Med Chem Lett 21: 5757-61 (2011) Article DOI: 10.1016/j.bmcl.2011.08.017 BindingDB Entry DOI: 10.7270/Q2H132DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Mus musculus) | BDBM50354718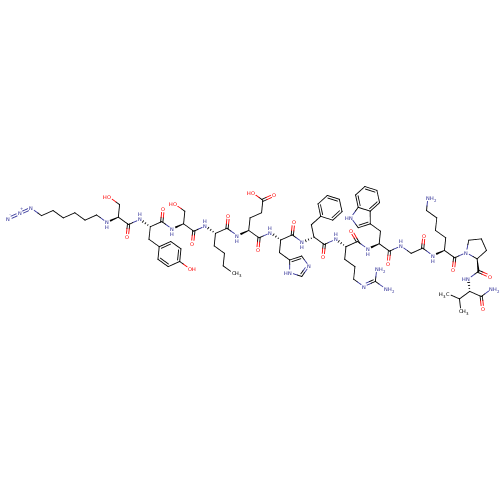 (CHEMBL1834392) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle4-D-Phe7]-alpha-MSH from MC1R in mouse B16-F10 cells after 1.5 hrs by gamma counting | Bioorg Med Chem Lett 21: 5757-61 (2011) Article DOI: 10.1016/j.bmcl.2011.08.017 BindingDB Entry DOI: 10.7270/Q2H132DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9055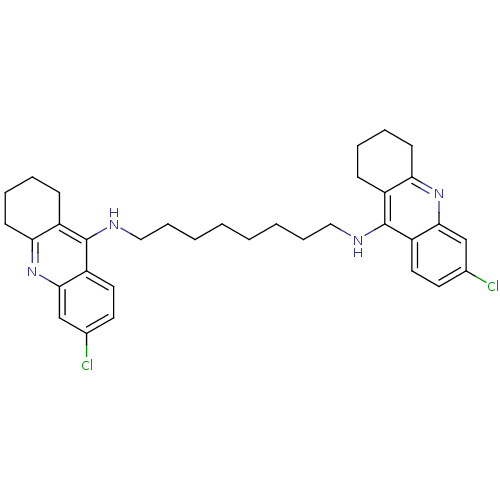 (6-chloro-N-{8-[(6-chloro-1,2,3,4-tetrahydroacridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Mus musculus) | BDBM50354717 (CHEMBL1834393) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle4-D-Phe7]-alpha-MSH from MC1R in mouse B16-F10 cells after 1.5 hrs by gamma counting | Bioorg Med Chem Lett 21: 5757-61 (2011) Article DOI: 10.1016/j.bmcl.2011.08.017 BindingDB Entry DOI: 10.7270/Q2H132DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9051 (6-fluoro-N-{7-[(6-fluoro-1,2,3,4-tetrahydroacridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9053 (6-chloro-N-{6-[(6-chloro-1,2,3,4-tetrahydroacridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9052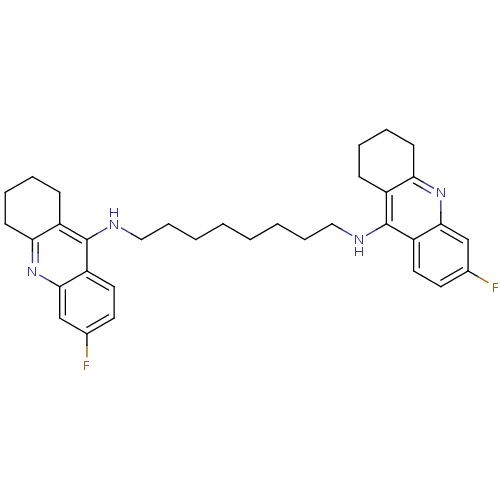 (6-fluoro-N-{8-[(6-fluoro-1,2,3,4-tetrahydroacridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Mus musculus) | BDBM50354716 (CHEMBL1834394) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle4-D-Phe7]-alpha-MSH from MC1R in mouse B16-F10 cells after 1.5 hrs by gamma counting | Bioorg Med Chem Lett 21: 5757-61 (2011) Article DOI: 10.1016/j.bmcl.2011.08.017 BindingDB Entry DOI: 10.7270/Q2H132DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Mus musculus) | BDBM50354715 (CHEMBL1834395) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa Curated by ChEMBL | Assay Description Displacement of [125I]-[Nle4-D-Phe7]-alpha-MSH from MC1R in mouse B16-F10 cells after 1.5 hrs by gamma counting | Bioorg Med Chem Lett 21: 5757-61 (2011) Article DOI: 10.1016/j.bmcl.2011.08.017 BindingDB Entry DOI: 10.7270/Q2H132DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9050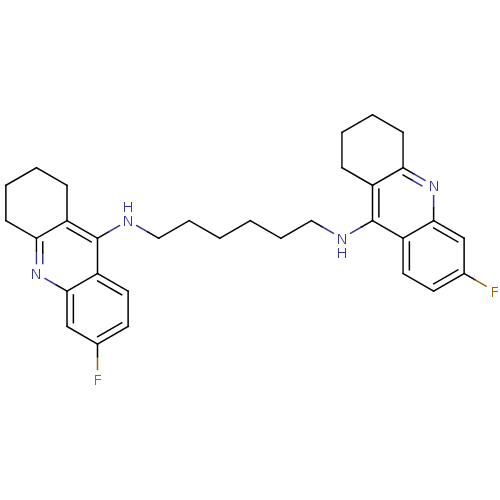 (6-fluoro-N-{6-[(6-fluoro-1,2,3,4-tetrahydroacridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9057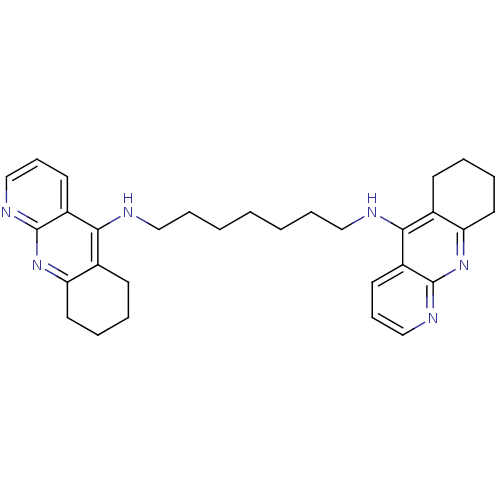 (Homodimeric Tacrine Analog 3k | N,N-Bis-(1,2,3,4-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9047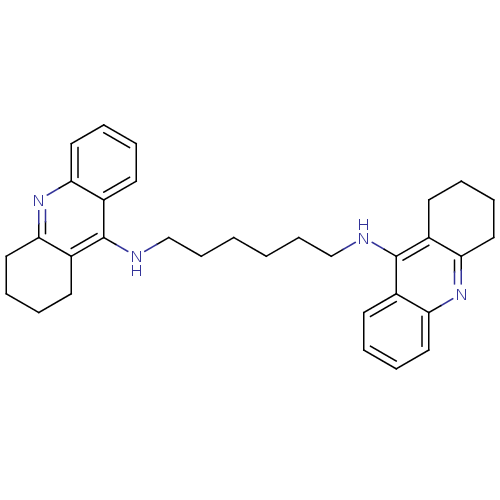 (Bis-THA inhibitor 5 | CHEMBL73800 | Hexylene-Linke...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9061 (Homodimeric Tacrine Analog 4c | N,N-Bis-(2,3,4,5-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 112 total ) | Next | Last >> |