

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ketohexokinase (Homo sapiens (Human)) | BDBM319585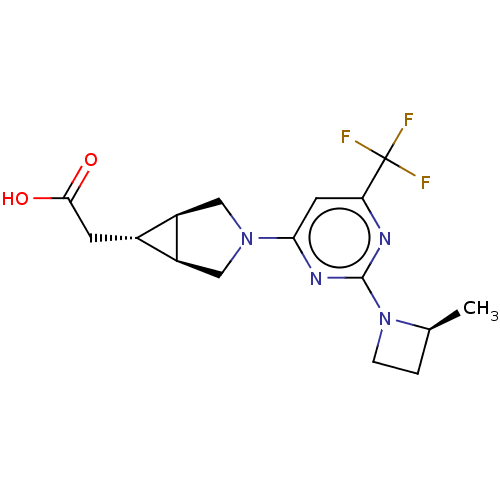 (US10174007, Example 4 | US10787438, Example 4 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed noncompetitive inhibition of recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319582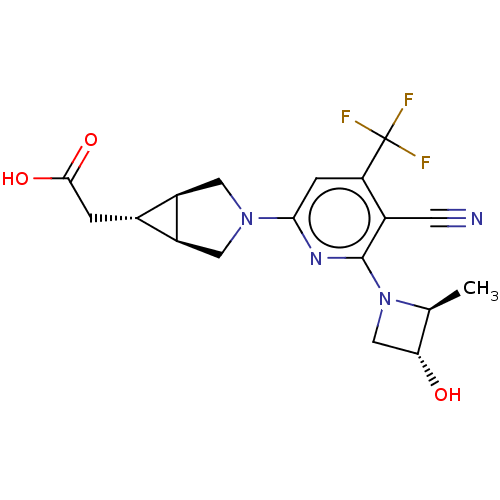 (US10174007, Example 1 | US10787438, Example 1 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 1 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated for... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00944 BindingDB Entry DOI: 10.7270/Q2QC074M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319582 (US10174007, Example 1 | US10787438, Example 1 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2B2807K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319583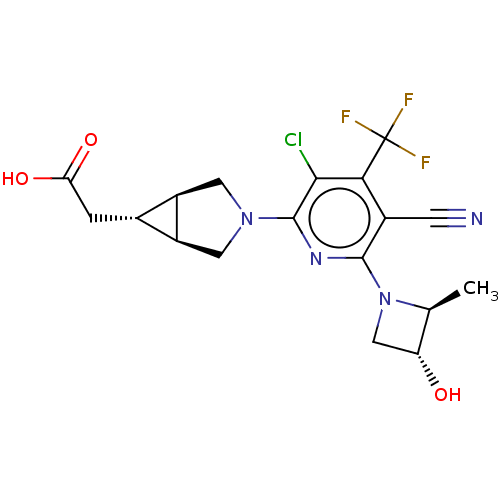 (US10174007, Example 2 | US10787438, Example 2 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2B2807K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM601403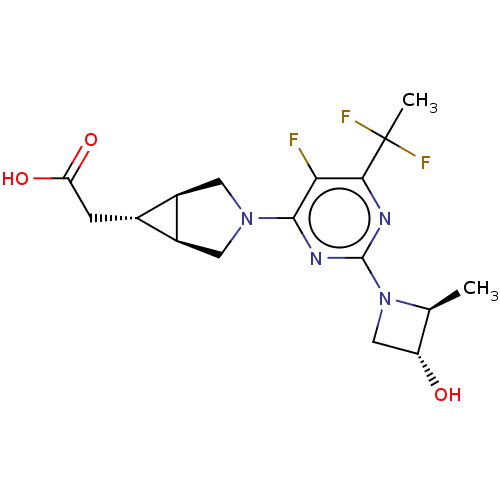 (US11634410, Example 50 | [(1R,5S,6R)-3-{5-cyano-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2B2807K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319583 (US10174007, Example 2 | US10787438, Example 2 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds having an IC50 value less than 20 nM were examined in a second KHK assay, referred to as Assay B, using 10-fold less enzyme and measuring a... | US Patent US10174007 (2019) BindingDB Entry DOI: 10.7270/Q20867DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319583 (US10174007, Example 2 | US10787438, Example 2 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay B, using 10-fold less enzyme and measuring absorbance for 3 hours to obtain IC50 values below the 10 nM lower limit of Assay A. Compounds were ... | US Patent US10988463 (2021) BindingDB Entry DOI: 10.7270/Q2SJ1PRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM494884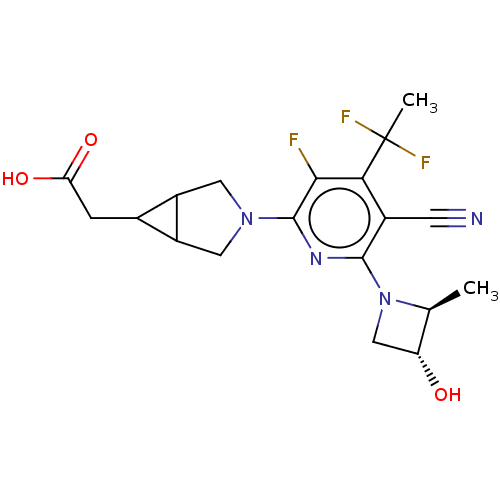 (US10988463, Example 50 | [(1R,5S,6R)-3-{5-cyano-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay B, using 10-fold less enzyme and measuring absorbance for 3 hours to obtain IC50 values below the 10 nM lower limit of Assay A. Compounds were ... | US Patent US10988463 (2021) BindingDB Entry DOI: 10.7270/Q2SJ1PRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319583 (US10174007, Example 2 | US10787438, Example 2 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay B, using 10-fold less enzyme and measuring absorbance for 3 hours to obtain IC50 values below the 10 nM lower limit of Assay A. Compounds were ... | US Patent US10787438 (2020) BindingDB Entry DOI: 10.7270/Q2VQ35RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319582 (US10174007, Example 1 | US10787438, Example 1 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay B, using 10-fold less enzyme and measuring absorbance for 3 hours to obtain IC50 values below the 10 nM lower limit of Assay A. Compounds were ... | US Patent US10787438 (2020) BindingDB Entry DOI: 10.7270/Q2VQ35RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM464138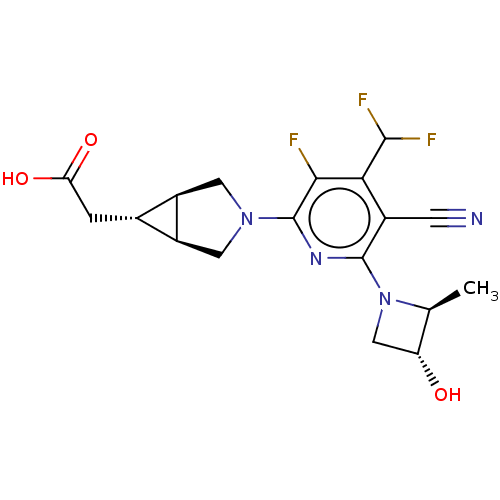 (US10787438, Example 50 | [(1R,5S,6R)-3-{5-cyano-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay B, using 10-fold less enzyme and measuring absorbance for 3 hours to obtain IC50 values below the 10 nM lower limit of Assay A. Compounds were ... | US Patent US10787438 (2020) BindingDB Entry DOI: 10.7270/Q2VQ35RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319582 (US10174007, Example 1 | US10787438, Example 1 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay B, using 10-fold less enzyme and measuring absorbance for 3 hours to obtain IC50 values below the 10 nM lower limit of Assay A. Compounds were ... | US Patent US10988463 (2021) BindingDB Entry DOI: 10.7270/Q2SJ1PRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319582 (US10174007, Example 1 | US10787438, Example 1 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds having an IC50 value less than 20 nM were examined in a second KHK assay, referred to as Assay B, using 10-fold less enzyme and measuring a... | US Patent US10174007 (2019) BindingDB Entry DOI: 10.7270/Q20867DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319605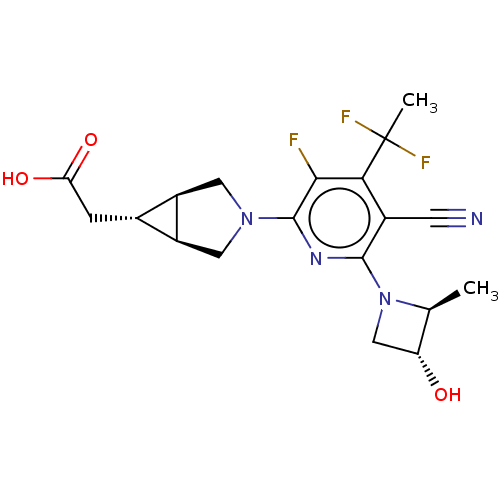 (US10174007, Example 50 | [(1R,5S,6R)-3-{5-cyano-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds having an IC50 value less than 20 nM were examined in a second KHK assay, referred to as Assay B, using 10-fold less enzyme and measuring a... | US Patent US10174007 (2019) BindingDB Entry DOI: 10.7270/Q20867DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439646 (CHEMBL2419600 | US8993586, 110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439646 (CHEMBL2419600 | US8993586, 110) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439642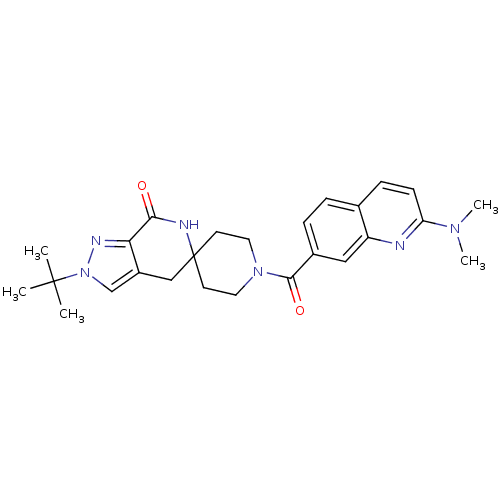 (CHEMBL2419589 | US8993586, 105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM494882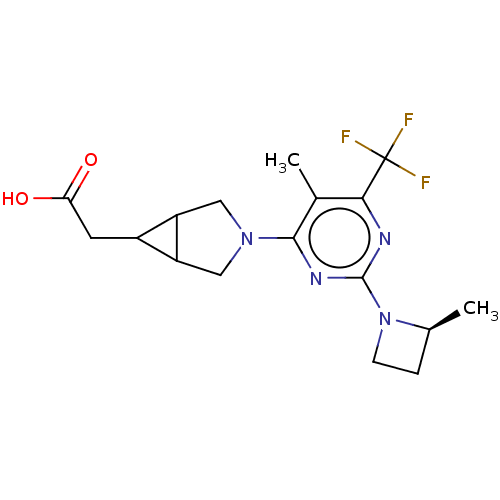 (US10988463, Example 42 | [(1R,5S,6R)-3-{5-methyl-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay B, using 10-fold less enzyme and measuring absorbance for 3 hours to obtain IC50 values below the 10 nM lower limit of Assay A. Compounds were ... | US Patent US10988463 (2021) BindingDB Entry DOI: 10.7270/Q2SJ1PRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319602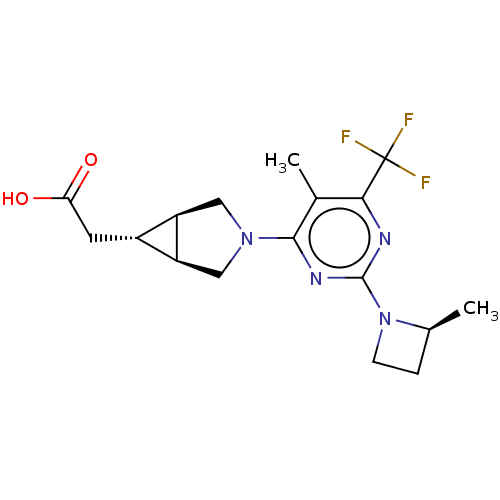 (US10174007, Example 42 | US10787438, Example 42 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2B2807K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319602 (US10174007, Example 42 | US10787438, Example 42 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay B, using 10-fold less enzyme and measuring absorbance for 3 hours to obtain IC50 values below the 10 nM lower limit of Assay A. Compounds were ... | US Patent US10787438 (2020) BindingDB Entry DOI: 10.7270/Q2VQ35RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319602 (US10174007, Example 42 | US10787438, Example 42 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds having an IC50 value less than 20 nM were examined in a second KHK assay, referred to as Assay B, using 10-fold less enzyme and measuring a... | US Patent US10174007 (2019) BindingDB Entry DOI: 10.7270/Q20867DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM464138 (US10787438, Example 50 | [(1R,5S,6R)-3-{5-cyano-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay C, was performed at high fructose and ATP concentrations, conditions that would be more consistent with physiological concentrations of the nat... | US Patent US10787438 (2020) BindingDB Entry DOI: 10.7270/Q2VQ35RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319605 (US10174007, Example 50 | [(1R,5S,6R)-3-{5-cyano-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A third KHK assay, referred to as Assay C, was performed at high fructose and ATP concentrations, conditions that would be more consistent with physi... | US Patent US10174007 (2019) BindingDB Entry DOI: 10.7270/Q20867DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM601403 (US11634410, Example 50 | [(1R,5S,6R)-3-{5-cyano-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2B2807K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM494884 (US10988463, Example 50 | [(1R,5S,6R)-3-{5-cyano-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay C, was performed at high fructose and ATP concentrations, conditions that would be more consistent with physiological concentrations of the nat... | US Patent US10988463 (2021) BindingDB Entry DOI: 10.7270/Q2SJ1PRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439644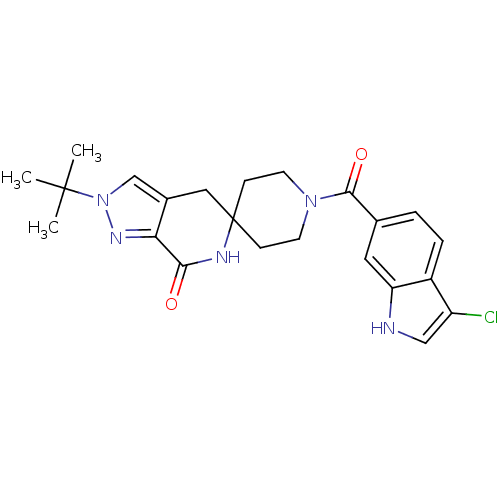 (CHEMBL2419593 | US8993586, 86) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319600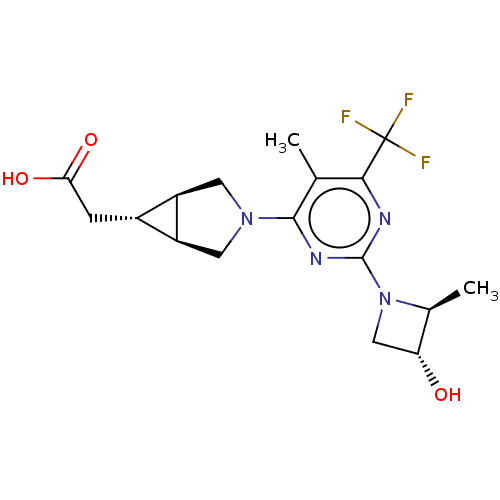 (US10174007, Example 24 | US10787438, Example 24 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay B, using 10-fold less enzyme and measuring absorbance for 3 hours to obtain IC50 values below the 10 nM lower limit of Assay A. Compounds were ... | US Patent US10787438 (2020) BindingDB Entry DOI: 10.7270/Q2VQ35RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319582 (US10174007, Example 1 | US10787438, Example 1 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay C, was performed at high fructose and ATP concentrations, conditions that would be more consistent with physiological concentrations of the nat... | US Patent US10787438 (2020) BindingDB Entry DOI: 10.7270/Q2VQ35RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319600 (US10174007, Example 24 | US10787438, Example 24 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2B2807K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319600 (US10174007, Example 24 | US10787438, Example 24 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds having an IC50 value less than 20 nM were examined in a second KHK assay, referred to as Assay B, using 10-fold less enzyme and measuring a... | US Patent US10174007 (2019) BindingDB Entry DOI: 10.7270/Q20867DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319582 (US10174007, Example 1 | US10787438, Example 1 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A third KHK assay, referred to as Assay C, was performed at high fructose and ATP concentrations, conditions that would be more consistent with physi... | US Patent US10174007 (2019) BindingDB Entry DOI: 10.7270/Q20867DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319582 (US10174007, Example 1 | US10787438, Example 1 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2B2807K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM494880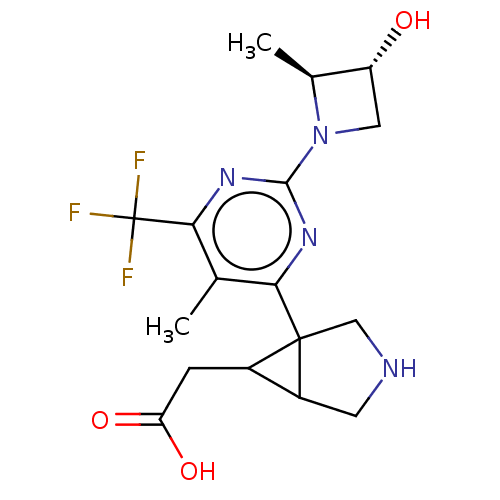 (US10988463, Example 24 | [(1R,5S,6R)-3-{2-[(2S,3R)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay B, using 10-fold less enzyme and measuring absorbance for 3 hours to obtain IC50 values below the 10 nM lower limit of Assay A. Compounds were ... | US Patent US10988463 (2021) BindingDB Entry DOI: 10.7270/Q2SJ1PRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319582 (US10174007, Example 1 | US10787438, Example 1 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay C, was performed at high fructose and ATP concentrations, conditions that would be more consistent with physiological concentrations of the nat... | US Patent US10988463 (2021) BindingDB Entry DOI: 10.7270/Q2SJ1PRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319583 (US10174007, Example 2 | US10787438, Example 2 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay C, was performed at high fructose and ATP concentrations, conditions that would be more consistent with physiological concentrations of the nat... | US Patent US10787438 (2020) BindingDB Entry DOI: 10.7270/Q2VQ35RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319583 (US10174007, Example 2 | US10787438, Example 2 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay C, was performed at high fructose and ATP concentrations, conditions that would be more consistent with physiological concentrations of the nat... | US Patent US10988463 (2021) BindingDB Entry DOI: 10.7270/Q2SJ1PRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319583 (US10174007, Example 2 | US10787438, Example 2 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2B2807K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319583 (US10174007, Example 2 | US10787438, Example 2 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A third KHK assay, referred to as Assay C, was performed at high fructose and ATP concentrations, conditions that would be more consistent with physi... | US Patent US10174007 (2019) BindingDB Entry DOI: 10.7270/Q20867DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM276763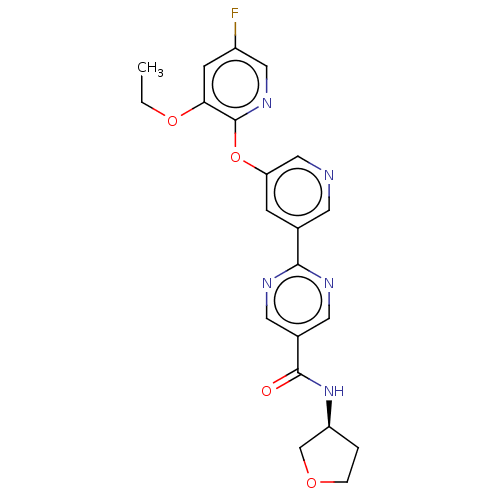 (US10071992, Example 8 | US11034678, Example 8 | US...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc. US Patent | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white Polyplates (Perkin Elmer) in a total volume of 20 μL. To 1 &#... | US Patent US10071992 (2018) BindingDB Entry DOI: 10.7270/Q2RJ4MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM276763 (US10071992, Example 8 | US11034678, Example 8 | US...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For determination of IC50 values, the reactions were carried out in 384-well white Polyplates (Perkin Elmer) in a total volume of 20 μL. To 1 &#... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC64BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319601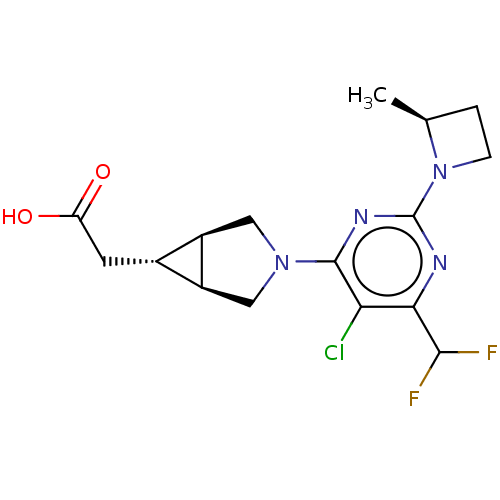 (US10174007, Example 40 | US10787438, Example 40 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Compounds having an IC50 value less than 20 nM were examined in a second KHK assay, referred to as Assay B, using 10-fold less enzyme and measuring a... | US Patent US10174007 (2019) BindingDB Entry DOI: 10.7270/Q20867DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319601 (US10174007, Example 40 | US10787438, Example 40 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2B2807K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM494881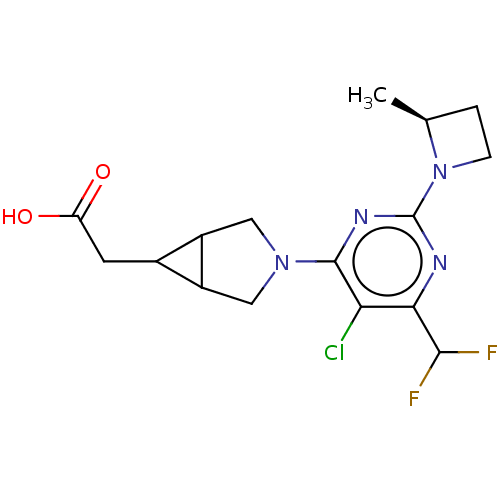 (US10988463, Example 40 | [(1R,5S,6R)-3-{5-chloro-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay B, using 10-fold less enzyme and measuring absorbance for 3 hours to obtain IC50 values below the 10 nM lower limit of Assay A. Compounds were ... | US Patent US10988463 (2021) BindingDB Entry DOI: 10.7270/Q2SJ1PRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319601 (US10174007, Example 40 | US10787438, Example 40 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay B, using 10-fold less enzyme and measuring absorbance for 3 hours to obtain IC50 values below the 10 nM lower limit of Assay A. Compounds were ... | US Patent US10787438 (2020) BindingDB Entry DOI: 10.7270/Q2VQ35RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439634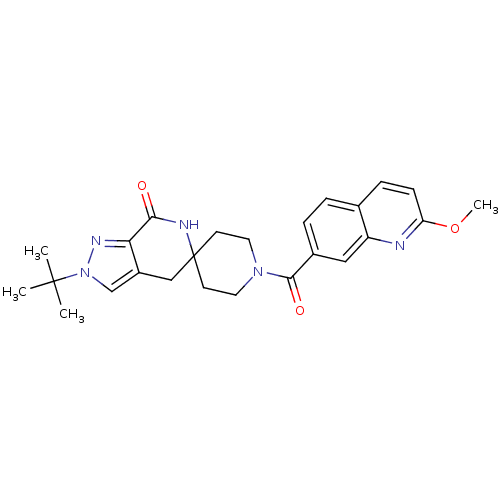 (CHEMBL2419596 | US8993586, 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439643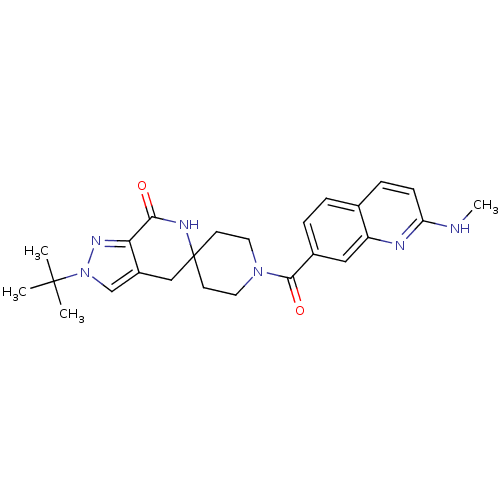 (CHEMBL2419598 | US8993586, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439641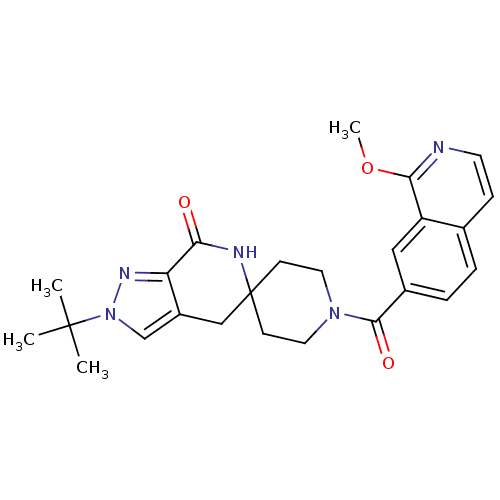 (CHEMBL2419597 | US8993586, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM494884 (US10988463, Example 50 | [(1R,5S,6R)-3-{5-cyano-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay A, a 384-well format on a Corning 3653 assay plate is used, and monitored by UV-vis spectroscopy in continuous mode at rt. Compounds were prepa... | US Patent US10988463 (2021) BindingDB Entry DOI: 10.7270/Q2SJ1PRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM464138 (US10787438, Example 50 | [(1R,5S,6R)-3-{5-cyano-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Assay A, a 384-well format on a Corning 3653 assay plate is used, and monitored by UV-vis spectroscopy in continuous mode at rt. Compounds were prepa... | US Patent US10787438 (2020) BindingDB Entry DOI: 10.7270/Q2VQ35RP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ketohexokinase (Homo sapiens (Human)) | BDBM319605 (US10174007, Example 50 | [(1R,5S,6R)-3-{5-cyano-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A 384-well format on a Corning 3653 assay plate is used, and monitored by UV-vis spectroscopy in continuous mode at rt. Compounds were prepared in DM... | US Patent US10174007 (2019) BindingDB Entry DOI: 10.7270/Q20867DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1231 total ) | Next | Last >> |