

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (RAT) | BDBM50087691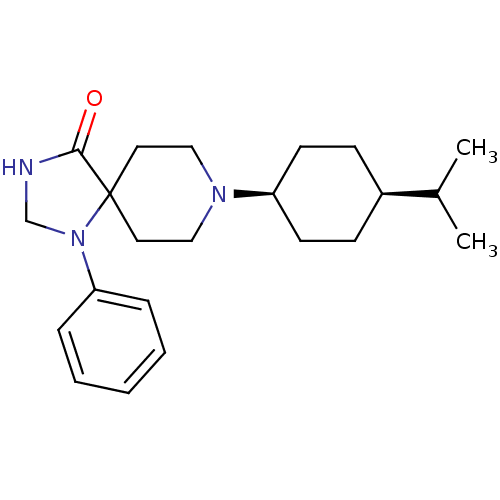 (8-(4-Isopropyl-cyclohexyl)-1-phenyl-1,3,8-triaza-s...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087692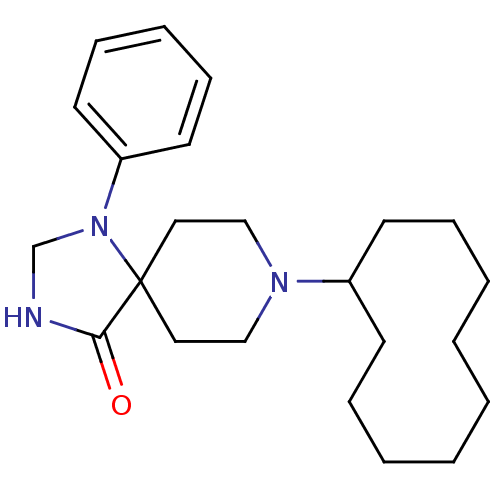 (8-Cyclodecyl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50027473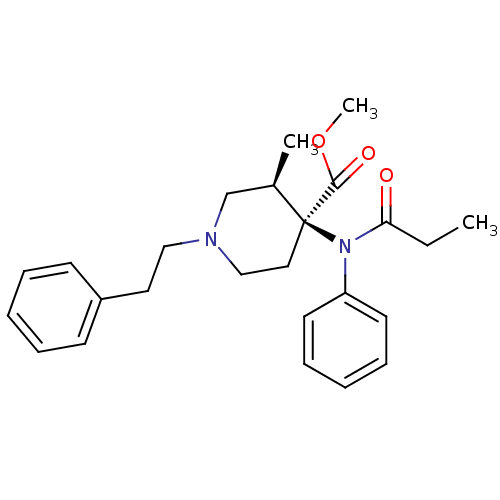 (3-Methyl-1-phenethyl-4-(phenyl-propionyl-amino)-pi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding displacement analyses was performed from permanently transfected HEK293 cells expressing Opioid receptor mu 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50057010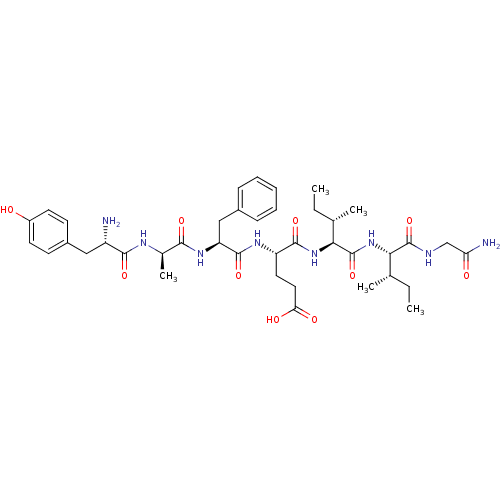 ((S)-4-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [Ile5,6-3H]-deltorphin to membrane from baby hamster kidney cells infected with forest virus encoding the ... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087697 (8-Cycloundecyl-1-phenyl-1,3,8-triaza-spiro[4.5]dec...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087687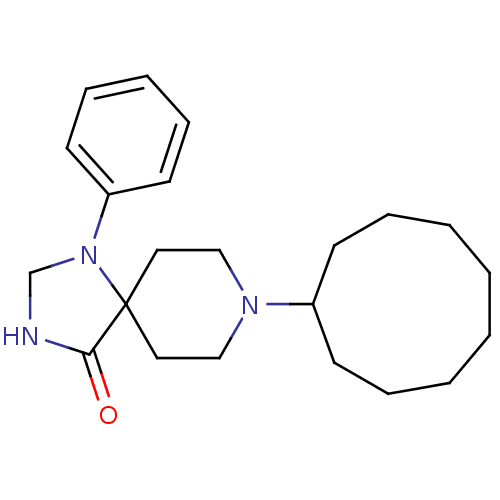 (8-Cyclononyl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087685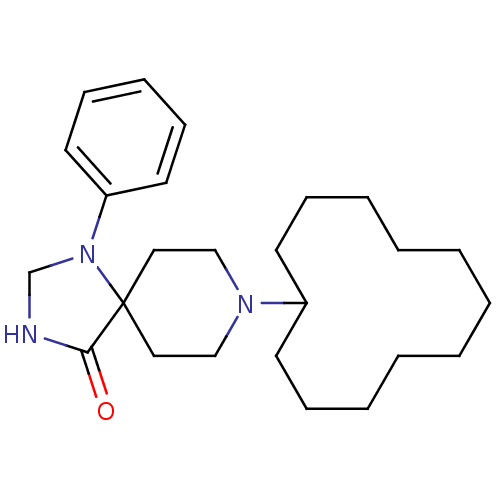 (8-Cyclododecyl-1-phenyl-1,3,8-triaza-spiro[4.5]dec...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50471324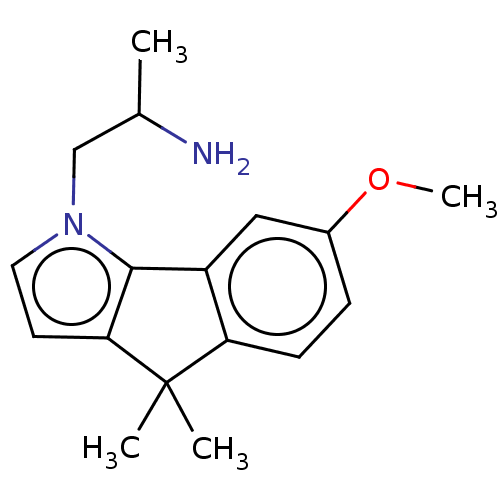 (CHEMBL318639) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Inc Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 2C receptor using displacement of [3H]DOB | J Med Chem 40: 2762-9 (1997) Article DOI: 10.1021/jm970030l BindingDB Entry DOI: 10.7270/Q270845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087698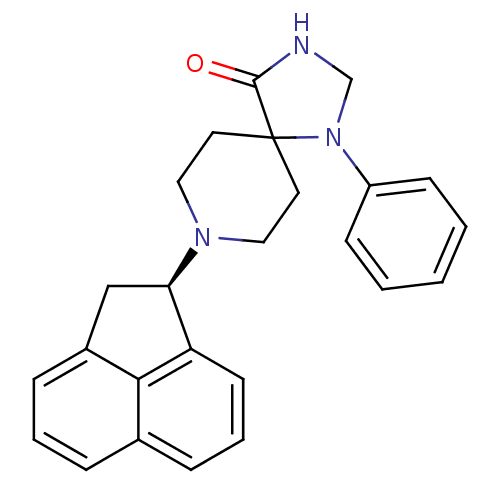 (8-(R)-Acenaphthen-1-yl-1-phenyl-1,3,8-triaza-spiro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50474329 (CHEMBL315551) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of N/OFQ binding to human Orphanin FQ receptor (Nociceptin/Orphanin) | J Med Chem 46: 255-64 (2003) Article DOI: 10.1021/jm0209174 BindingDB Entry DOI: 10.7270/Q2GH9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087697 (8-Cycloundecyl-1-phenyl-1,3,8-triaza-spiro[4.5]dec...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087016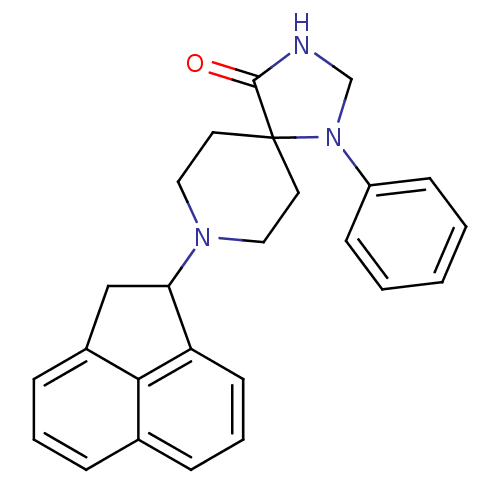 (8-Acenaphthen-1-yl-1-phenyl-1,3,8-triaza-spiro[4.5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding affinity against transfected HEK293 cells expressing human Opioid receptor like 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50474331 (CHEMBL316086) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of N/OFQ binding to human Orphanin FQ receptor (Nociceptin/Orphanin) | J Med Chem 46: 255-64 (2003) Article DOI: 10.1021/jm0209174 BindingDB Entry DOI: 10.7270/Q2GH9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50474328 (CHEMBL330291) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of N/OFQ binding to human Orphanin FQ receptor (Nociceptin/Orphanin) | J Med Chem 46: 255-64 (2003) Article DOI: 10.1021/jm0209174 BindingDB Entry DOI: 10.7270/Q2GH9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087692 (8-Cyclodecyl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50474330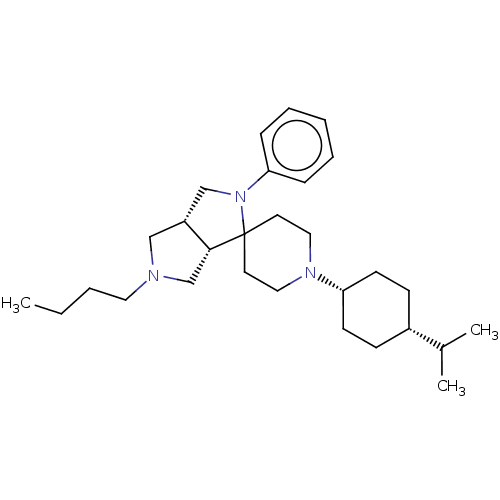 (CHEMBL2112342) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of N/OFQ binding to human Orphanin FQ receptor (Nociceptin/Orphanin) | J Med Chem 46: 255-64 (2003) Article DOI: 10.1021/jm0209174 BindingDB Entry DOI: 10.7270/Q2GH9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087008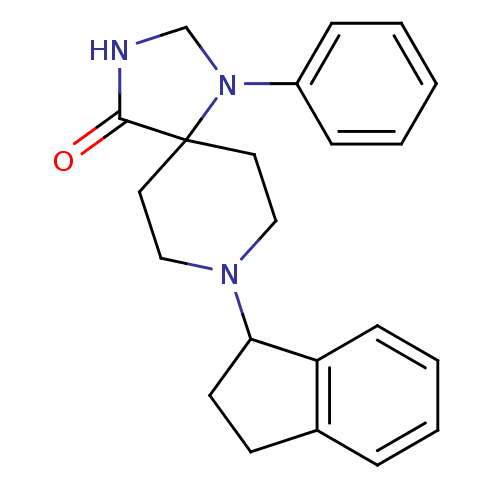 (8-indan-1-yl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding affinity against transfected HEK293 cells expressing Opioid receptor like 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50474321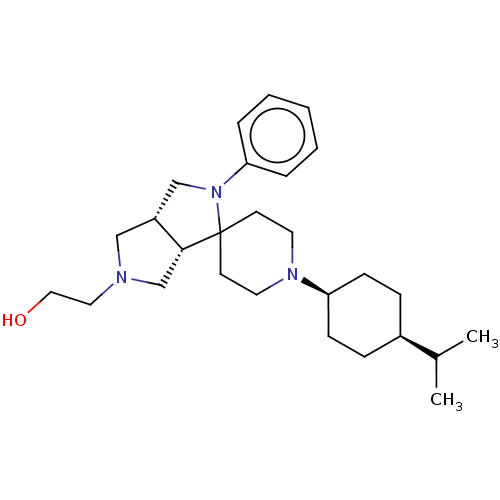 (CHEMBL2112348) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of N/OFQ binding to human Orphanin FQ receptor (Nociceptin/Orphanin) | J Med Chem 46: 255-64 (2003) Article DOI: 10.1021/jm0209174 BindingDB Entry DOI: 10.7270/Q2GH9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087697 (8-Cycloundecyl-1-phenyl-1,3,8-triaza-spiro[4.5]dec...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087685 (8-Cyclododecyl-1-phenyl-1,3,8-triaza-spiro[4.5]dec...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50108306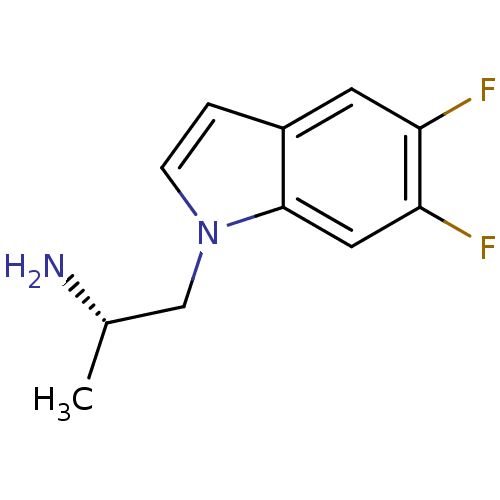 ((S)-1-(5,6-difluoro-1H-indol-1-yl)propan-2-amine |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Inc Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 2C receptor using displacement of [3H]DOB | J Med Chem 40: 2762-9 (1997) Article DOI: 10.1021/jm970030l BindingDB Entry DOI: 10.7270/Q270845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50471326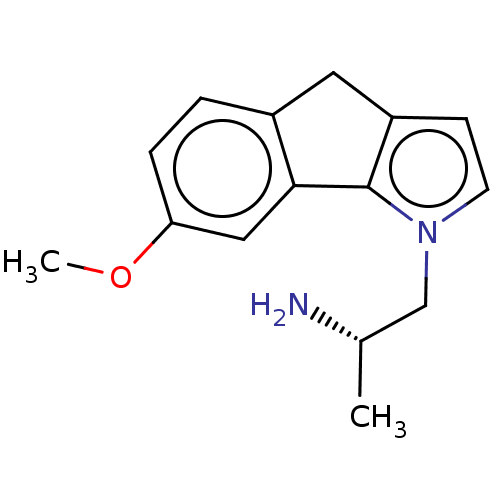 (CHEMBL2114265) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Inc Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 2C receptor using displacement of [3H]DOB | J Med Chem 40: 2762-9 (1997) Article DOI: 10.1021/jm970030l BindingDB Entry DOI: 10.7270/Q270845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50471314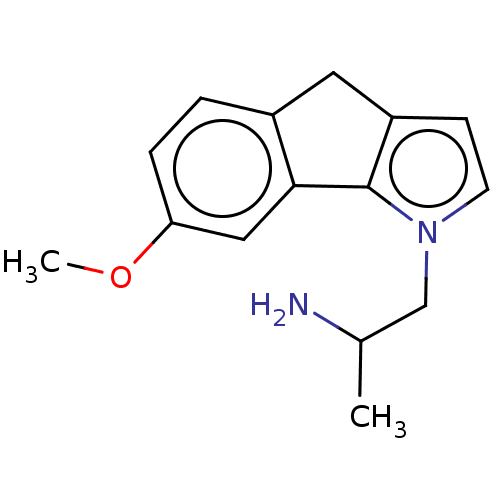 (CHEMBL414163) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Inc Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 2C receptor using displacement of [3H]DOB | J Med Chem 40: 2762-9 (1997) Article DOI: 10.1021/jm970030l BindingDB Entry DOI: 10.7270/Q270845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50232703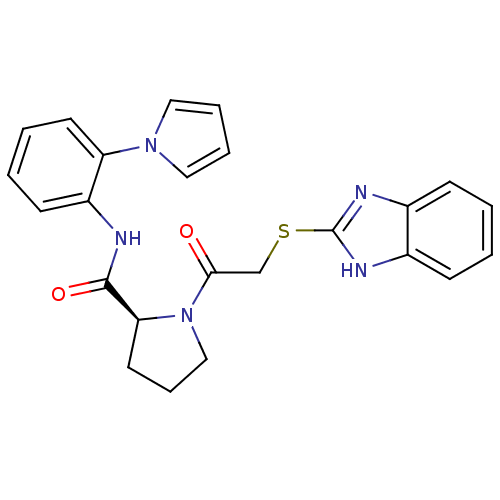 ((S)-1-(2-(1H-benzo[d]imidazol-2-ylthio)acetyl)-N-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor | J Med Chem 52: 891-903 (2009) Article DOI: 10.1021/jm801296d BindingDB Entry DOI: 10.7270/Q2TM7B3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50474322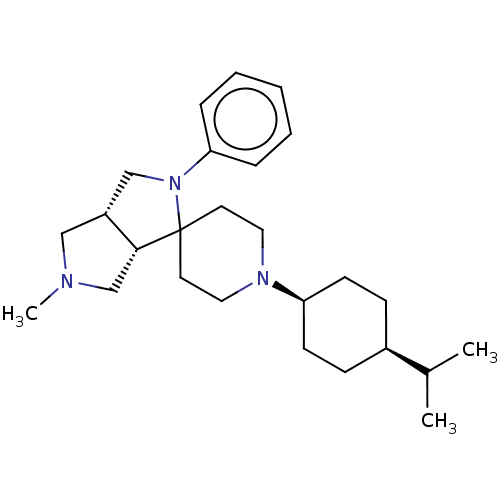 (CHEMBL2112345) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of N/OFQ binding to human Orphanin FQ receptor (Nociceptin/Orphanin) | J Med Chem 46: 255-64 (2003) Article DOI: 10.1021/jm0209174 BindingDB Entry DOI: 10.7270/Q2GH9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50474322 (CHEMBL2112345) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of N/OFQ binding to human Orphanin FQ receptor (Nociceptin/Orphanin) | J Med Chem 46: 255-64 (2003) Article DOI: 10.1021/jm0209174 BindingDB Entry DOI: 10.7270/Q2GH9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50471318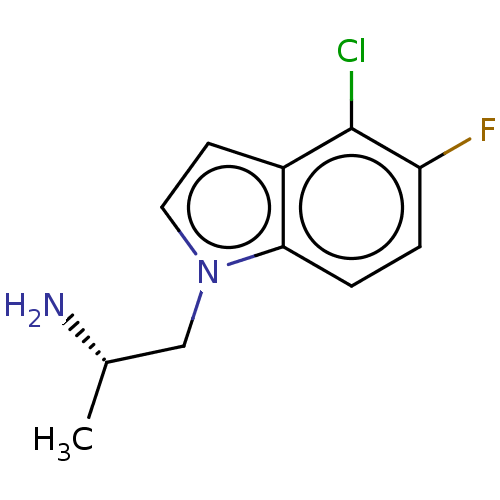 (CHEMBL2114266) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Inc Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 2C receptor using displacement of [3H]DOB | J Med Chem 40: 2762-9 (1997) Article DOI: 10.1021/jm970030l BindingDB Entry DOI: 10.7270/Q270845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50144841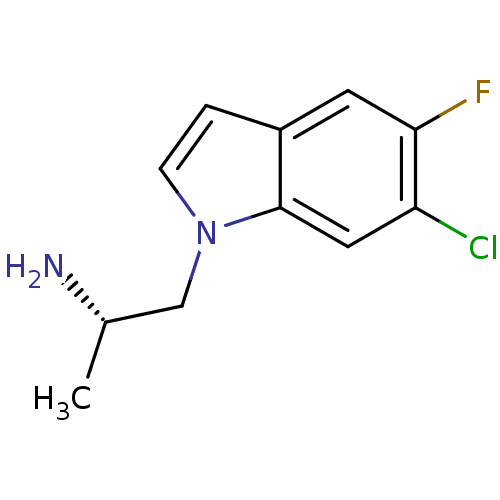 ((S)-1-(6-chloro-5-fluoro-1H-indol-1-yl)propan-2-am...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Inc Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 2C receptor using displacement of [3H]DOB | J Med Chem 40: 2762-9 (1997) Article DOI: 10.1021/jm970030l BindingDB Entry DOI: 10.7270/Q270845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50471329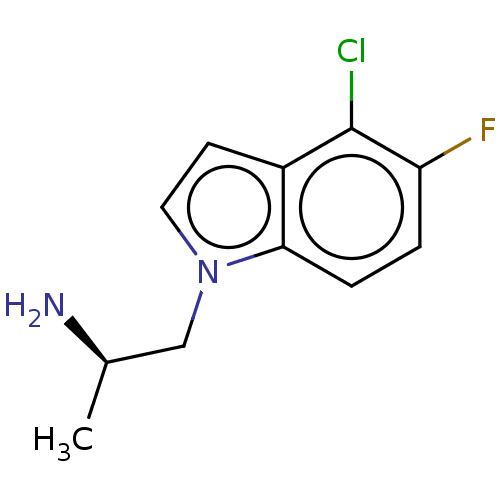 (CHEMBL2115254) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Inc Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 2C receptor using displacement of [3H]DOB | J Med Chem 40: 2762-9 (1997) Article DOI: 10.1021/jm970030l BindingDB Entry DOI: 10.7270/Q270845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087011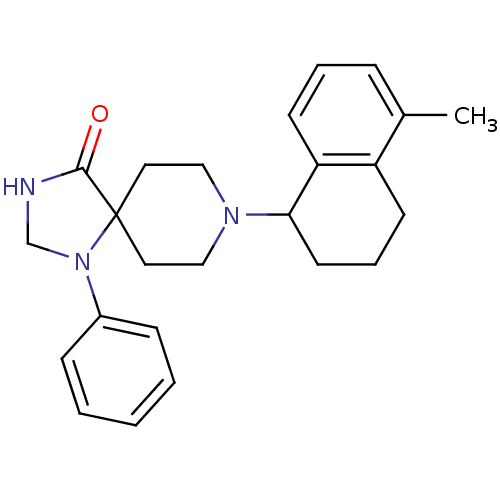 (8-(5-Methyl-1,2,3,4-tetrahydro-naphthalen-1-yl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding affinity against transfected HEK293 cells expressing human Opioid receptor like 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087690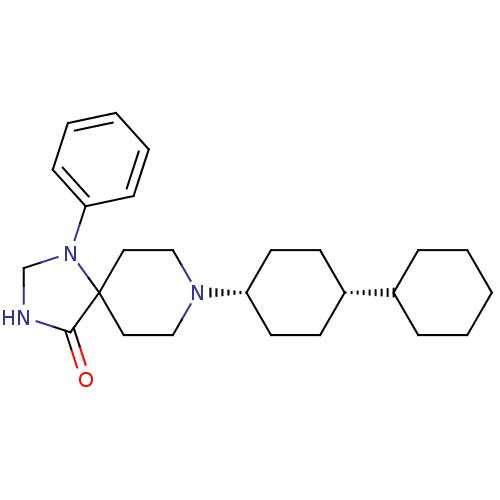 (8-Bicyclohexyl-4-yl-1-phenyl-1,3,8-triaza-spiro[4....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087690 (8-Bicyclohexyl-4-yl-1-phenyl-1,3,8-triaza-spiro[4....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50474324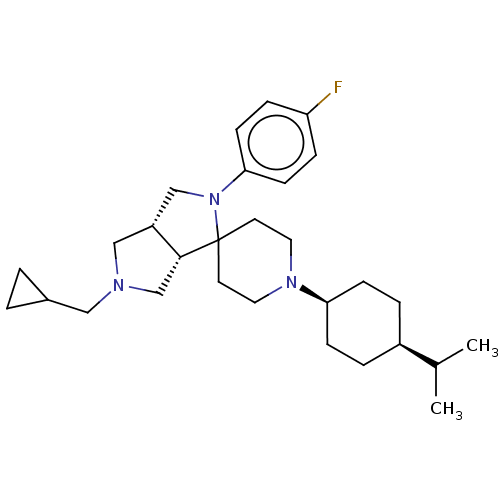 (CHEMBL2112346) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of N/OFQ binding to human Orphanin FQ receptor (Nociceptin/Orphanin) | J Med Chem 46: 255-64 (2003) Article DOI: 10.1021/jm0209174 BindingDB Entry DOI: 10.7270/Q2GH9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50474326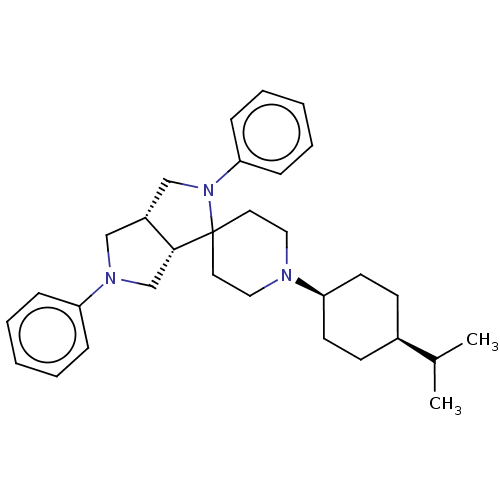 (CHEMBL2112347) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of N/OFQ binding to human Orphanin FQ receptor (Nociceptin/Orphanin) | J Med Chem 46: 255-64 (2003) Article DOI: 10.1021/jm0209174 BindingDB Entry DOI: 10.7270/Q2GH9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087684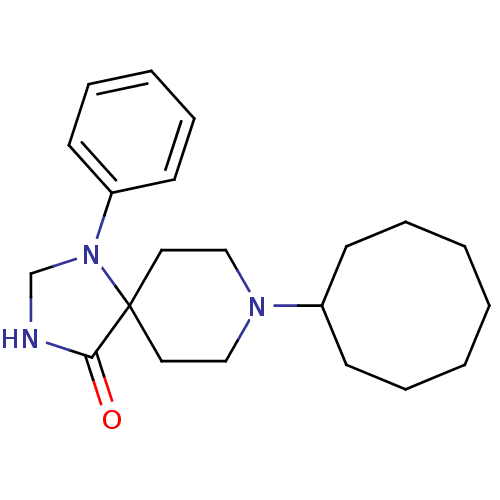 (8-Cyclooctyl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (RAT) | BDBM50087693 (1-Phenyl-8-(4-propyl-cyclohexyl)-1,3,8-triaza-spir...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50471312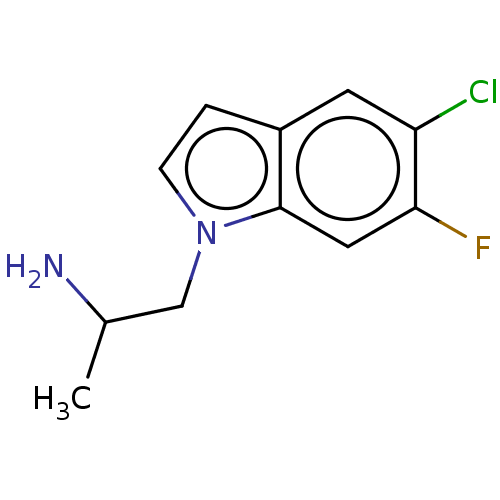 (CHEMBL313856) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Inc Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 2C receptor using displacement of [3H]DOB | J Med Chem 40: 2762-9 (1997) Article DOI: 10.1021/jm970030l BindingDB Entry DOI: 10.7270/Q270845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50474323 (CHEMBL2112341) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of N/OFQ binding to human Orphanin FQ receptor (Nociceptin/Orphanin) | J Med Chem 46: 255-64 (2003) Article DOI: 10.1021/jm0209174 BindingDB Entry DOI: 10.7270/Q2GH9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50474325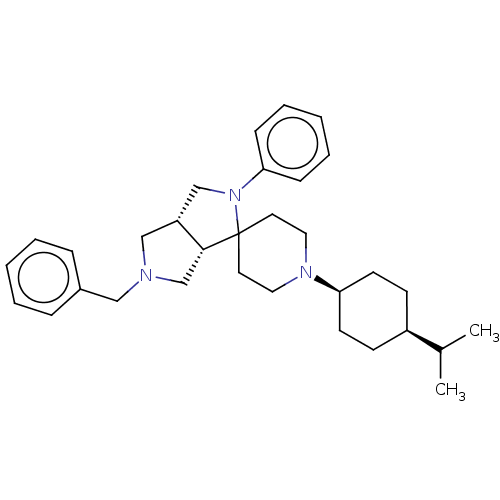 (CHEMBL2112343) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of N/OFQ binding to human Orphanin FQ receptor (Nociceptin/Orphanin) | J Med Chem 46: 255-64 (2003) Article DOI: 10.1021/jm0209174 BindingDB Entry DOI: 10.7270/Q2GH9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087692 (8-Cyclodecyl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087015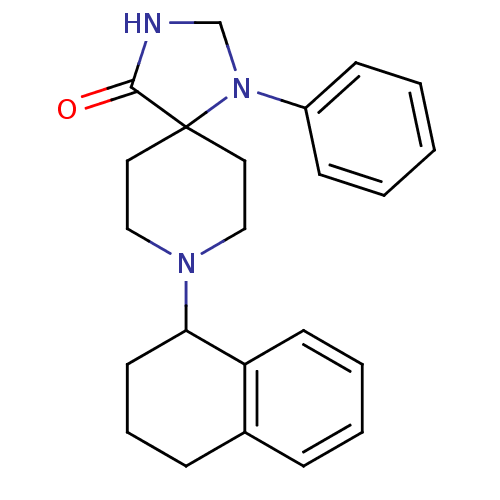 (1-Phenyl-8-(1,2,3,4-tetrahydro-naphthalen-1-yl)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding affinity against transfected HEK293 cells expressing Opioid receptor like 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50474327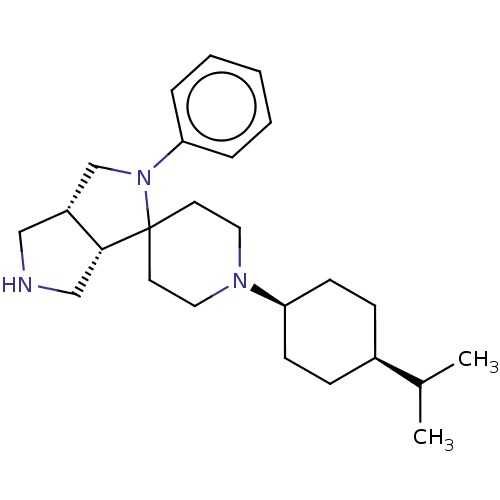 (CHEMBL2112344) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of N/OFQ binding to human Orphanin FQ receptor (Nociceptin/Orphanin) | J Med Chem 46: 255-64 (2003) Article DOI: 10.1021/jm0209174 BindingDB Entry DOI: 10.7270/Q2GH9MQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087002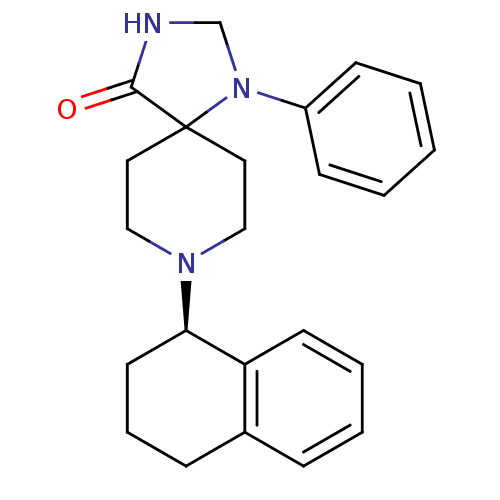 (1-Phenyl-8-(1,2,3,4-tetrahydro-naphthalen-1-yl)-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding affinity against transfected HEK293 cells expressing Opioid receptor like 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087022 (8-Indan-2-yl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding affinity against transfected HEK293 cells expressing human Opioid receptor like 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50087004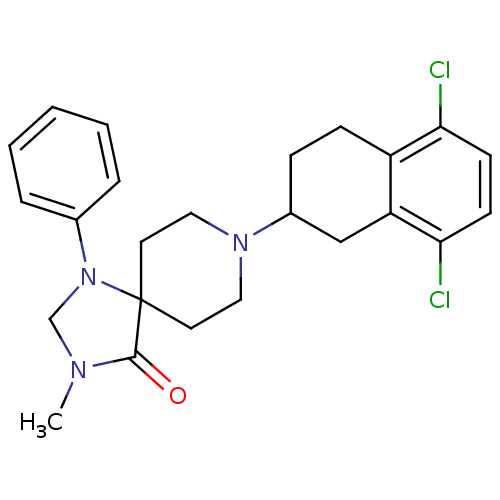 (8-(5,8-Dichloro-1,2,3,4-tetrahydro-naphthalen-2-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Competitive binding displacement analyses was performed from permanently transfected HEK293 cells expressing human Opioid receptor mu 1 | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087020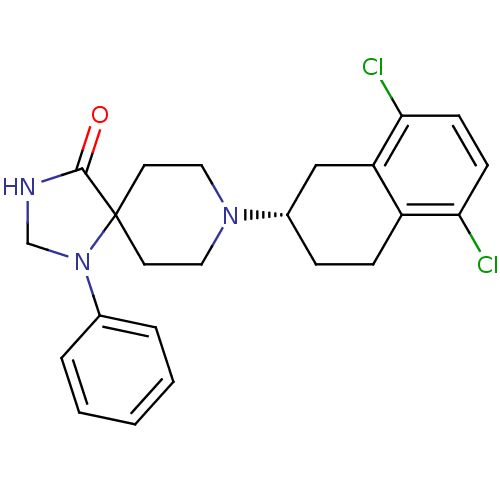 (8-(5,8-Dichloro-1,2,3,4-tetrahydro-naphthalen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Effective concentration required to stimulate binding of GTPgammaS to Opioid receptor like 1 was determined using scintillation proximity assay | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50087012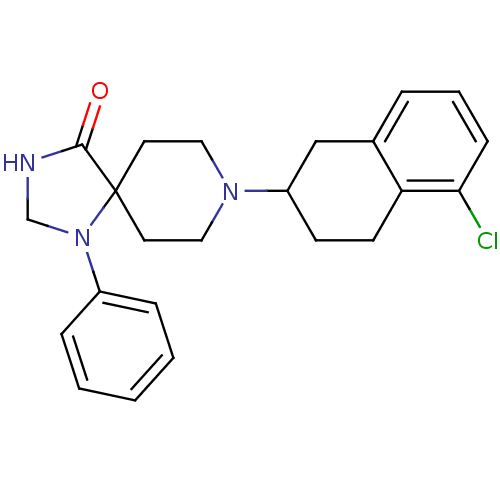 (8-(5-Chloro-1,2,3,4-tetrahydro-naphthalen-2-yl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Effective concentration required to stimulate binding of GTPgammaS to Opioid receptor like 1 was determined using scintillation proximity assay | J Med Chem 43: 1329-38 (2001) BindingDB Entry DOI: 10.7270/Q2ZG6SZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087685 (8-Cyclododecyl-1-phenyl-1,3,8-triaza-spiro[4.5]dec...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087687 (8-Cyclononyl-1-phenyl-1,3,8-triaza-spiro[4.5]decan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM85863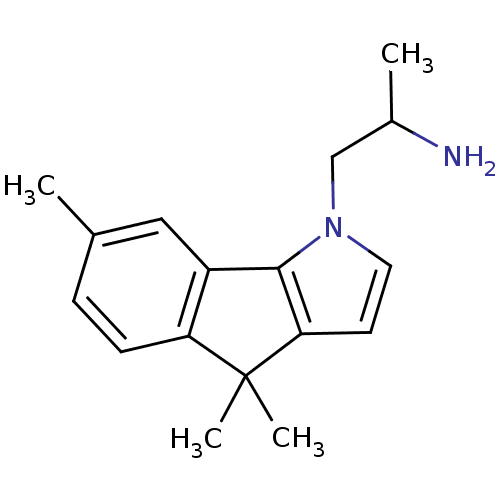 (CAS_170493-63-7 | NSC_3045228 | Ro 600332) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Inc Curated by ChEMBL | Assay Description Binding affinity against human 5-hydroxytryptamine 2C receptor using displacement of [3H]DOB | J Med Chem 40: 2762-9 (1997) Article DOI: 10.1021/jm970030l BindingDB Entry DOI: 10.7270/Q270845Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 919 total ) | Next | Last >> |