

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matripase (unknown origin) using Boc-QAR-AMC as substrate incubated for 30 mins prior to substrate addition by fluorescence assay | Bioorg Med Chem 23: 2328-43 (2015) Article DOI: 10.1016/j.bmc.2015.03.072 BindingDB Entry DOI: 10.7270/Q23F4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032934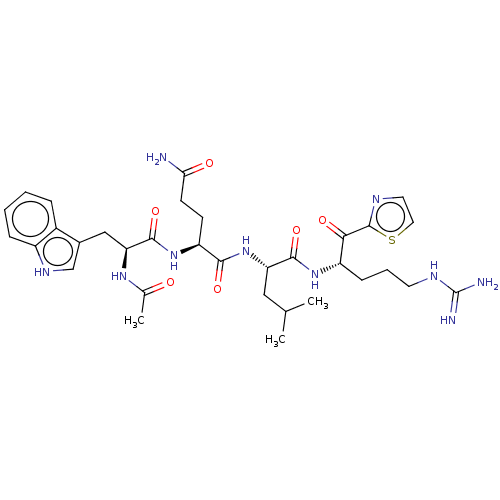 (CHEMBL3356591) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032999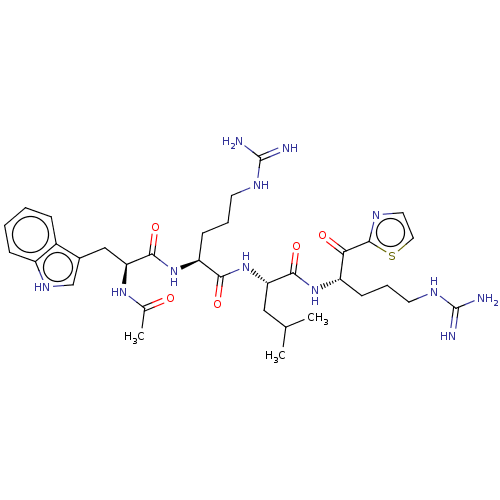 (CHEMBL3356597) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032936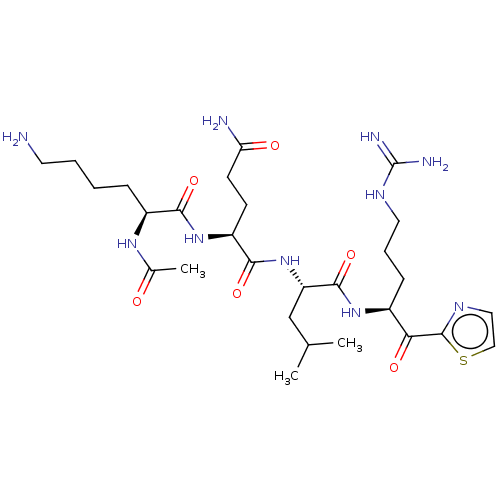 (CHEMBL3356589) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50018730 (3-(2-Phenethyl-octahydro-isoquinolin-4a-yl)-phenol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor mu 1 in rat brain using [3H]-NAL as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627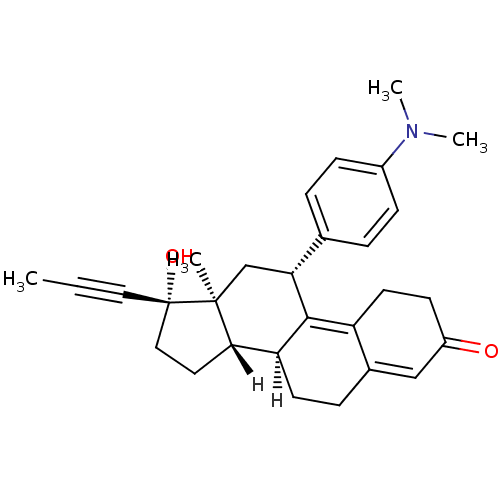 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 4 nM progesterone-stimulated transactivation of MMTV-Luc reporter in CV-1 cells expressing PR-B | Bioorg Med Chem Lett 15: 3203-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.001 BindingDB Entry DOI: 10.7270/Q2J67KP9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032931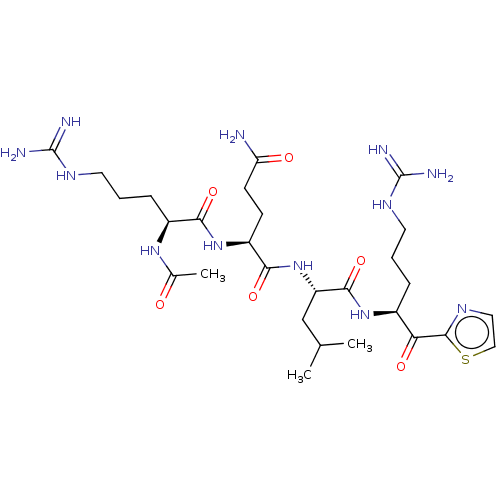 (CHEMBL3356594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324835 ((R)-4-hydroxy-7-(1-hydroxy-2-(3-(methyl(phenyl)ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta2 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032931 (CHEMBL3356594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032930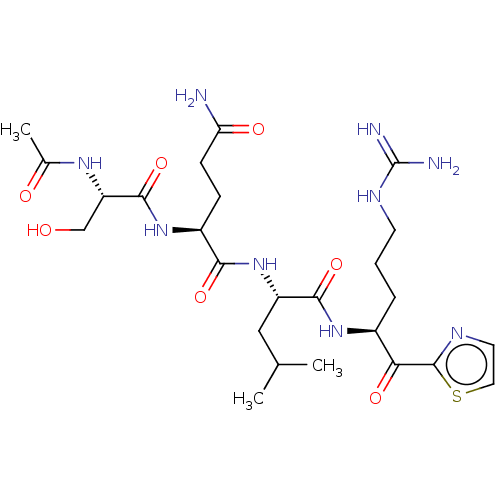 (CHEMBL3356595) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055260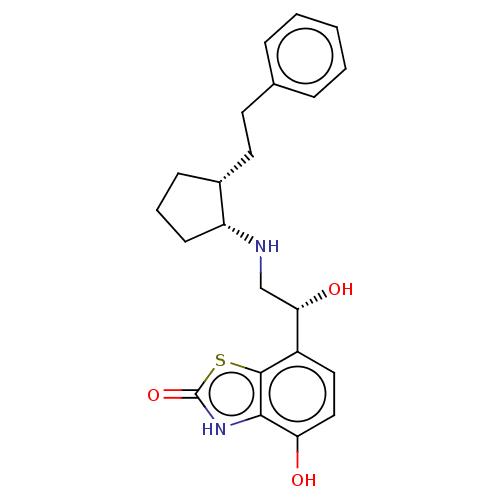 (CHEMBL3323658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324847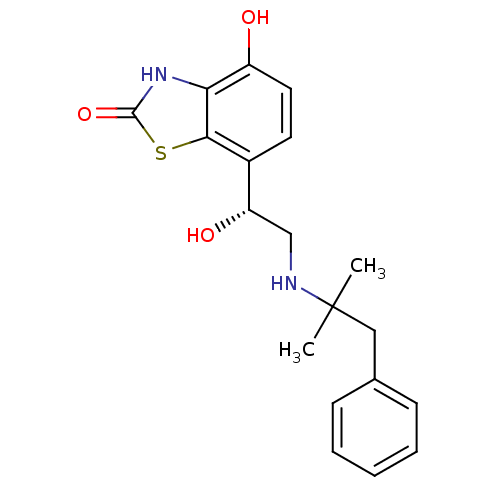 ((R)-4-hydroxy-7-(1-hydroxy-2-(2-methyl-1-phenylpro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta2 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50288303 ((R)-3-((S)-2-Amino-3-methyl-pentanoyl)-thiazolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human Dipeptidyl peptidase IV | Bioorg Med Chem Lett 6: 2745-2748 (1996) Article DOI: 10.1016/S0960-894X(96)00491-X BindingDB Entry DOI: 10.7270/Q2MP537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032935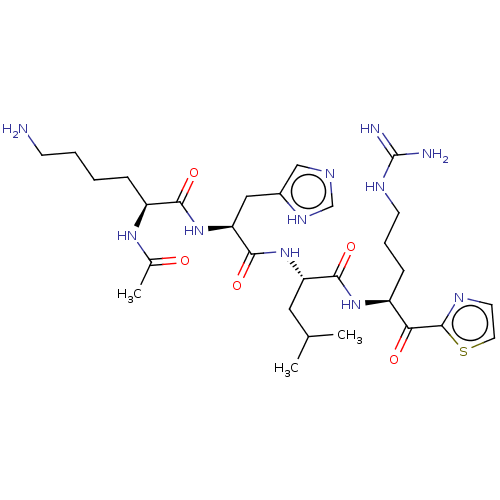 (CHEMBL3356590) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032947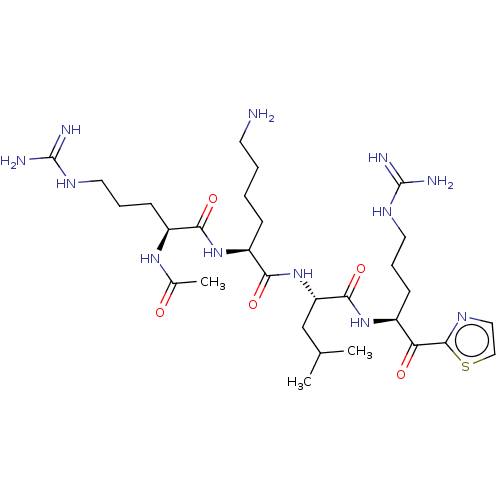 (CHEMBL3356606) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50288308 ((R)-3-(2-Amino-2-cyclopentyl-acetyl)-thiazolidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human Dipeptidyl peptidase IV | Bioorg Med Chem Lett 6: 2745-2748 (1996) Article DOI: 10.1016/S0960-894X(96)00491-X BindingDB Entry DOI: 10.7270/Q2MP537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324854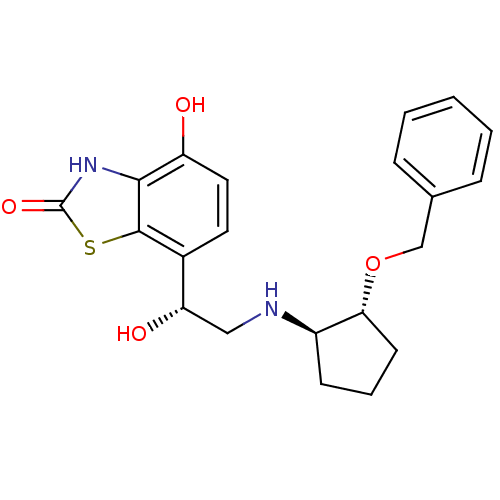 (7-((R)-2-((1R,2R)-2-(benzyloxy)cyclopentylamino)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta2 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of hepsin (unknown origin) using Boc-QAR-AMC as substrate after 30 mins prior to substrate addition by fluorescence assay | Bioorg Med Chem 23: 2328-43 (2015) Article DOI: 10.1016/j.bmc.2015.03.072 BindingDB Entry DOI: 10.7270/Q23F4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032941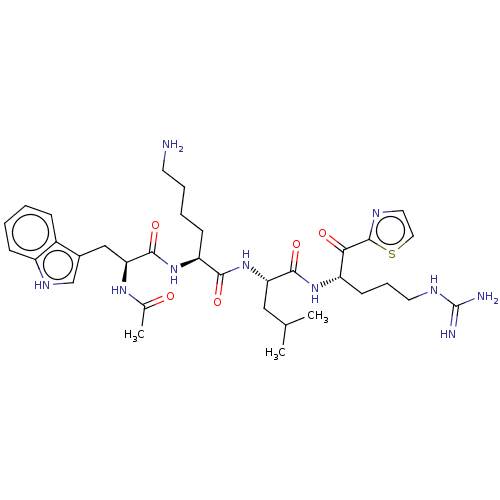 (CHEMBL3356600) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032991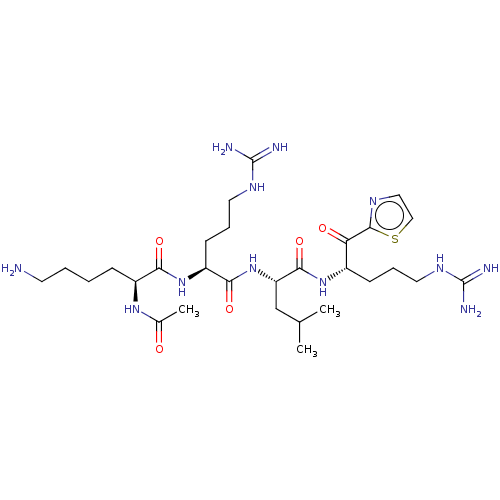 (CHEMBL3356596) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032933 (CHEMBL3356592) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055263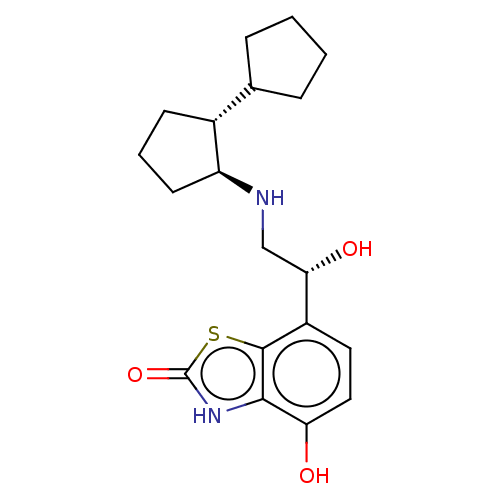 (CHEMBL3323654) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324856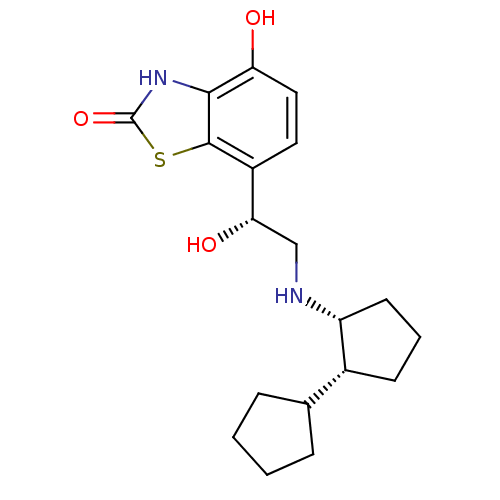 (7-((R)-2-((cis)-bi(cyclopentan)-2-ylamino)-1-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta2 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032940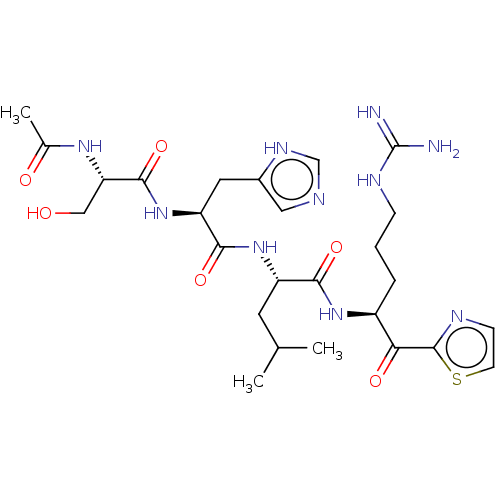 (CHEMBL3356599) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032945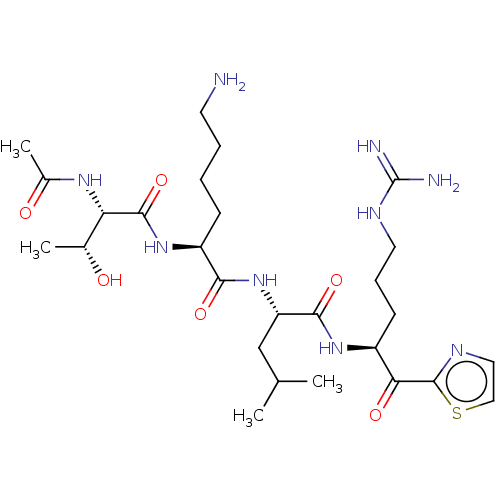 (CHEMBL3356604) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032944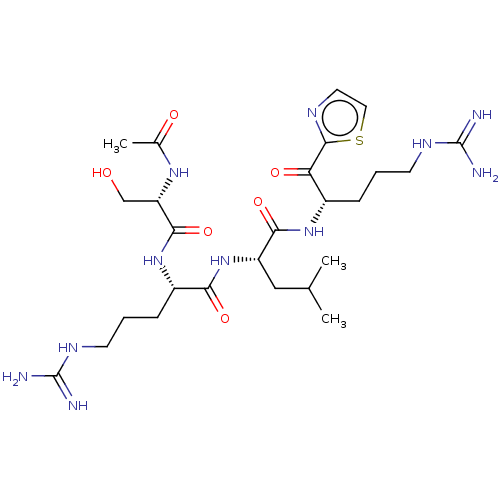 (CHEMBL3356603) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032933 (CHEMBL3356592) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM285708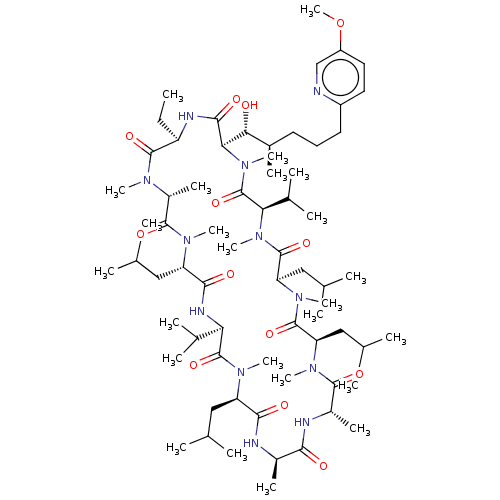 (US10077289, Compound 16 | [(2S,3R,4R)-3-Hydroxy-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | 7.8 | n/a |
Allergan, Inc. US Patent | Assay Description The protease-free PPIase assay measures the rate of cis to trans conversion of a peptide substrate catalyzed by the enzyme cyclophilin A. Addition of... | US Patent US10077289 (2018) BindingDB Entry DOI: 10.7270/Q25B04JF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50288307 ((R)-3-(2-Amino-2-cyclohexyl-acetyl)-thiazolidine-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human Dipeptidyl peptidase IV | Bioorg Med Chem Lett 6: 2745-2748 (1996) Article DOI: 10.1016/S0960-894X(96)00491-X BindingDB Entry DOI: 10.7270/Q2MP537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50055260 (CHEMBL3323658) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta1 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324856 (7-((R)-2-((cis)-bi(cyclopentan)-2-ylamino)-1-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324857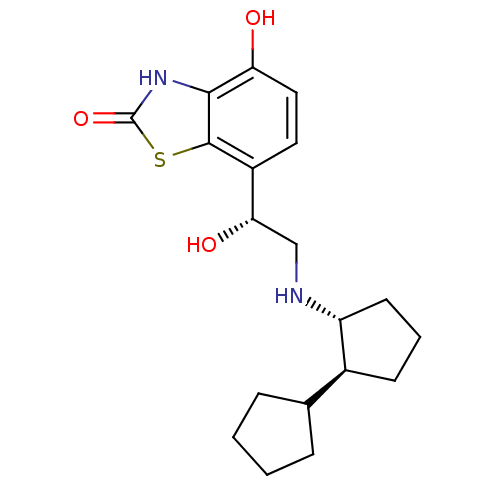 (7-((R)-2-((trans)-bi(cyclopentan)-2-ylamino)-1-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta2 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032947 (CHEMBL3356606) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50324854 (7-((R)-2-((1R,2R)-2-(benzyloxy)cyclopentylamino)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta-1 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032936 (CHEMBL3356589) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50018728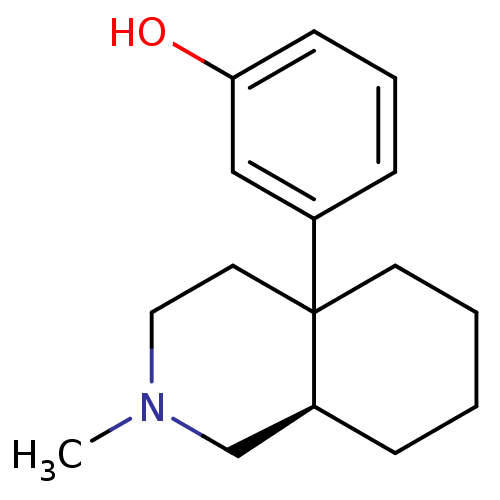 (3-(2-Methyl-octahydro-isoquinolin-4a-yl)-phenol | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor mu 1 in rat brain using [3H]-NAL as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50018728 (3-(2-Methyl-octahydro-isoquinolin-4a-yl)-phenol | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor mu 1 in rat brain using [3H]-NAL as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324840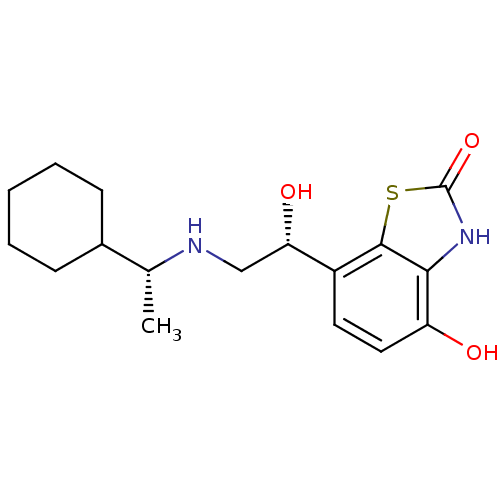 (7-((R)-2-((R)-1-cyclohexylethylamino)-1-hydroxyeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta2 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11644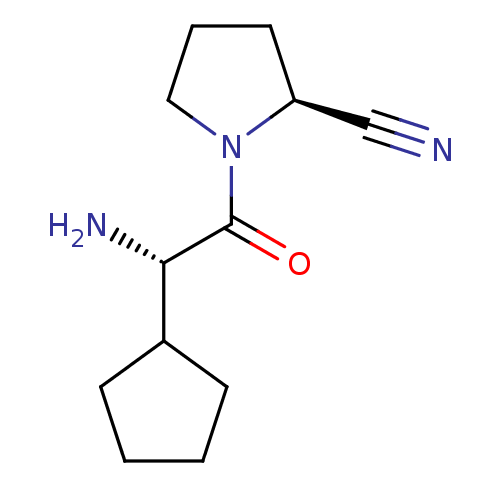 ((2S)-1-[(2S)-2-amino-2-cyclopentylacetyl]pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human Dipeptidyl peptidase IV | Bioorg Med Chem Lett 6: 2745-2748 (1996) Article DOI: 10.1016/S0960-894X(96)00491-X BindingDB Entry DOI: 10.7270/Q2MP537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein (Homo sapiens (Human)) | BDBM50032991 (CHEMBL3356596) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of matriptase (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11644 ((2S)-1-[(2S)-2-amino-2-cyclopentylacetyl]pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro test for inhibitory activity against human dipeptidyl peptidase IV. | Bioorg Med Chem Lett 6: 1163-1166 (1996) Article DOI: 10.1016/0960-894X(96)00190-4 BindingDB Entry DOI: 10.7270/Q279455Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032946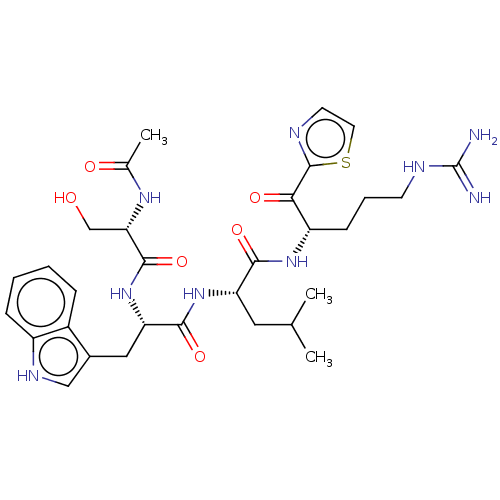 (CHEMBL3356605) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50324846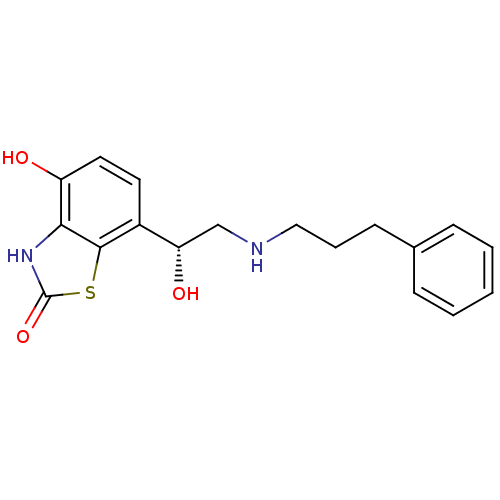 ((R)-4-hydroxy-7-(1-hydroxy-2-(3-phenylpropylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta2 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032939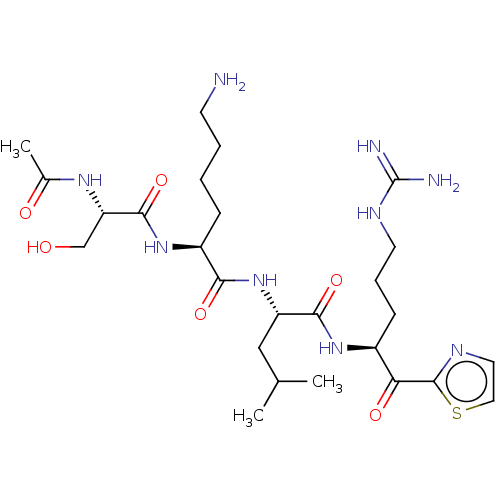 (CHEMBL3356598) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant hepsin (unknown origin) using Boc-QAR-AMC as substrate by fluorescence assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001023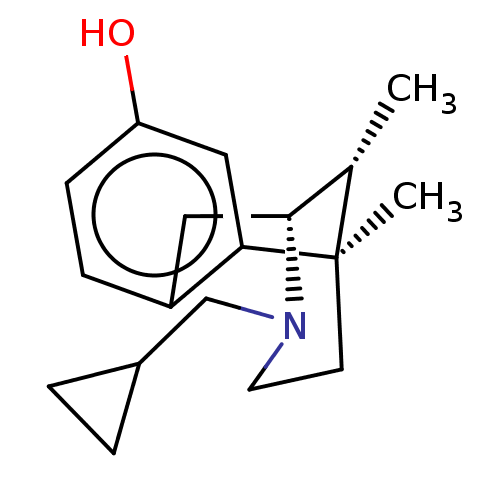 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor mu 1 in rat brain using [3H]-NAL as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity to Opioid receptor kappa 1 in guinea pig cortex using [3H]EKC as radioligand | J Med Chem 31: 555-60 (1988) BindingDB Entry DOI: 10.7270/Q2HQ40HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50324851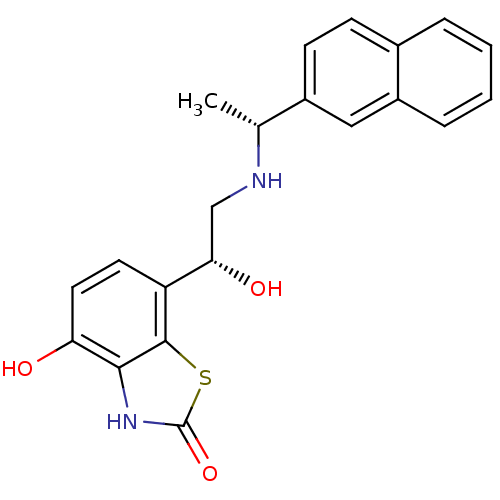 (4-hydroxy-7-((R)-1-hydroxy-2-((R)-1-(naphthalen-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CGP12177 from human beta-1 adrenoceptor | Bioorg Med Chem Lett 20: 5302-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.136 BindingDB Entry DOI: 10.7270/Q2HD7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50055245 (CHEMBL3323668) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]iodo-(+/-)-cyanopindolol from human adrenergic beta2 receptor expressed in CHO cells after 3 hrs by radio-ligand binding assay | Bioorg Med Chem Lett 24: 4341-7 (2014) Article DOI: 10.1016/j.bmcl.2014.06.014 BindingDB Entry DOI: 10.7270/Q2Z89F2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 6308 total ) | Next | Last >> |