

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM13976 (Aminobenzoic acid analog 5 | CHEMBL116605) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant of the compound was determined against Protein-tyrosine phosphatase 1B (PTB1B) | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM13976 (Aminobenzoic acid analog 5 | CHEMBL116605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant of compound against T cell protein tyrosine phosphatase was determined | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13997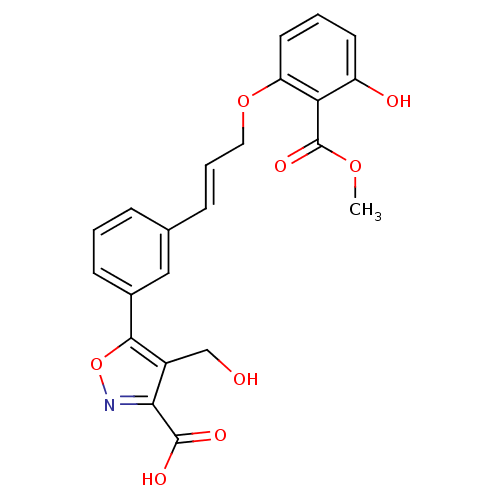 (5-{3-[(1E)-3-[3-hydroxy-2-(methoxycarbonyl)phenoxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | 920 | -34.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13996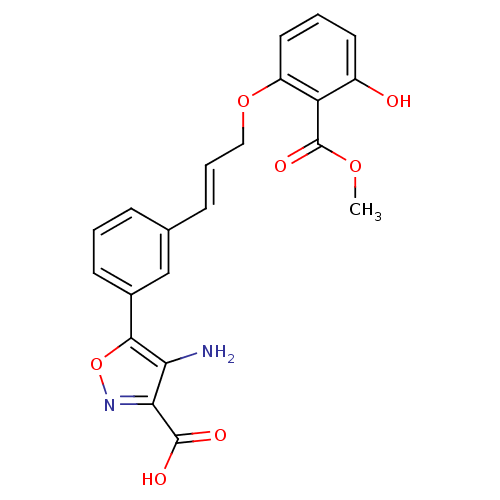 (4-amino-5-{3-[(1E)-3-[3-hydroxy-2-(methoxycarbonyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | -32.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50133280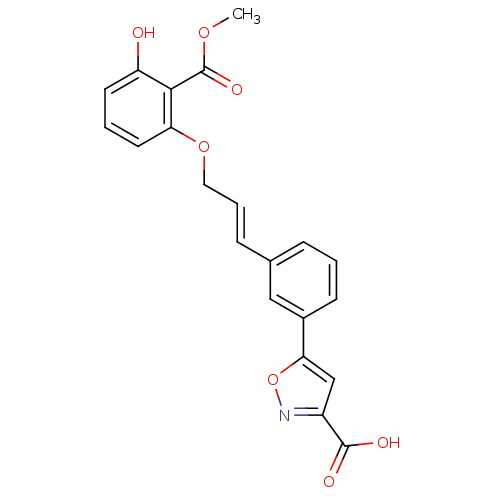 (5-(3-(3-(3-hydroxy-2-(methoxycarbonyl)phenoxy)prop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against protein tyrosine phosphatase PTB1B | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13990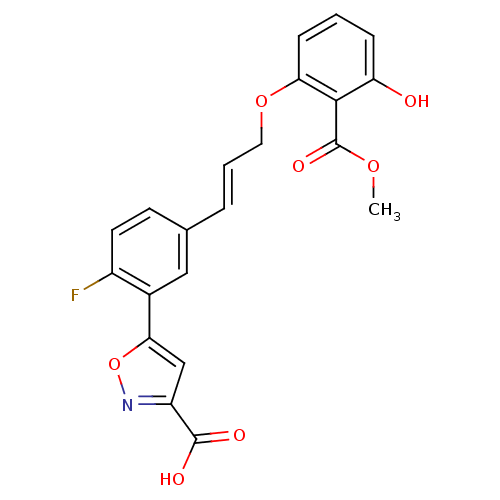 (5-{2-fluoro-5-[(1E)-3-[3-hydroxy-2-(methoxycarbony...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | 6.90E+3 | -29.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13995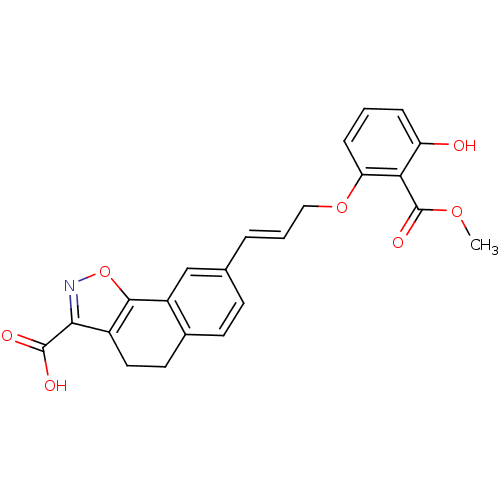 (12-[(1E)-3-[3-hydroxy-2-(methoxycarbonyl)phenoxy]p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.15E+4 | -27.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13997 (5-{3-[(1E)-3-[3-hydroxy-2-(methoxycarbonyl)phenoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.92E+4 | -26.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13994 (5-(3-{2-[3-hydroxy-2-(methoxycarbonyl)phenoxymethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13996 (4-amino-5-{3-[(1E)-3-[3-hydroxy-2-(methoxycarbonyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | >-25.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13993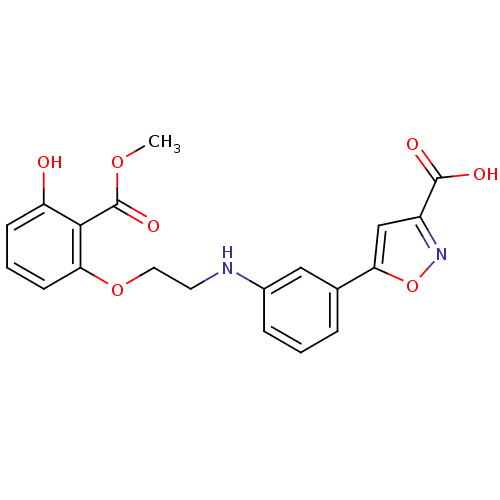 (5-[3-({2-[3-hydroxy-2-(methoxycarbonyl)phenoxy]eth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.03E+4 | -23.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13992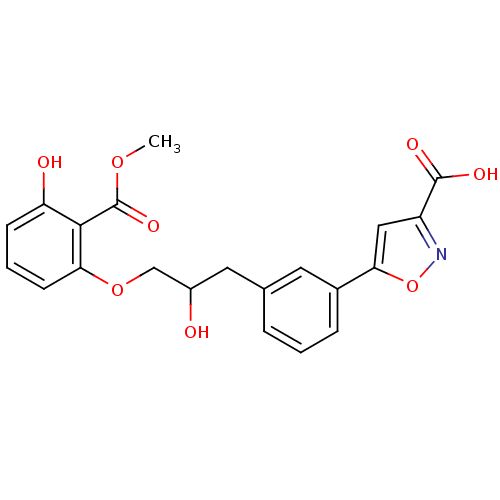 (5-(3-{2-hydroxy-3-[3-hydroxy-2-(methoxycarbonyl)ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.22E+5 | -22.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50133279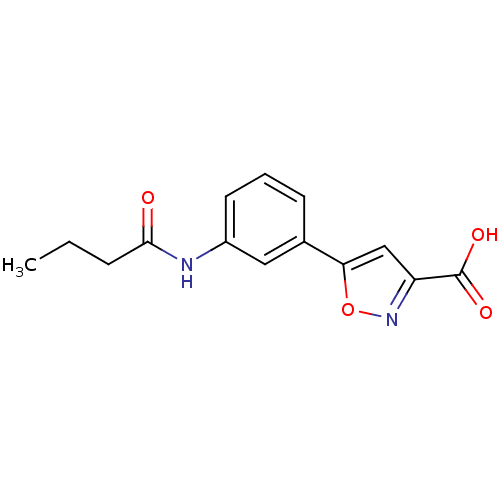 (5-(3-Butyrylamino-phenyl)-isoxazole-3-carboxylic a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against protein tyrosine phosphatase PTB1B | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13990 (5-{2-fluoro-5-[(1E)-3-[3-hydroxy-2-(methoxycarbony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.64E+5 | -21.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13990 (5-{2-fluoro-5-[(1E)-3-[3-hydroxy-2-(methoxycarbony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound was determined against T cell protein tyrosine phosphatase (TCPTP) | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50133280 (5-(3-(3-(3-hydroxy-2-(methoxycarbonyl)phenoxy)prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant of compound against T cell protein tyrosine phosphatase was determined | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13991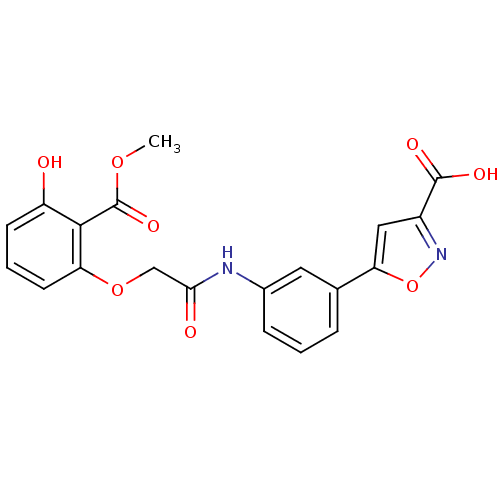 (5-(3-{2-[3-hydroxy-2-(methoxycarbonyl)phenoxy]acet...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.16E+5 | -20.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50133279 (5-(3-Butyrylamino-phenyl)-isoxazole-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant of compound against T cell protein tyrosine phosphatase was determined | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM19386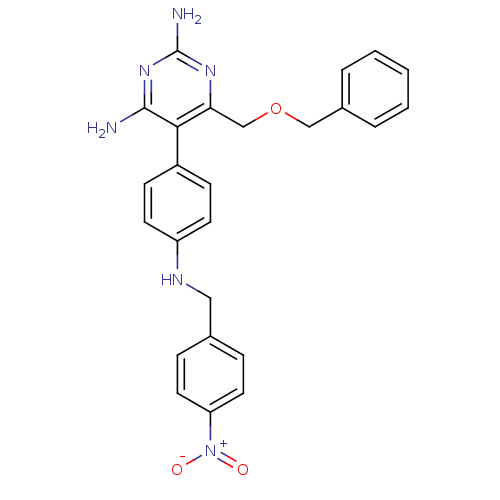 (2,4-diaminopyrimidine derivative, 8b | 6-[(benzylo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | 5.80 | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... | J Med Chem 49: 2568-78 (2006) Article DOI: 10.1021/jm0510934 BindingDB Entry DOI: 10.7270/Q27W69G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM19387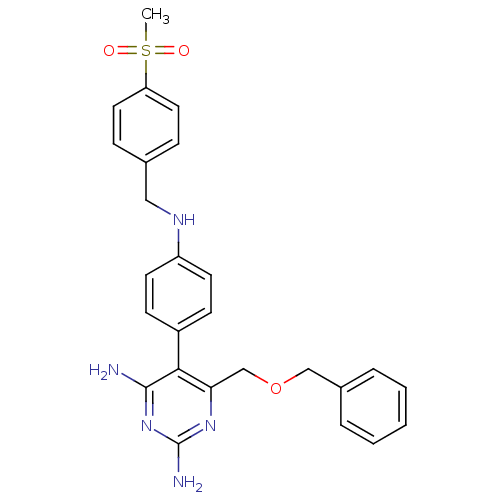 (2,4-diaminopyrimidine derivative, 8c | 6-[(benzylo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by binding assay | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM19387 (2,4-diaminopyrimidine derivative, 8c | 6-[(benzylo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | 7.20 | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... | J Med Chem 49: 2568-78 (2006) Article DOI: 10.1021/jm0510934 BindingDB Entry DOI: 10.7270/Q27W69G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50181180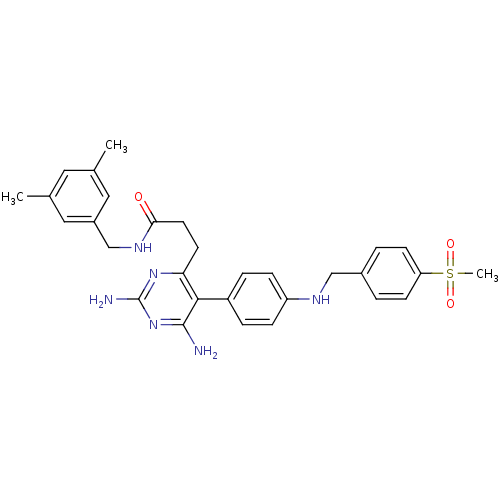 (CHEMBL377514 | N-(3,5-dimethylbenzyl)-3-(5-(4-(4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by binding assay | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50181184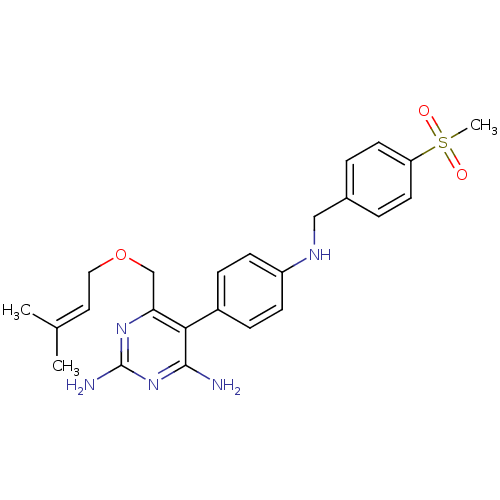 (5-(4-(4-(methylsulfonyl)benzylamino)phenyl)-6-((3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by binding assay | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM19403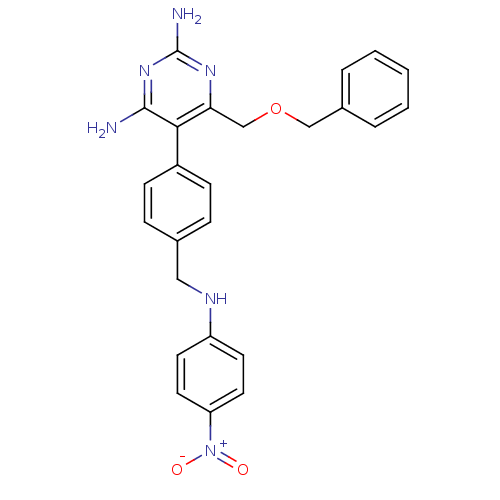 (2,4-diaminopyrimidine derivative, 11a | 6-[(benzyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | 16 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... | J Med Chem 49: 2568-78 (2006) Article DOI: 10.1021/jm0510934 BindingDB Entry DOI: 10.7270/Q27W69G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM19388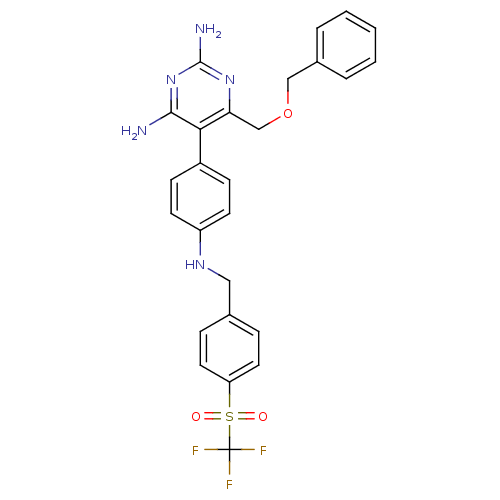 (2,4-diaminopyrimidine derivative, 8d | 6-[(benzylo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | 85 | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... | J Med Chem 49: 2568-78 (2006) Article DOI: 10.1021/jm0510934 BindingDB Entry DOI: 10.7270/Q27W69G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM19389 (1-(4-{[(4-{2,4-diamino-6-[(benzyloxy)methyl]pyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | 10 | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... | J Med Chem 49: 2568-78 (2006) Article DOI: 10.1021/jm0510934 BindingDB Entry DOI: 10.7270/Q27W69G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50181214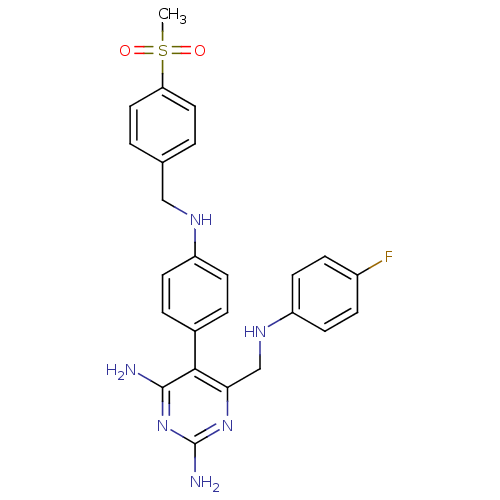 (5-(4-(4-(methylsulfonyl)benzylamino)phenyl)-6-((4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by binding assay | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM19390 (2,4-diaminopyrimidine derivative, 8f | 6-[(benzylo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | 3.60 | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... | J Med Chem 49: 2568-78 (2006) Article DOI: 10.1021/jm0510934 BindingDB Entry DOI: 10.7270/Q27W69G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50181184 (5-(4-(4-(methylsulfonyl)benzylamino)phenyl)-6-((3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by FLIPR | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50181191 (CHEMBL205711 | N-(3-chlorobenzyl)-3-(5-(4-(4-(meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by binding assay | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50158330 ((+/-)isobutyl 8-chloro-2-((4-(diethylamino)phenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to GHSR | J Med Chem 47: 6655-7 (2004) Article DOI: 10.1021/jm0491750 BindingDB Entry DOI: 10.7270/Q237786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM19377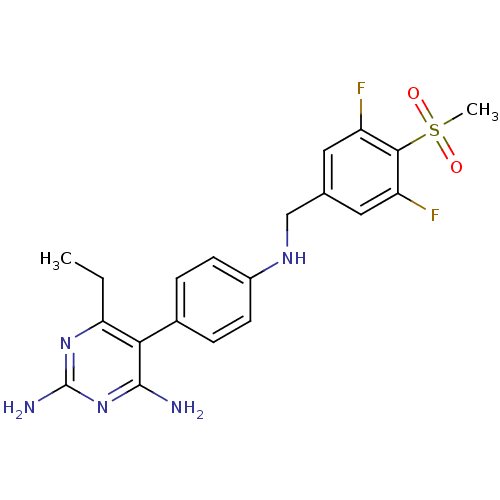 (2,4-diaminopyrimidine derivative, 7c | 5-(4-{[(3,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | 45 | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... | J Med Chem 49: 2568-78 (2006) Article DOI: 10.1021/jm0510934 BindingDB Entry DOI: 10.7270/Q27W69G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50181217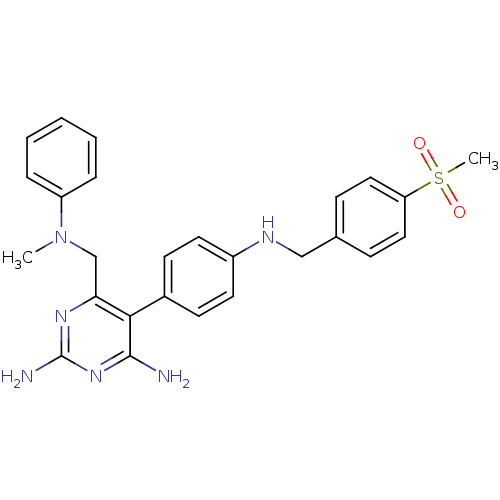 (5-(4-(4-(methylsulfonyl)benzylamino)phenyl)-6-((me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by binding assay | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50181182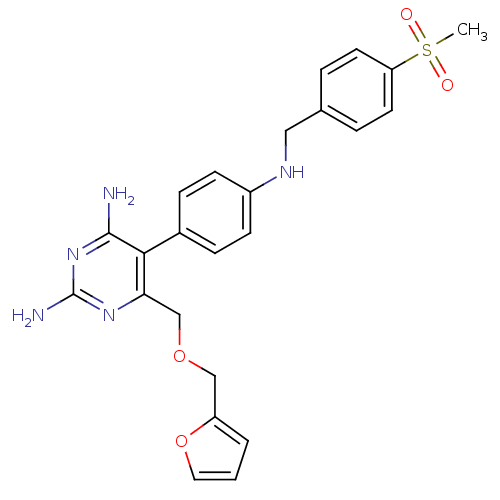 (5-(4-(4-(methylsulfonyl)benzylamino)phenyl)-6-((fu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by binding assay | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM19391 (2,4-diaminopyrimidine derivative, 8g | 4-{[(4-{2,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | 19 | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... | J Med Chem 49: 2568-78 (2006) Article DOI: 10.1021/jm0510934 BindingDB Entry DOI: 10.7270/Q27W69G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50181201 (5-(4-(4-(methylsulfonyl)benzylamino)phenyl)-6-(iso...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by binding assay | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM19392 (2,4-diaminopyrimidine derivative, 8h | 6-[(benzylo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | 97 | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... | J Med Chem 49: 2568-78 (2006) Article DOI: 10.1021/jm0510934 BindingDB Entry DOI: 10.7270/Q27W69G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50181180 (CHEMBL377514 | N-(3,5-dimethylbenzyl)-3-(5-(4-(4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by FLIPR | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50153531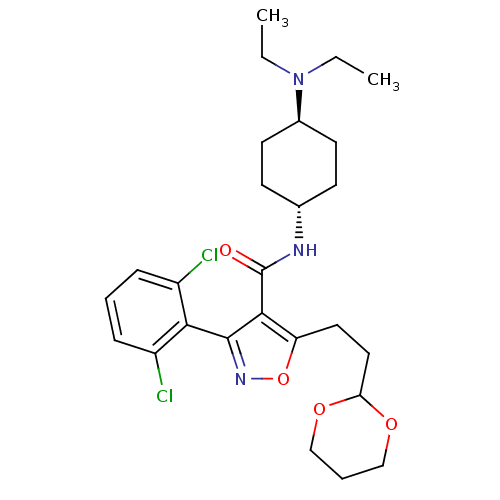 (3-(2,6-Dichloro-phenyl)-5-(2-[1,3]dioxan-2-yl-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Ability to inhibit ghrelin induced increase in intracellular [Ca2+] in CHO-K cells | Bioorg Med Chem Lett 14: 5223-6 (2004) Article DOI: 10.1016/j.bmcl.2004.06.060 BindingDB Entry DOI: 10.7270/Q2Z60NJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50181229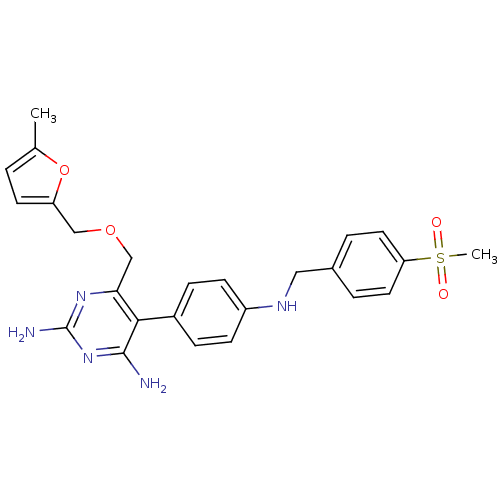 (5-[4-(4-methanesulfonyl-benzylamino)-phenyl]-6-(5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by binding assay | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM19409 (2,4-diaminopyrimidine derivative, 13 | 6-ethyl-5-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | 32 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... | J Med Chem 49: 2568-78 (2006) Article DOI: 10.1021/jm0510934 BindingDB Entry DOI: 10.7270/Q27W69G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50181225 (5-(4-(4-(methylsulfonyl)benzylamino)phenyl)-6-((be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by binding assay | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM19407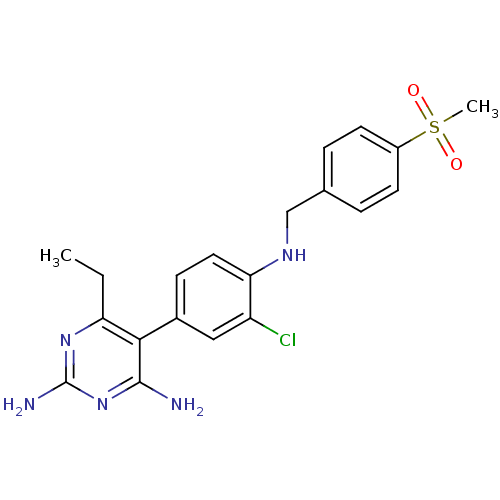 (2,4-diaminopyrimidine derivative, 12a | 5-(3-chlor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | 11 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... | J Med Chem 49: 2568-78 (2006) Article DOI: 10.1021/jm0510934 BindingDB Entry DOI: 10.7270/Q27W69G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50181192 (5-(4-(4-(methylsulfonyl)benzylamino)phenyl)-6-octy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by binding assay | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50181191 (CHEMBL205711 | N-(3-chlorobenzyl)-3-(5-(4-(4-(meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by FLIPR | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50181204 (5-(4-(4-(methylsulfonyl)benzylamino)phenyl)-6-isop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by binding assay | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50181179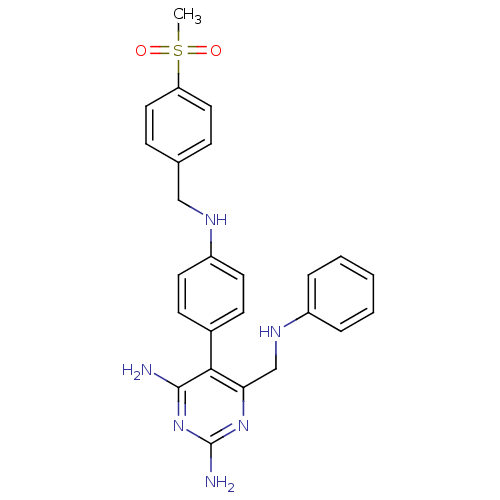 (5-(4-(4-(methylsulfonyl)benzylamino)phenyl)-6-((ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human GHS receptor by binding assay | Bioorg Med Chem Lett 16: 1864-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.012 BindingDB Entry DOI: 10.7270/Q2610ZW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM19408 (2,4-diaminopyrimidine derivative, 12b | 5-(3-bromo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | 42 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing ... | J Med Chem 49: 2568-78 (2006) Article DOI: 10.1021/jm0510934 BindingDB Entry DOI: 10.7270/Q27W69G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50153534 (3-(2,6-Dichloro-phenyl)-5-(2-[1,3]dioxan-2-yl-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of binding to human growth hormone secretagogue receptor | Bioorg Med Chem Lett 15: 1201-4 (2005) Article DOI: 10.1016/j.bmcl.2004.11.075 BindingDB Entry DOI: 10.7270/Q24Q7THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50153534 (3-(2,6-Dichloro-phenyl)-5-(2-[1,3]dioxan-2-yl-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]-ghrelin from cloned human GHS-R expressed in CHO-K cells was determined (Kd of ghrelin is 0.4 nM) | Bioorg Med Chem Lett 14: 5223-6 (2004) Article DOI: 10.1016/j.bmcl.2004.06.060 BindingDB Entry DOI: 10.7270/Q2Z60NJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 274 total ) | Next | Last >> |