

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50059889 ((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged LRRK2 after 1 hr by TR-FRET assay | Bioorg Med Chem Lett 23: 3690-6 (2013) Article DOI: 10.1016/j.bmcl.2013.04.086 BindingDB Entry DOI: 10.7270/Q27P90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50436423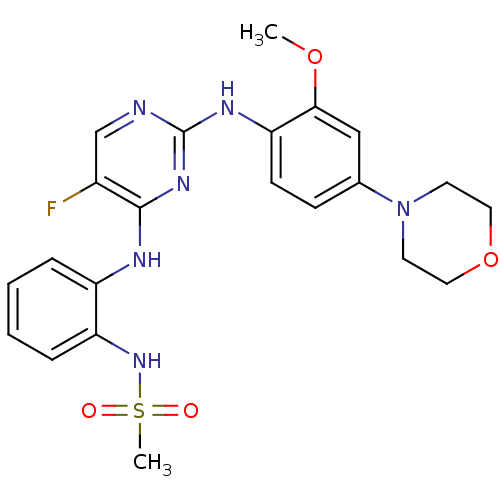 (CHEMBL2397014) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of human recombinant wild type LRRK2 using biotin-LRRKtide as substrate preincubated for 15 mins prior to substrate addition measured afte... | Bioorg Med Chem Lett 23: 3690-6 (2013) Article DOI: 10.1016/j.bmcl.2013.04.086 BindingDB Entry DOI: 10.7270/Q27P90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM482157 (BDBM50379529 | LRRK2-IN-1 | US11370796, Compound L...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM482157 (BDBM50379529 | LRRK2-IN-1 | US11370796, Compound L...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 ((1326 to 2517 amino acids) (unknown origin) | Bioorg Med Chem Lett 23: 3690-6 (2013) Article DOI: 10.1016/j.bmcl.2013.04.086 BindingDB Entry DOI: 10.7270/Q27P90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM482157 (BDBM50379529 | LRRK2-IN-1 | US11370796, Compound L...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of wild-type GST-tagged LRRK2 (1326 to 2527 aa)(unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by ... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM4814 (CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged LRRK2 after 1 hr by TR-FRET assay | Bioorg Med Chem Lett 23: 3690-6 (2013) Article DOI: 10.1016/j.bmcl.2013.04.086 BindingDB Entry DOI: 10.7270/Q27P90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50132420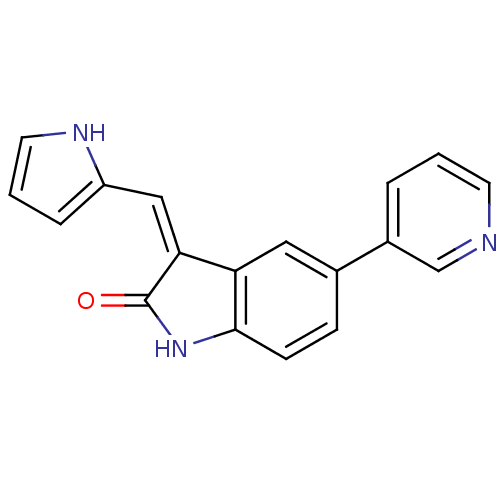 (3-((1H-pyrrol-2-yl)methylene)-5-(pyridin-3-yl)indo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged LRRK2 after 1 hr by TR-FRET assay | Bioorg Med Chem Lett 23: 3690-6 (2013) Article DOI: 10.1016/j.bmcl.2013.04.086 BindingDB Entry DOI: 10.7270/Q27P90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073840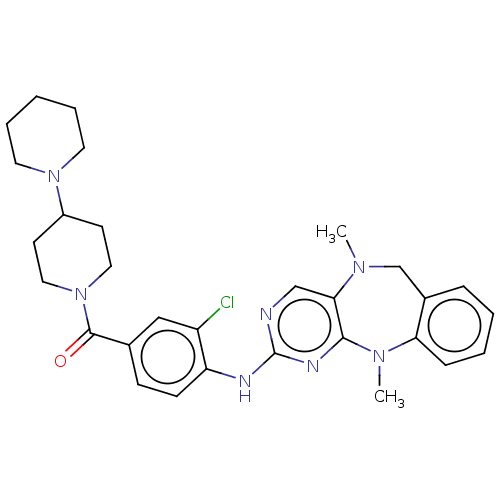 (CHEMBL3409459) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073813 (CHEMBL3409458) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM14028 ((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 244 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged LRRK2 after 1 hr by TR-FRET assay | Bioorg Med Chem Lett 23: 3690-6 (2013) Article DOI: 10.1016/j.bmcl.2013.04.086 BindingDB Entry DOI: 10.7270/Q27P90SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073919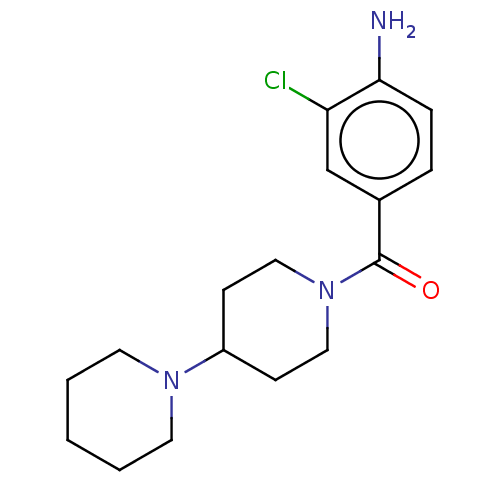 (CHEMBL3409462) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 654 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073926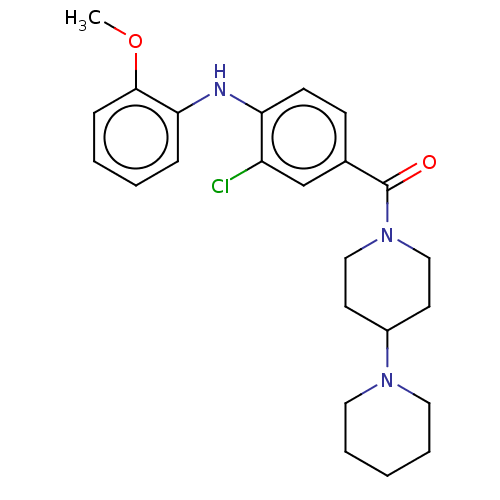 (CHEMBL3409468) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073927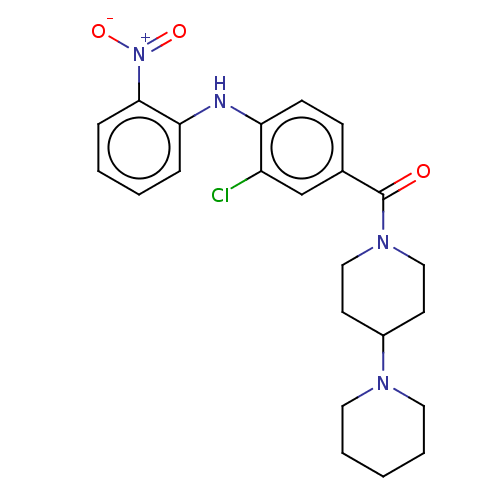 (CHEMBL3409469) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073928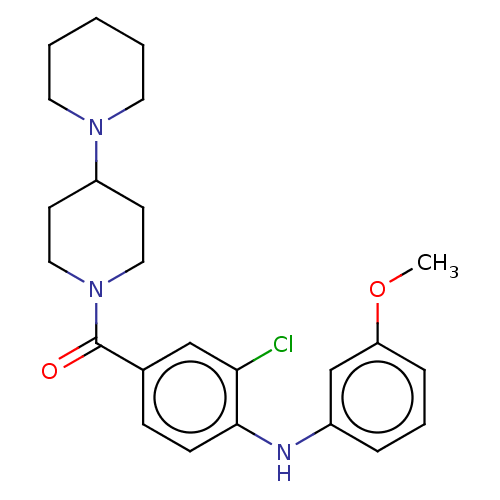 (CHEMBL3409470) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073929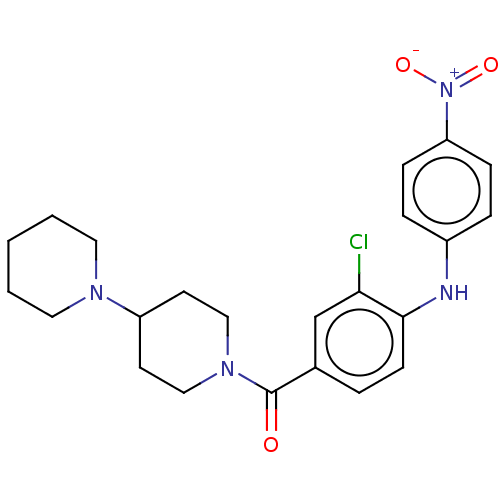 (CHEMBL3409471) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073931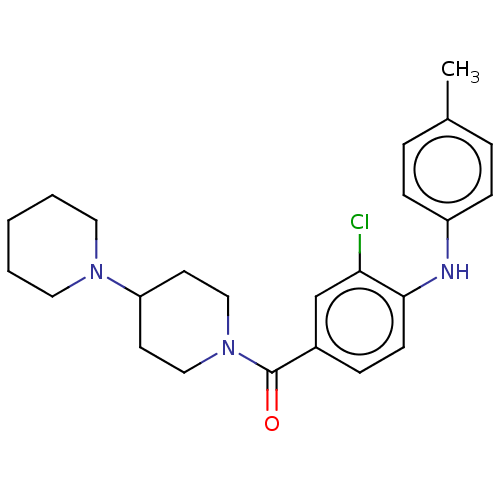 (CHEMBL3409472) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073932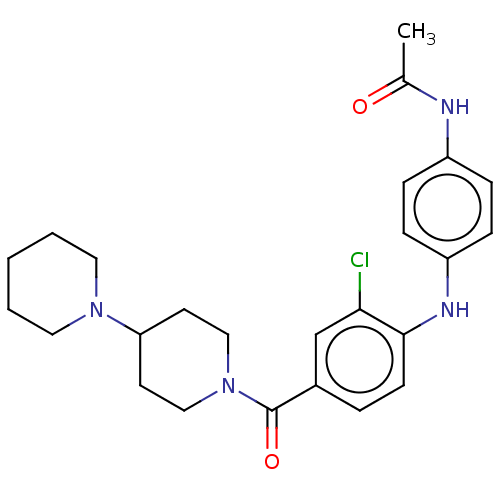 (CHEMBL3409473) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073933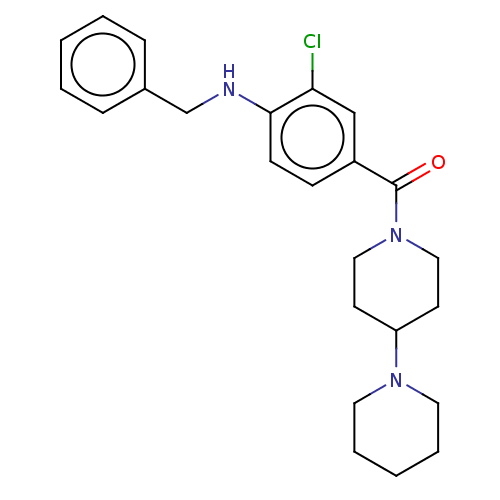 (CHEMBL3409474) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073934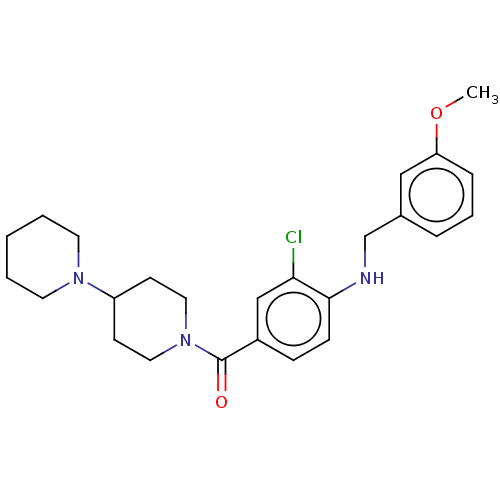 (CHEMBL3409475) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073935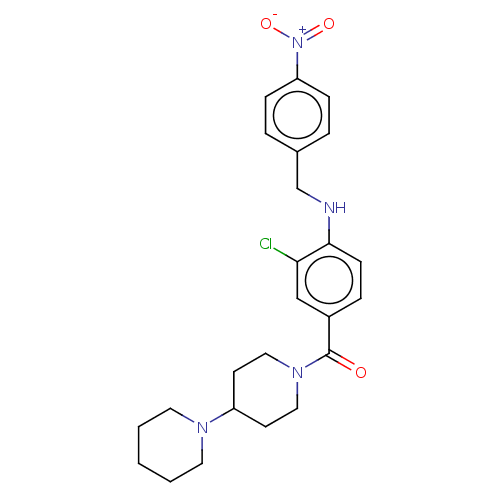 (CHEMBL3409476) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073936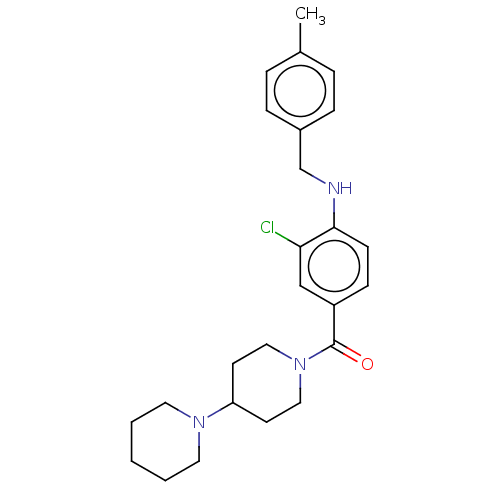 (CHEMBL3409477) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073937 (CHEMBL3409478) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073938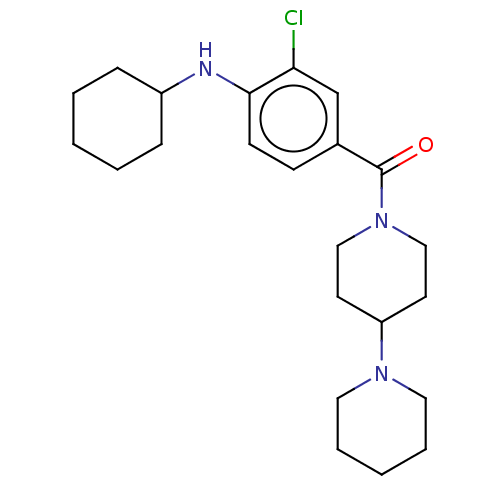 (CHEMBL3409479) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073939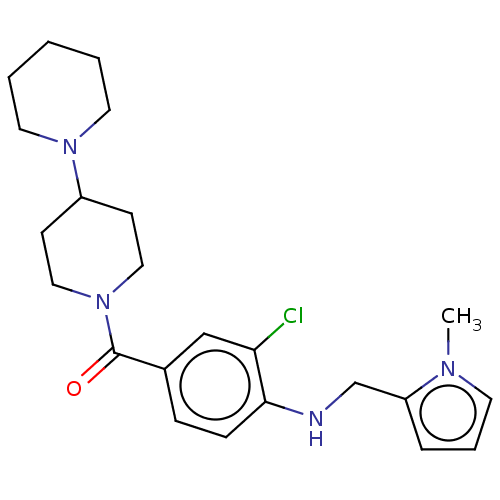 (CHEMBL3409480) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073940 (CHEMBL3409481) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073921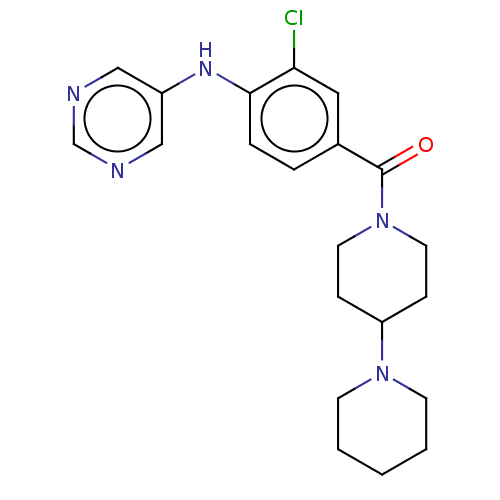 (CHEMBL3409464) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073923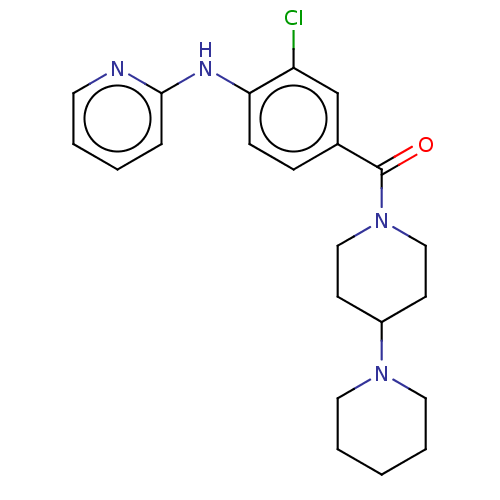 (CHEMBL3409465) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073924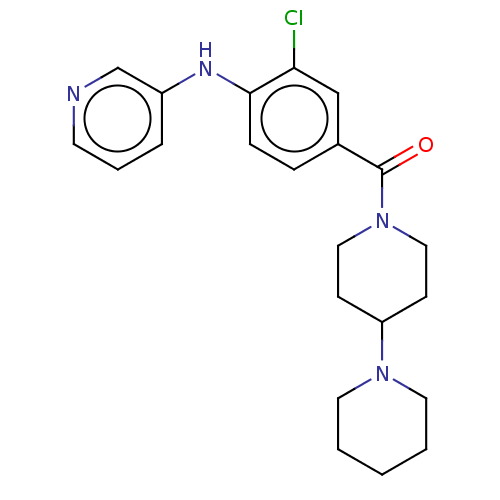 (CHEMBL3409466) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073925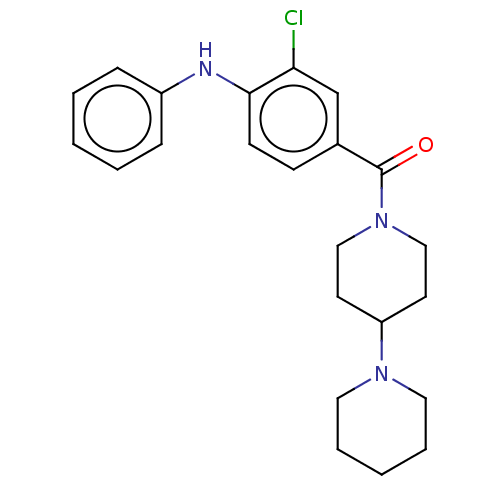 (CHEMBL3409467) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073879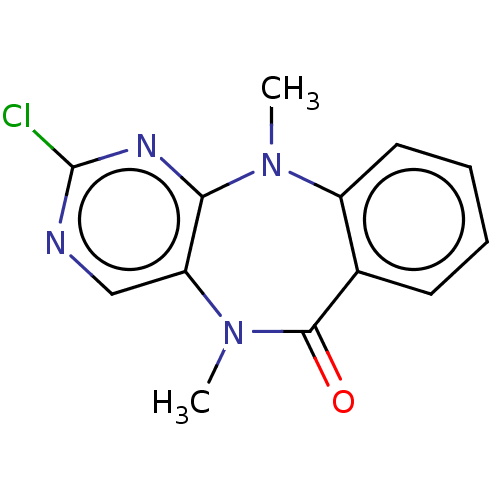 (CHEMBL3409460) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073907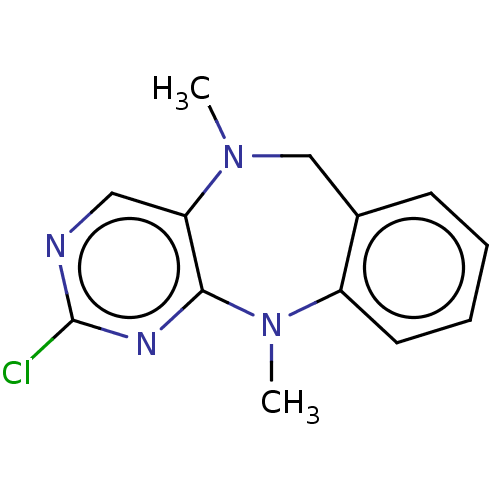 (CHEMBL3409461) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50073920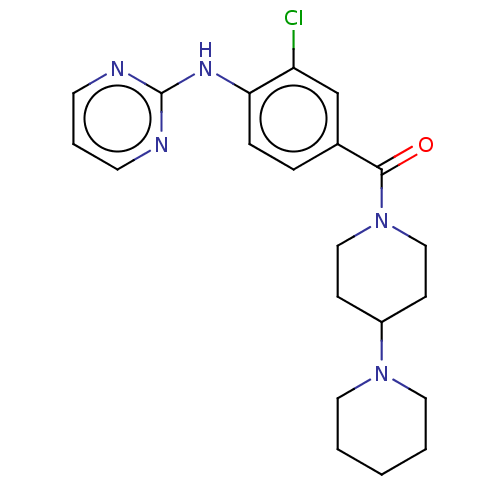 (CHEMBL3409463) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney Curated by ChEMBL | Assay Description Inhibition of LRRK2 G2019S mutant (1326 to 252 aa) (unknown origin) stably expressed in HEK293 cell lysate using [gamma-32P] after 15 mins by Cerenko... | Eur J Med Chem 95: 29-34 (2015) Article DOI: 10.1016/j.ejmech.2015.03.003 BindingDB Entry DOI: 10.7270/Q2TH8PDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50169700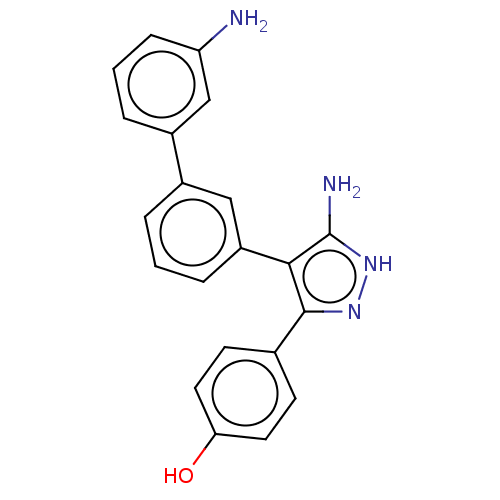 (CHEMBL3806115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate by LC-MS/MS analysis | J Med Chem 59: 3272-302 (2016) Article DOI: 10.1021/acs.jmedchem.6b00007 BindingDB Entry DOI: 10.7270/Q2MS3VN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50169700 (CHEMBL3806115) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis | J Med Chem 59: 3272-302 (2016) Article DOI: 10.1021/acs.jmedchem.6b00007 BindingDB Entry DOI: 10.7270/Q2MS3VN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50169702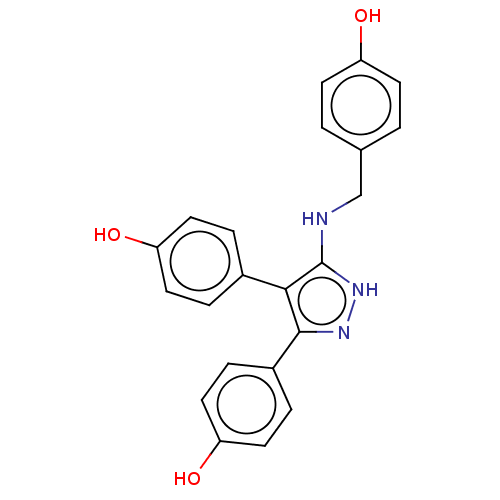 (CHEMBL3805279) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate by LC-MS/MS analysis | J Med Chem 59: 3272-302 (2016) Article DOI: 10.1021/acs.jmedchem.6b00007 BindingDB Entry DOI: 10.7270/Q2MS3VN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50169702 (CHEMBL3805279) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis | J Med Chem 59: 3272-302 (2016) Article DOI: 10.1021/acs.jmedchem.6b00007 BindingDB Entry DOI: 10.7270/Q2MS3VN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50169698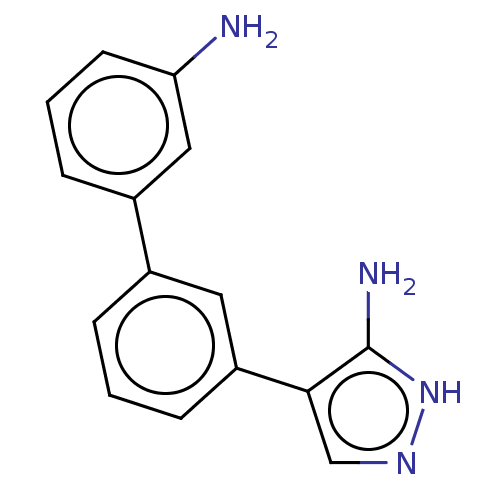 (CHEMBL3806191) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate by LC-MS/MS analysis | J Med Chem 59: 3272-302 (2016) Article DOI: 10.1021/acs.jmedchem.6b00007 BindingDB Entry DOI: 10.7270/Q2MS3VN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50169702 (CHEMBL3805279) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate by LC-MS/MS analysis | J Med Chem 59: 3272-302 (2016) Article DOI: 10.1021/acs.jmedchem.6b00007 BindingDB Entry DOI: 10.7270/Q2MS3VN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50169698 (CHEMBL3806191) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC-MS/MS analysis | J Med Chem 59: 3272-302 (2016) Article DOI: 10.1021/acs.jmedchem.6b00007 BindingDB Entry DOI: 10.7270/Q2MS3VN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50169700 (CHEMBL3806115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate by LC-MS/MS analysis | J Med Chem 59: 3272-302 (2016) Article DOI: 10.1021/acs.jmedchem.6b00007 BindingDB Entry DOI: 10.7270/Q2MS3VN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50169702 (CHEMBL3805279) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using mephenytoin as substrate by LC-MS/MS analysis | J Med Chem 59: 3272-302 (2016) Article DOI: 10.1021/acs.jmedchem.6b00007 BindingDB Entry DOI: 10.7270/Q2MS3VN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50169700 (CHEMBL3806115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using mephenytoin as substrate by LC-MS/MS analysis | J Med Chem 59: 3272-302 (2016) Article DOI: 10.1021/acs.jmedchem.6b00007 BindingDB Entry DOI: 10.7270/Q2MS3VN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50169698 (CHEMBL3806191) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using mephenytoin as substrate by LC-MS/MS analysis | J Med Chem 59: 3272-302 (2016) Article DOI: 10.1021/acs.jmedchem.6b00007 BindingDB Entry DOI: 10.7270/Q2MS3VN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50169698 (CHEMBL3806191) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate by LC-MS/MS analysis | J Med Chem 59: 3272-302 (2016) Article DOI: 10.1021/acs.jmedchem.6b00007 BindingDB Entry DOI: 10.7270/Q2MS3VN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 144 [31-434] (Mycobacterium tuberculosis) | BDBM222346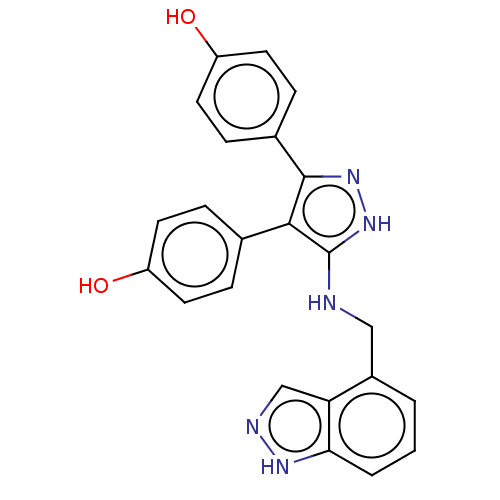 (CYP144A1 ligand, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 8.20E+4 | n/a | n/a | n/a | 7.0 | n/a |
University of Cambridge | Assay Description Optical titrations to determine the binding affinity (KD values) of ligands were carried out on a Cary 60 UV−visible spectrophotometer (Varian,... | Biochemistry 56: 1559-1572 (2017) Article DOI: 10.1021/acs.biochem.6b00954 BindingDB Entry DOI: 10.7270/Q2SJ1JF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 144 [31-434] (Mycobacterium tuberculosis) | BDBM222347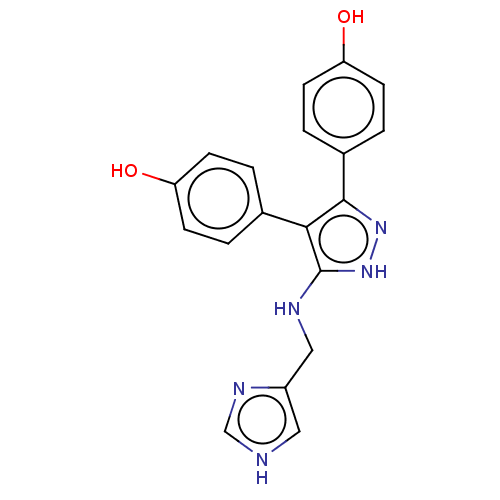 (CYP144A1 ligand, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | 7.0 | n/a |
University of Cambridge | Assay Description Optical titrations to determine the binding affinity (KD values) of ligands were carried out on a Cary 60 UV−visible spectrophotometer (Varian,... | Biochemistry 56: 1559-1572 (2017) Article DOI: 10.1021/acs.biochem.6b00954 BindingDB Entry DOI: 10.7270/Q2SJ1JF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 144 [31-434] (Mycobacterium tuberculosis) | BDBM222348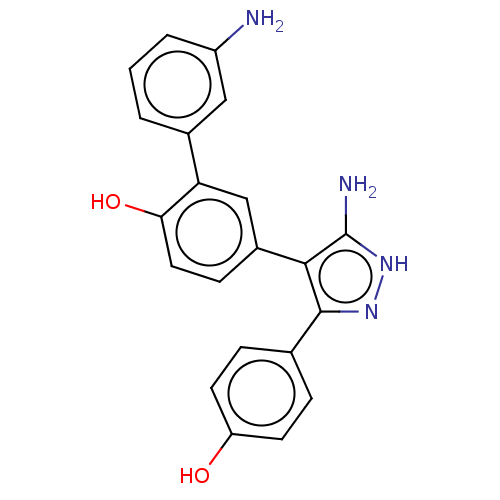 (CYP144A1 ligand, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | 7.5 | 25 |
University of Cambridge | Assay Description ITC binding isotherms were recorded on a MicroCal ITC200 instrument (MalvernInstruments). Titrations were conducted at 25 °C by injecting aliquots (2... | Biochemistry 56: 1559-1572 (2017) Article DOI: 10.1021/acs.biochem.6b00954 BindingDB Entry DOI: 10.7270/Q2SJ1JF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 144 [31-434] (Mycobacterium tuberculosis) | BDBM24656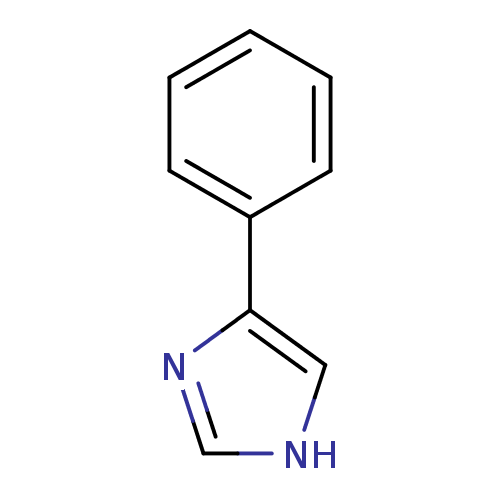 (4-PIM | 4-Phenylimidazole | 4-phenyl-1H-imidazole ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 2.98E+6 | n/a | n/a | n/a | 7.0 | n/a |
University of Cambridge | Assay Description Optical titrations to determine the binding affinity (KD values) of ligands were carried out on a Cary 60 UV−visible spectrophotometer (Varian,... | Biochemistry 56: 1559-1572 (2017) Article DOI: 10.1021/acs.biochem.6b00954 BindingDB Entry DOI: 10.7270/Q2SJ1JF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 144 [31-434] (Mycobacterium tuberculosis) | BDBM7886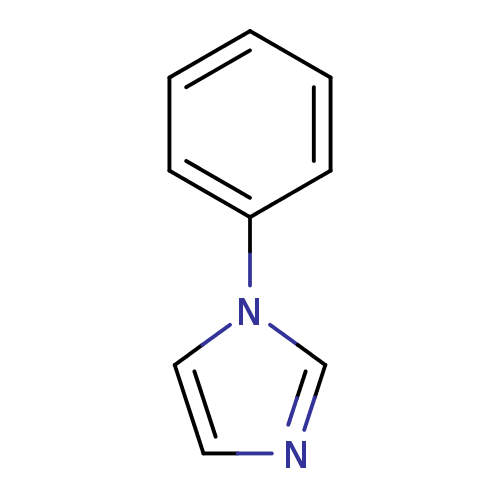 (1-PIM | 1-phenyl-1H-imidazole | 1-phenylimidazole ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 2.90E+5 | n/a | n/a | n/a | 7.0 | n/a |
University of Cambridge | Assay Description Optical titrations to determine the binding affinity (KD values) of ligands were carried out on a Cary 60 UV−visible spectrophotometer (Varian,... | Biochemistry 56: 1559-1572 (2017) Article DOI: 10.1021/acs.biochem.6b00954 BindingDB Entry DOI: 10.7270/Q2SJ1JF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 144 [31-434] (Mycobacterium tuberculosis) | BDBM222349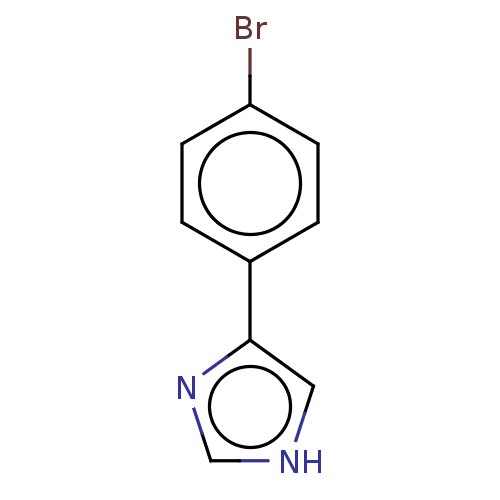 (CYP144A1 ligand, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 2.30E+5 | n/a | n/a | n/a | 7.0 | n/a |
University of Cambridge | Assay Description Optical titrations to determine the binding affinity (KD values) of ligands were carried out on a Cary 60 UV−visible spectrophotometer (Varian,... | Biochemistry 56: 1559-1572 (2017) Article DOI: 10.1021/acs.biochem.6b00954 BindingDB Entry DOI: 10.7270/Q2SJ1JF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 117 total ) | Next | Last >> |