 Found 153898 hits with Last Name = 'ke' and Initial = 'j'
Found 153898 hits with Last Name = 'ke' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548
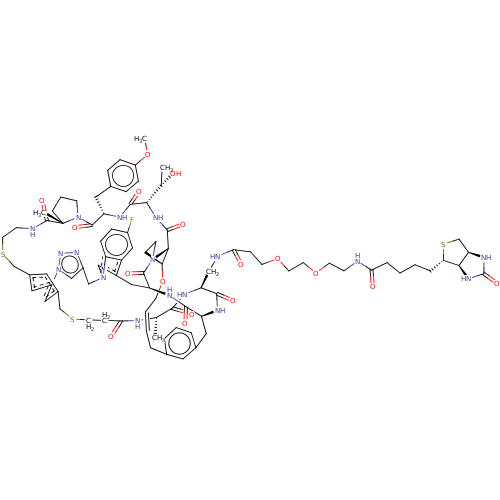
(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547
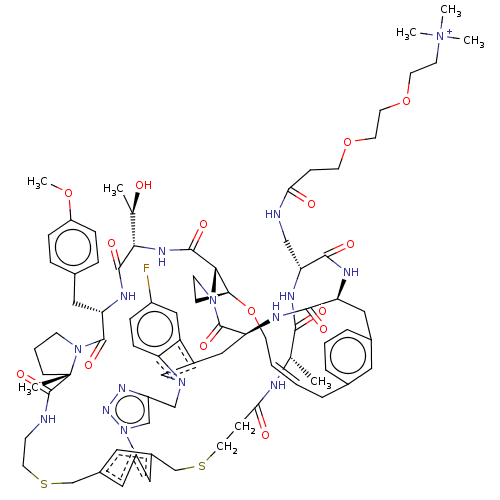
(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50099843
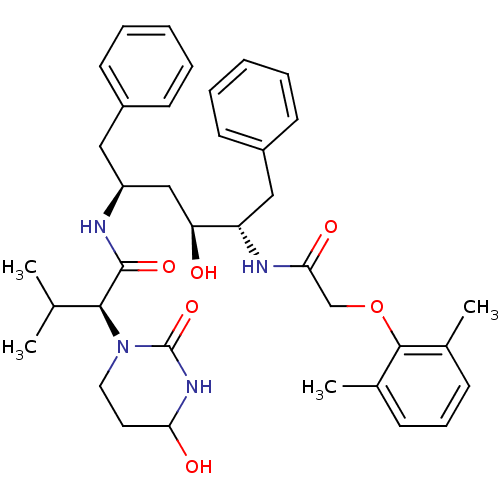
((S)-N-[(S)-4-[2-(2,6-Dimethyl-phenoxy)-acetylamino...)Show SMILES CC(C)[C@H](N1CCC(O)NC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 Show InChI InChI=1S/C37H48N4O6/c1-24(2)34(41-19-18-32(43)40-37(41)46)36(45)38-29(20-27-14-7-5-8-15-27)22-31(42)30(21-28-16-9-6-10-17-28)39-33(44)23-47-35-25(3)12-11-13-26(35)4/h5-17,24,29-32,34,42-43H,18-23H2,1-4H3,(H,38,45)(H,39,44)(H,40,46)/t29-,30-,31-,32?,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease |
Bioorg Med Chem Lett 11: 1351-3 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BX6 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM578

((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...)Show SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease |
Bioorg Med Chem Lett 11: 1351-3 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BX6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50099842

((S)-N-[(S)-4-[2-(2,6-Dimethyl-phenoxy)-acetylamino...)Show SMILES CC(C)[C@H](N1CCC(=O)NC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 Show InChI InChI=1S/C37H46N4O6/c1-24(2)34(41-19-18-32(43)40-37(41)46)36(45)38-29(20-27-14-7-5-8-15-27)22-31(42)30(21-28-16-9-6-10-17-28)39-33(44)23-47-35-25(3)12-11-13-26(35)4/h5-17,24,29-31,34,42H,18-23H2,1-4H3,(H,38,45)(H,39,44)(H,40,43,46)/t29-,30-,31-,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease |
Bioorg Med Chem Lett 11: 1351-3 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BX6 |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546
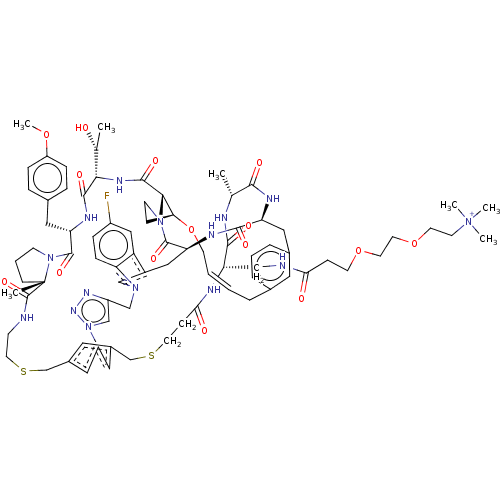
(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86041
(NFEP)Show SMILES Fc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13FN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930
(CHEMBL584130 | KNI-814)Show SMILES Cc1cccc(C)c1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C33H39N3O5S/c1-20-11-9-12-21(2)25(20)18-34-31(40)29-33(4,5)42-19-36(29)32(41)28(38)26(17-23-13-7-6-8-14-23)35-30(39)24-15-10-16-27(37)22(24)3/h6-16,26,28-29,37-38H,17-19H2,1-5H3,(H,34,40)(H,35,39)/t26-,28-,29+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay |
J Med Chem 52: 7604-17 (2009)
Article DOI: 10.1021/jm9005115
BindingDB Entry DOI: 10.7270/Q2FR00F2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50056769
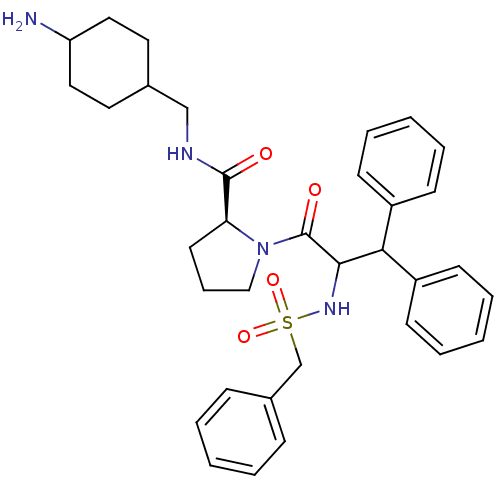
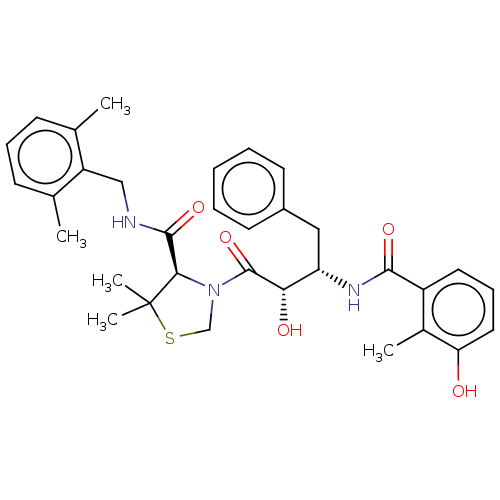
((S)-1-(3,3-Diphenyl-2-phenylmethanesulfonylamino-p...)Show SMILES NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)C(NS(=O)(=O)Cc2ccccc2)C(c2ccccc2)c2ccccc2)CC1 |wU:9.8,(16.05,-4.09,;16.82,-5.42,;16.81,-7.08,;17.34,-8.54,;16.12,-9.36,;16.57,-10.83,;15.52,-11.95,;14.02,-11.6,;12.97,-12.72,;13.56,-10.13,;14.5,-8.9,;13.63,-7.64,;12.11,-8.1,;12.12,-9.64,;10.86,-10.51,;10.97,-12.05,;9.46,-9.85,;8.2,-10.74,;6.87,-11.5,;6.09,-10.16,;7.64,-12.86,;5.51,-12.28,;4.18,-11.51,;4.18,-9.97,;2.85,-9.2,;1.52,-9.97,;1.52,-11.53,;2.85,-12.28,;9.33,-8.33,;7.93,-7.68,;7.8,-6.14,;6.4,-5.49,;5.14,-6.38,;5.28,-7.92,;6.68,-8.56,;10.59,-7.44,;9.81,-6.11,;10.59,-4.77,;12.13,-4.77,;12.89,-6.11,;12.13,-7.44,;16.29,-7.89,;15.73,-6.52,)| Show InChI InChI=1S/C34H42N4O4S/c35-29-20-18-25(19-21-29)23-36-33(39)30-17-10-22-38(30)34(40)32(37-43(41,42)24-26-11-4-1-5-12-26)31(27-13-6-2-7-14-27)28-15-8-3-9-16-28/h1-9,11-16,25,29-32,37H,10,17-24,35H2,(H,36,39)/t25?,29?,30-,32?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards thrombin |
J Med Chem 40: 830-2 (1997)
Article DOI: 10.1021/jm960762y
BindingDB Entry DOI: 10.7270/Q25H7GXW |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86042

(NEP)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2/t9?,10-,11?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86041

(NFEP)Show SMILES Fc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13FN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86040
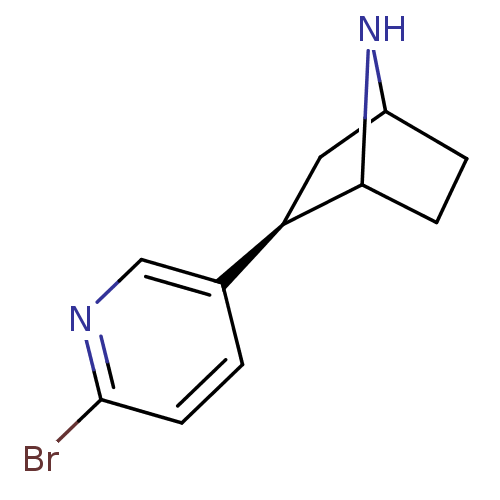
(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM200
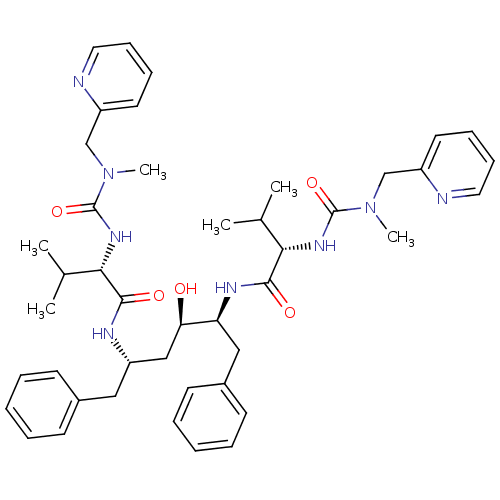
((2S)-N-[(2S,3R,5S)-3-hydroxy-5-[(2S)-3-methyl-2-{[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1ccccn1)C(=O)N[C@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)N(C)Cc1ccccn1)C(C)C)Cc1ccccc1 |r| Show InChI InChI=1S/C44H58N8O5/c1-30(2)39(49-43(56)51(5)28-34-21-13-15-23-45-34)41(54)47-36(25-32-17-9-7-10-18-32)27-38(53)37(26-33-19-11-8-12-20-33)48-42(55)40(31(3)4)50-44(57)52(6)29-35-22-14-16-24-46-35/h7-24,30-31,36-40,53H,25-29H2,1-6H3,(H,47,54)(H,48,55)(H,49,56)(H,50,57)/t36-,37-,38+,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 0.00400 | -66.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
NCI-FCRDC
| Assay Description
HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... |
J Am Chem Soc 116: 847-55 (1994)
Article DOI: 10.1021/ja00082a004
BindingDB Entry DOI: 10.7270/Q2KK98Z1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM194
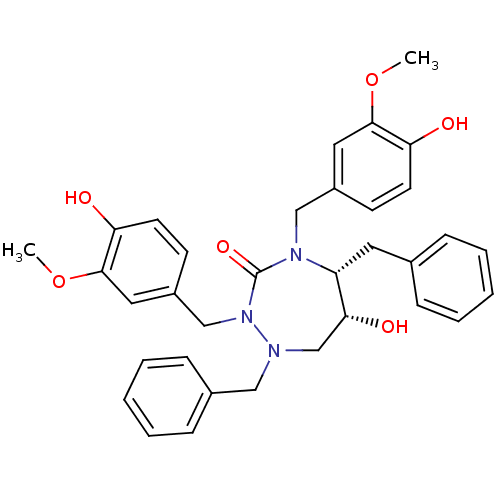
((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(4-hydroxy-...)Show SMILES COc1cc(CN2[C@H](Cc3ccccc3)[C@H](O)CN(Cc3ccccc3)N(Cc3ccc(O)c(OC)c3)C2=O)ccc1O |r| Show InChI InChI=1S/C34H37N3O6/c1-42-32-18-26(13-15-29(32)38)21-36-28(17-24-9-5-3-6-10-24)31(40)23-35(20-25-11-7-4-8-12-25)37(34(36)41)22-27-14-16-30(39)33(19-27)43-2/h3-16,18-19,28,31,38-40H,17,20-23H2,1-2H3/t28-,31-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00500 | -65.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories
| Assay Description
HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... |
J Med Chem 39: 392-7 (1996)
Article DOI: 10.1021/jm9507183
BindingDB Entry DOI: 10.7270/Q2V40SC6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.00545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of dihydrofolate reductase (DHFR) isolated from L1210 cells |
J Med Chem 31: 2164-9 (1988)
BindingDB Entry DOI: 10.7270/Q25H7F8Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547

(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00736 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581545
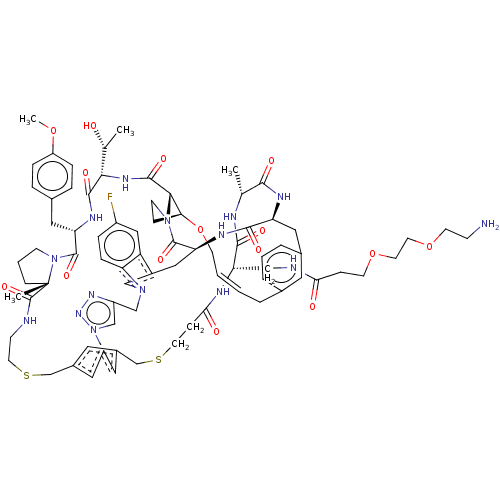
(CHEMBL5084902)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCCN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:94| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544
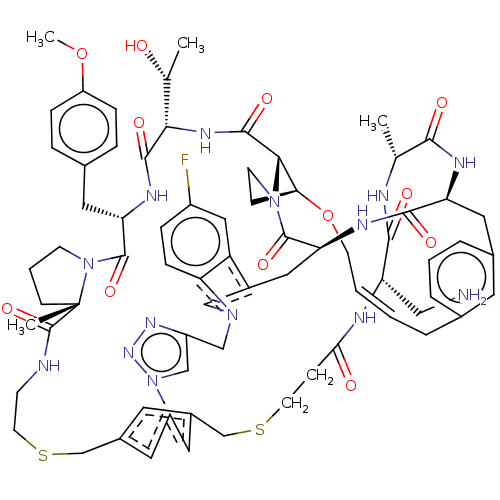
(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00826 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86042

(NEP)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2/t9?,10-,11?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86041

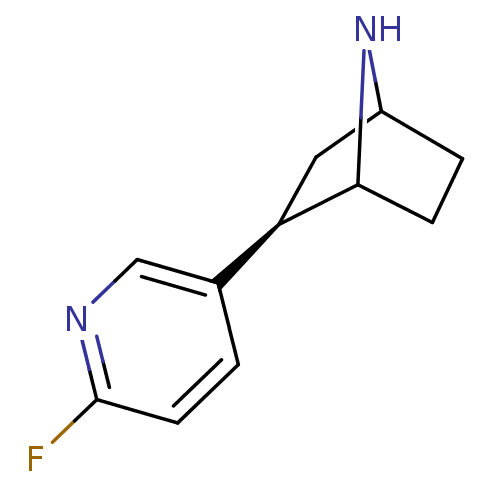
(NFEP)Show SMILES Fc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13FN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544

(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19770
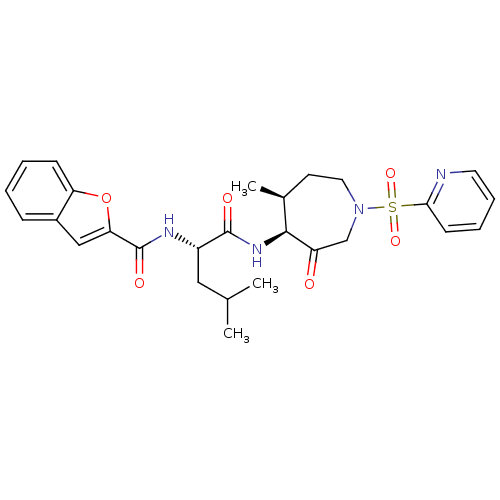
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1[C@@H](C)CCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-20(29-27(34)23-15-19-8-4-5-9-22(19)37-23)26(33)30-25-18(3)11-13-31(16-21(25)32)38(35,36)24-10-6-7-12-28-24/h4-10,12,15,17-18,20,25H,11,13-14,16H2,1-3H3,(H,29,34)(H,30,33)/t18-,20-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00990 | -62.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM299749

(4-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...)Show SMILES Cn1cnc(c1)-c1cnc(N)c(OCC2CCN(CC2)c2nc(OCC3(CC3)C#N)nc(n2)C(=O)NC23CC(C2)C3)c1 Show InChI InChI=1S/C29H34N10O3/c1-38-13-21(33-17-38)20-8-22(23(31)32-12-20)41-14-18-2-6-39(7-3-18)26-34-24(25(40)37-29-9-19(10-29)11-29)35-27(36-26)42-16-28(15-30)4-5-28/h8,12-13,17-19H,2-7,9-11,14,16H2,1H3,(H2,31,32)(H,37,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... |
US Patent US9593097 (2017)
BindingDB Entry DOI: 10.7270/Q2GM89BD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50474150
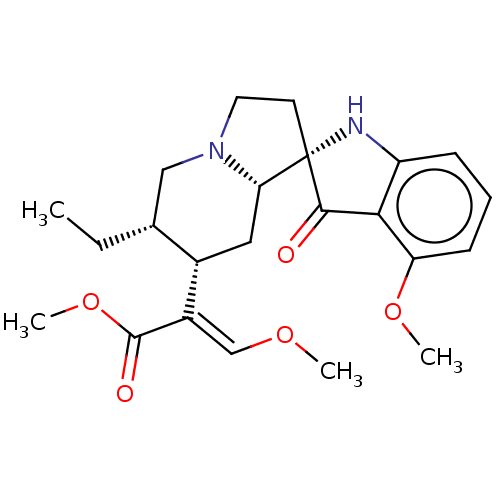
(CHEMBL58362)Show SMILES [H][C@@]12C[C@]([H])(C(=C/OC)\C(=O)OC)[C@]([H])(CC)CN1CC[C@]21Nc2cccc(OC)c2C1=O Show InChI InChI=1S/C23H30N2O5/c1-5-14-12-25-10-9-23(19(25)11-15(14)16(13-28-2)22(27)30-4)21(26)20-17(24-23)7-6-8-18(20)29-3/h6-8,13-15,19,24H,5,9-12H2,1-4H3/b16-13+/t14-,15+,19+,23+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor (unknown origin) |
J Med Chem 56: 4840-8 (2013)
Article DOI: 10.1021/jm400143z
BindingDB Entry DOI: 10.7270/Q2FT8PZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548

(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609446

(US11702418, Example 10-5)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCNCC(F)(F)C1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86040

(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609420
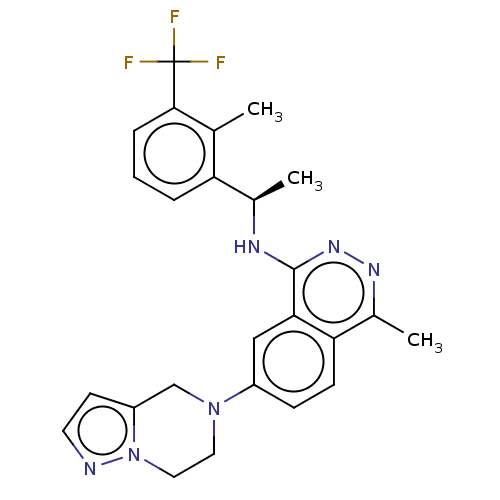
(US11702418, Example 6-10)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCn2nccc2C1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609526

(US11702418, Example 11-5)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCN(CC1)C(=O)[C@H]1CCOC1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50150028

((5R,6R)-1-Benzoyl-5-benzyl-6-hydroxy-2,4-bis-(4-hy...)Show SMILES O[C@@H]1CN(N(Cc2ccc(O)cc2)C(=O)N(Cc2ccc(O)cc2)[C@@H]1Cc1ccccc1)C(=O)c1ccccc1 Show InChI InChI=1S/C32H31N3O5/c36-27-15-11-24(12-16-27)20-33-29(19-23-7-3-1-4-8-23)30(38)22-34(31(39)26-9-5-2-6-10-26)35(32(33)40)21-25-13-17-28(37)18-14-25/h1-18,29-30,36-38H,19-22H2/t29-,30-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Tested for inhibition of HIV protease |
Bioorg Med Chem Lett 14: 4075-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.036
BindingDB Entry DOI: 10.7270/Q25B01ZK |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251]
(Staphylococcus aureus) | BDBM97445
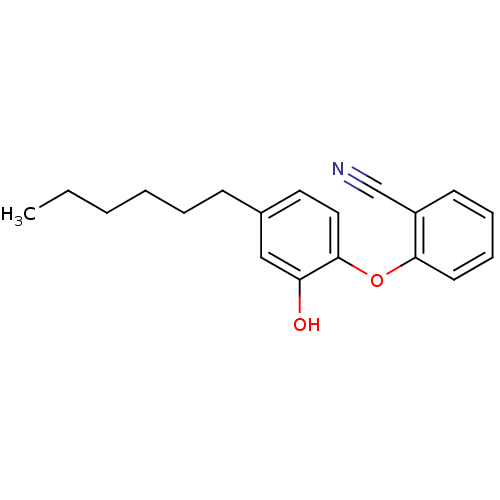
(PT119)Show InChI InChI=1S/C19H21NO2/c1-2-3-4-5-8-15-11-12-19(17(21)13-15)22-18-10-7-6-9-16(18)14-20/h6-7,9-13,21H,2-5,8H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus enoyl ACP reductase |
Eur J Med Chem 88: 66-73 (2014)
Article DOI: 10.1016/j.ejmech.2014.09.008
BindingDB Entry DOI: 10.7270/Q25T3N3S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Son of sevenless homolog 1 [564-1049]
() | BDBM609452

(US11702418, Example 10-11)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCn2c(C)nc(C)c2C1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609525

(US11702418, Example 11-4)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCN(CC1)C(=O)[C@@H]1CCOC1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50365814
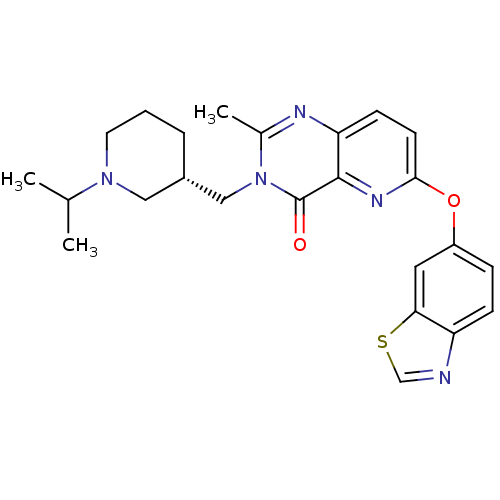
(CHEMBL1956993)Show SMILES CC(C)N1CCC[C@H](Cn2c(C)nc3ccc(Oc4ccc5ncsc5c4)nc3c2=O)C1 |r| Show InChI InChI=1S/C24H27N5O2S/c1-15(2)28-10-4-5-17(12-28)13-29-16(3)26-20-8-9-22(27-23(20)24(29)30)31-18-6-7-19-21(11-18)32-14-25-19/h6-9,11,14-15,17H,4-5,10,12-13H2,1-3H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Prosidion Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ghrelin from human GHSR membranes overexpressing GSH-R1a by scintillation counting |
Bioorg Med Chem Lett 22: 2271-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.078
BindingDB Entry DOI: 10.7270/Q20G3KMP |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50365815
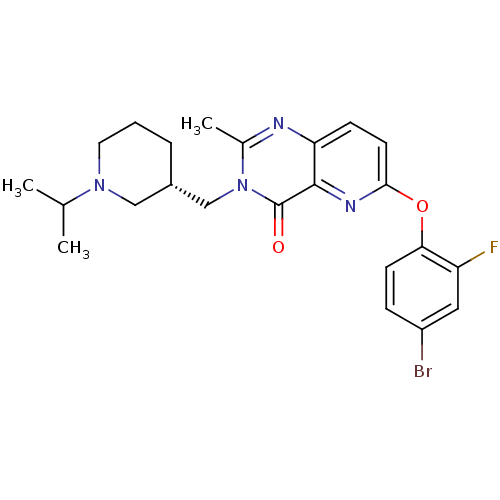
(CHEMBL1956994)Show SMILES CC(C)N1CCC[C@H](Cn2c(C)nc3ccc(Oc4ccc(Br)cc4F)nc3c2=O)C1 |r| Show InChI InChI=1S/C23H26BrFN4O2/c1-14(2)28-10-4-5-16(12-28)13-29-15(3)26-19-7-9-21(27-22(19)23(29)30)31-20-8-6-17(24)11-18(20)25/h6-9,11,14,16H,4-5,10,12-13H2,1-3H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Prosidion Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Ghrelin from human GHSR membranes overexpressing GSH-R1a by scintillation counting |
Bioorg Med Chem Lett 22: 2271-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.078
BindingDB Entry DOI: 10.7270/Q20G3KMP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM29611

(2-Iodomelatonin | CHEMBL289233 | Melatonin,2-Iodo)Show InChI InChI=1S/C13H15IN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 1239-45 (1998)
BindingDB Entry DOI: 10.7270/Q2PK0DPM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Son of sevenless homolog 1 [564-1049]
() | BDBM609524
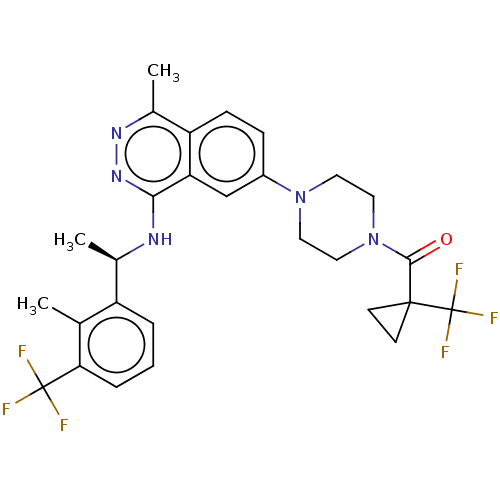
(US11702418, Example 11-3)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCN(CC1)C(=O)C1(CC1)C(F)(F)F)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609451

(US11702418, Example 10-10)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCN(CC1)c1cnn(C)c1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609523

(US11702418, Example 11-2)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCN(CC1)C(=O)C1CCOCC1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM609522

((R)-(4-(1-methyl-4-((1-(2-methyl-3-(trifluoromethy...)Show SMILES C[C@@H](Nc1nnc(C)c2ccc(cc12)N1CCN(CC1)C(=O)C1COC1)c1cccc(c1C)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2C251J6 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM198
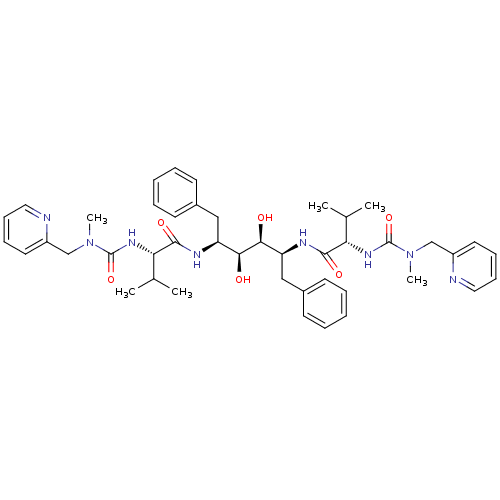
((2S)-N-[(2S,3S,4S,5S)-3,4-dihydroxy-5-[(2S)-3-meth...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1ccccn1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)N(C)Cc1ccccn1)C(C)C |r| Show InChI InChI=1S/C44H58N8O6/c1-29(2)37(49-43(57)51(5)27-33-21-13-15-23-45-33)41(55)47-35(25-31-17-9-7-10-18-31)39(53)40(54)36(26-32-19-11-8-12-20-32)48-42(56)38(30(3)4)50-44(58)52(6)28-34-22-14-16-24-46-34/h7-24,29-30,35-40,53-54H,25-28H2,1-6H3,(H,47,55)(H,48,56)(H,49,57)(H,50,58)/t35-,36-,37-,38-,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 0.0110 | -63.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
NCI-FCRDC
| Assay Description
HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... |
J Am Chem Soc 116: 847-55 (1994)
Article DOI: 10.1021/ja00082a004
BindingDB Entry DOI: 10.7270/Q2KK98Z1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546

(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM299975

(4-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...)Show SMILES Cn1cnc(c1)-c1cnc(N)c(OCC2CCN(CC2)c2nc(OCC3(CC3)C#N)nc(n2)C(=O)NC2(C)CCC2)c1 Show InChI InChI=1S/C29H36N10O3/c1-28(6-3-7-28)37-25(40)24-34-26(36-27(35-24)42-17-29(16-30)8-9-29)39-10-4-19(5-11-39)15-41-22-12-20(13-32-23(22)31)21-14-38(2)18-33-21/h12-14,18-19H,3-11,15,17H2,1-2H3,(H2,31,32)(H,37,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... |
US Patent US9593097 (2017)
BindingDB Entry DOI: 10.7270/Q2GM89BD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM299902

(6-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...)Show SMILES C[C@H](CO)NC(=O)c1nc(OC[C@H]2CCCO2)nc(n1)N1CCC(COc2cc(cnc2N)-c2cn(C)cn2)CC1 |r| Show InChI InChI=1S/C27H37N9O5/c1-17(13-37)31-25(38)24-32-26(34-27(33-24)41-15-20-4-3-9-39-20)36-7-5-18(6-8-36)14-40-22-10-19(11-29-23(22)28)21-12-35(2)16-30-21/h10-12,16-18,20,37H,3-9,13-15H2,1-2H3,(H2,28,29)(H,31,38)/t17-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... |
US Patent US9593097 (2017)
BindingDB Entry DOI: 10.7270/Q2GM89BD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142111
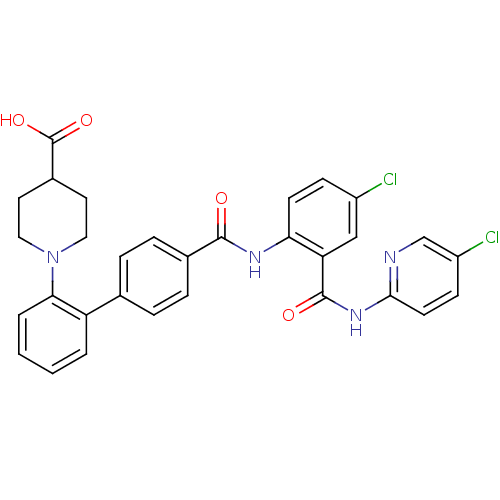
(1-{4'-[4-Chloro-2-(5-chloro-pyridin-2-ylcarbamoyl)...)Show SMILES OC(=O)C1CCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C31H26Cl2N4O4/c32-22-9-11-26(25(17-22)30(39)36-28-12-10-23(33)18-34-28)35-29(38)20-7-5-19(6-8-20)24-3-1-2-4-27(24)37-15-13-21(14-16-37)31(40)41/h1-12,17-18,21H,13-16H2,(H,35,38)(H,40,41)(H,34,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50379086
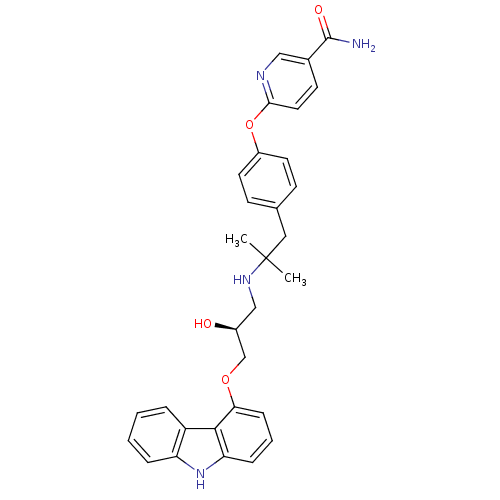
(CHEMBL2012521 | CHEMBL2012522 | LY-377604)Show SMILES CC(C)(Cc1ccc(Oc2ccc(cn2)C(N)=O)cc1)NC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C31H32N4O4/c1-31(2,16-20-10-13-23(14-11-20)39-28-15-12-21(17-33-28)30(32)37)34-18-22(36)19-38-27-9-5-8-26-29(27)24-6-3-4-7-25(24)35-26/h3-15,17,22,34-36H,16,18-19H2,1-2H3,(H2,32,37)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]Iodocyanopindolol from human adrenergic beta2 receptor expressed in insect sf9 cells by scintillation counting |
ACS Med Chem Lett 2: 583-586 (2011)
Article DOI: 10.1021/ml200071k
BindingDB Entry DOI: 10.7270/Q20R9QDP |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50379086

(CHEMBL2012521 | CHEMBL2012522 | LY-377604)Show SMILES CC(C)(Cc1ccc(Oc2ccc(cn2)C(N)=O)cc1)NC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C31H32N4O4/c1-31(2,16-20-10-13-23(14-11-20)39-28-15-12-21(17-33-28)30(32)37)34-18-22(36)19-38-27-9-5-8-26-29(27)24-6-3-4-7-25(24)35-26/h3-15,17,22,34-36H,16,18-19H2,1-2H3,(H2,32,37)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]Iodocyanopindolol from human adrenergic beta2 receptor expressed in insect sf9 cells by scintillation counting |
ACS Med Chem Lett 2: 583-586 (2011)
Article DOI: 10.1021/ml200071k
BindingDB Entry DOI: 10.7270/Q20R9QDP |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM199
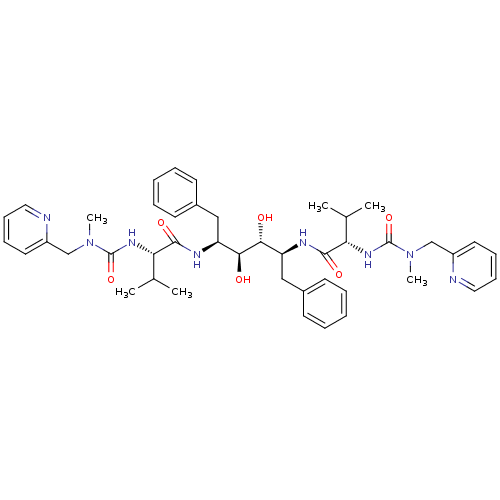
((2S)-N-[(2S,3R,4S,5S)-3,4-dihydroxy-5-[(2S)-3-meth...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1ccccn1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)N(C)Cc1ccccn1)C(C)C |r| Show InChI InChI=1S/C44H58N8O6/c1-29(2)37(49-43(57)51(5)27-33-21-13-15-23-45-33)41(55)47-35(25-31-17-9-7-10-18-31)39(53)40(54)36(26-32-19-11-8-12-20-32)48-42(56)38(30(3)4)50-44(58)52(6)28-34-22-14-16-24-46-34/h7-24,29-30,35-40,53-54H,25-28H2,1-6H3,(H,47,55)(H,48,56)(H,49,57)(H,50,58)/t35-,36-,37-,38-,39-,40+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 0.0120 | -63.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
NCI-FCRDC
| Assay Description
HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... |
J Am Chem Soc 116: 847-55 (1994)
Article DOI: 10.1021/ja00082a004
BindingDB Entry DOI: 10.7270/Q2KK98Z1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM300011
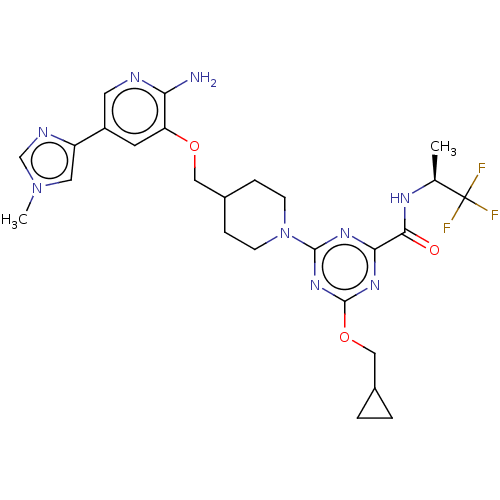
(4-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...)Show SMILES C[C@H](NC(=O)c1nc(OCC2CC2)nc(n1)N1CCC(COc2cc(cnc2N)-c2cn(C)cn2)CC1)C(F)(F)F |r| Show InChI InChI=1S/C26H32F3N9O3/c1-15(26(27,28)29)33-23(39)22-34-24(36-25(35-22)41-13-16-3-4-16)38-7-5-17(6-8-38)12-40-20-9-18(10-31-21(20)30)19-11-37(2)14-32-19/h9-11,14-17H,3-8,12-13H2,1-2H3,(H2,30,31)(H,33,39)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... |
US Patent US9593097 (2017)
BindingDB Entry DOI: 10.7270/Q2GM89BD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM300077
(N-(3-amino-3- methylbutan- 2-yl)-2- (cyclo- propyl...)Show SMILES COc1ccnc2[nH]cc(C3CCN(CC3)c3cc(nc(OCC4CC4)n3)C(=O)NC(C)C(C)(C)N)c12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... |
US Patent US9593097 (2017)
BindingDB Entry DOI: 10.7270/Q2GM89BD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data