

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50439332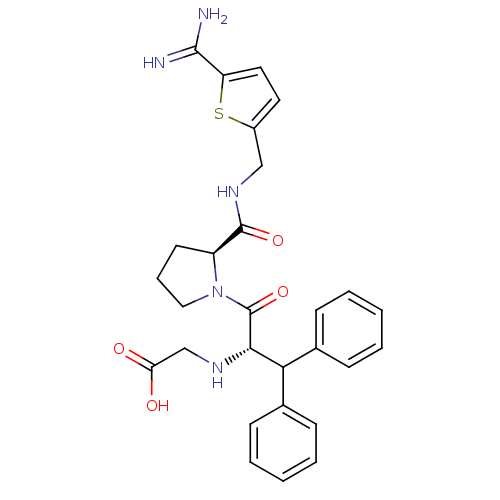 (CHEMBL2419745) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Curated by ChEMBL | Assay Description Inhibition of human t-PA | Bioorg Med Chem Lett 23: 4779-84 (2013) Article DOI: 10.1016/j.bmcl.2013.07.008 BindingDB Entry DOI: 10.7270/Q2X92CR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM29388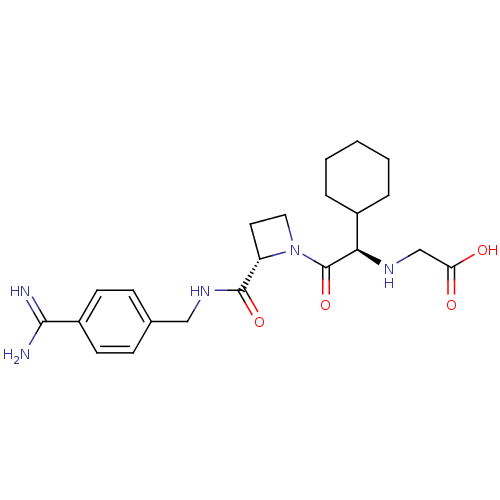 (Exanta | Melagatran | US11584714, Compound 999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Curated by ChEMBL | Assay Description Inhibition of human t-PA | Bioorg Med Chem Lett 23: 4779-84 (2013) Article DOI: 10.1016/j.bmcl.2013.07.008 BindingDB Entry DOI: 10.7270/Q2X92CR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM29388 (Exanta | Melagatran | US11584714, Compound 999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Curated by ChEMBL | Assay Description Inhibition of human plasmin | Bioorg Med Chem Lett 23: 4779-84 (2013) Article DOI: 10.1016/j.bmcl.2013.07.008 BindingDB Entry DOI: 10.7270/Q2X92CR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50439332 (CHEMBL2419745) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 23: 4779-84 (2013) Article DOI: 10.1016/j.bmcl.2013.07.008 BindingDB Entry DOI: 10.7270/Q2X92CR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM29388 (Exanta | Melagatran | US11584714, Compound 999) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 23: 4779-84 (2013) Article DOI: 10.1016/j.bmcl.2013.07.008 BindingDB Entry DOI: 10.7270/Q2X92CR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50439332 (CHEMBL2419745) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Curated by ChEMBL | Assay Description Inhibition of human plasmin | Bioorg Med Chem Lett 23: 4779-84 (2013) Article DOI: 10.1016/j.bmcl.2013.07.008 BindingDB Entry DOI: 10.7270/Q2X92CR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50038001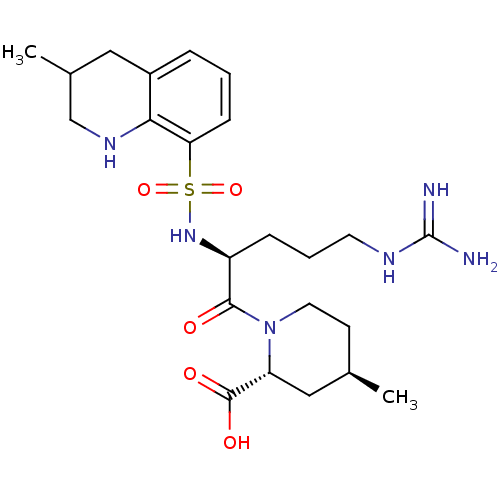 ((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 23: 4779-84 (2013) Article DOI: 10.1016/j.bmcl.2013.07.008 BindingDB Entry DOI: 10.7270/Q2X92CR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50038001 ((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 8.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Curated by ChEMBL | Assay Description Inhibition of human t-PA | Bioorg Med Chem Lett 23: 4779-84 (2013) Article DOI: 10.1016/j.bmcl.2013.07.008 BindingDB Entry DOI: 10.7270/Q2X92CR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50038001 ((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Curated by ChEMBL | Assay Description Inhibition of human plasmin | Bioorg Med Chem Lett 23: 4779-84 (2013) Article DOI: 10.1016/j.bmcl.2013.07.008 BindingDB Entry DOI: 10.7270/Q2X92CR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121552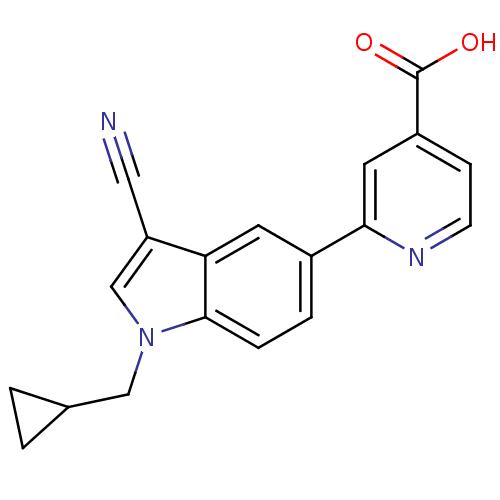 (US8729273, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121532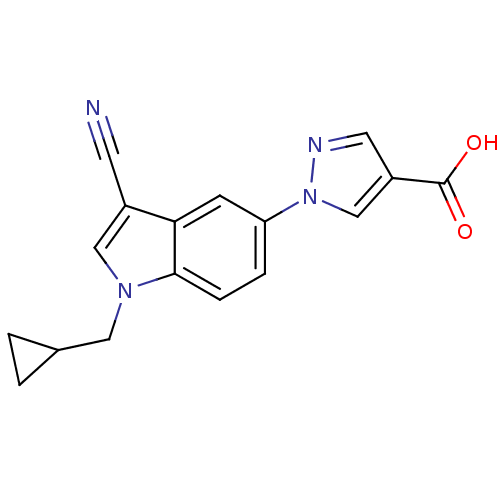 (US8729273, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121531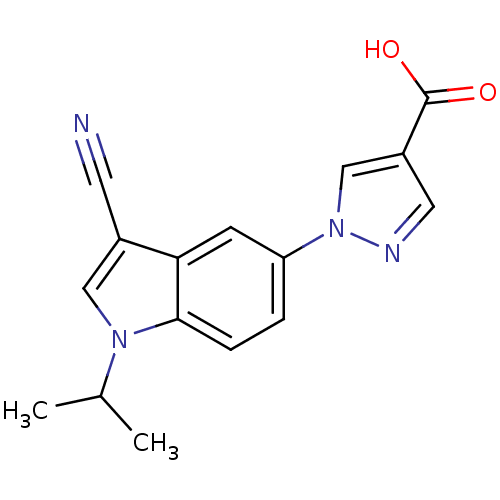 (US8729273, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121536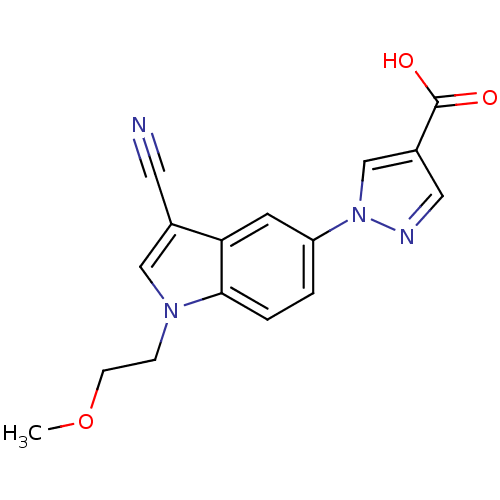 (US8729273, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121534 (US8729273, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121547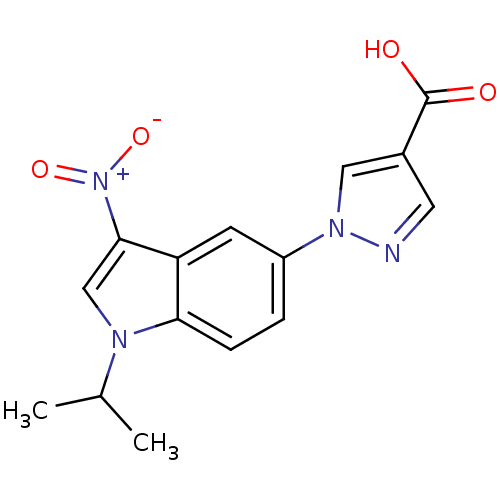 (US8729273, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121539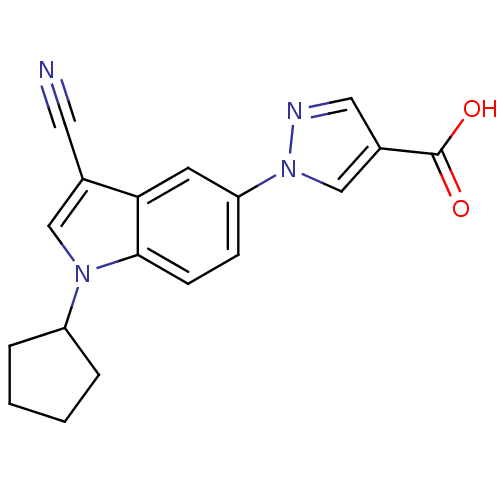 (US8729273, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121550 (US8729273, 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121543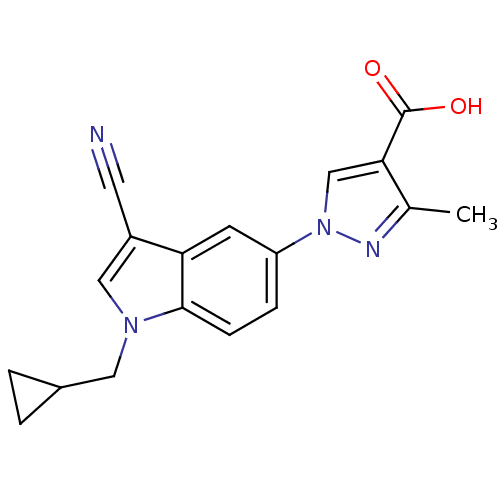 (US8729273, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121535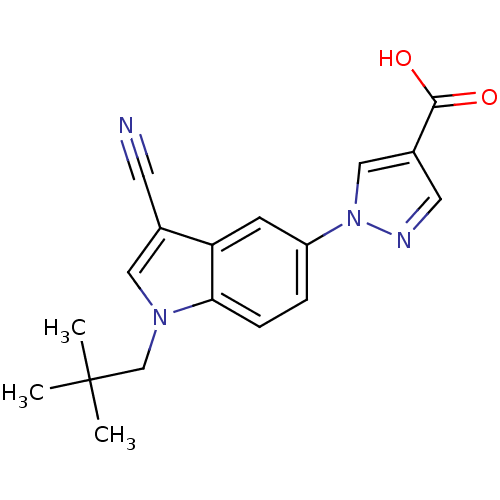 (US8729273, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121559 (US8729273, 29) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121540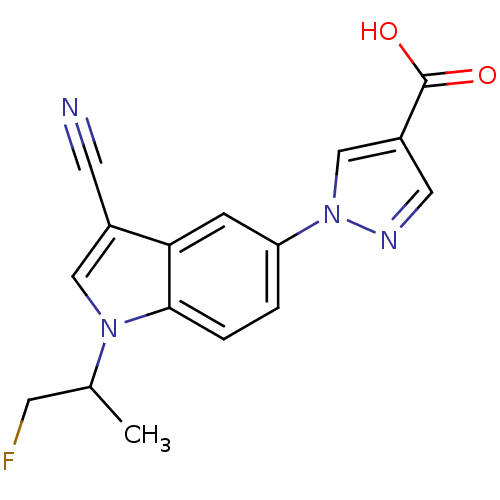 (US8729273, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121541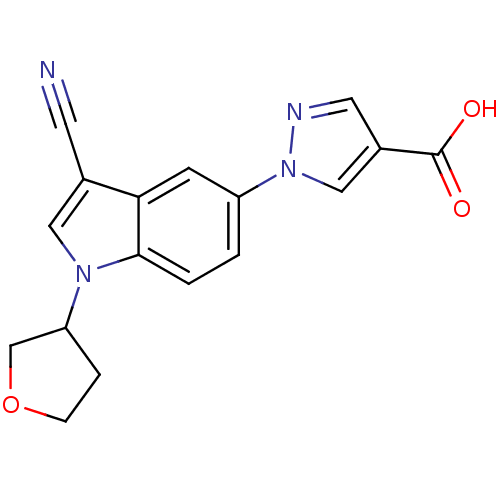 (US8729273, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121533 (US8729273, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121554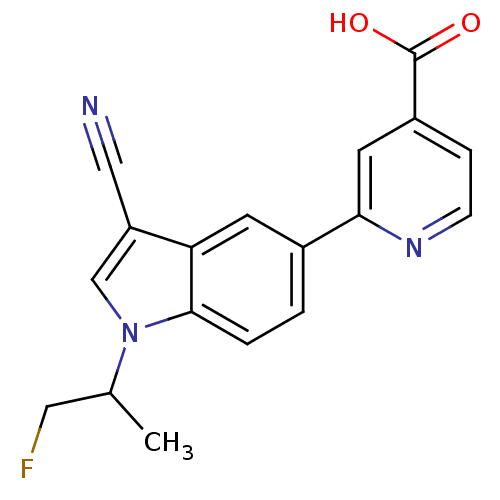 (US8729273, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121551 (US8729273, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121537 (US8729273, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121538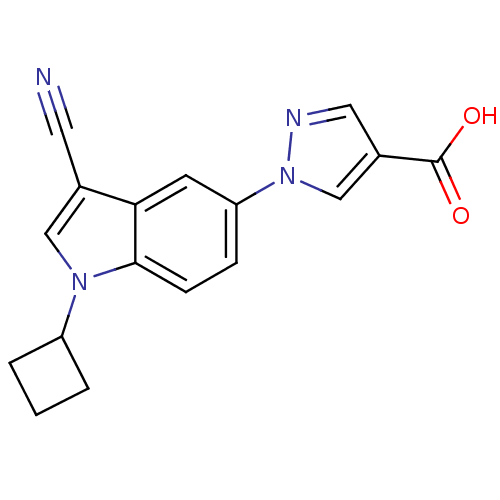 (US8729273, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121555 (US8729273, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121553 (US8729273, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121557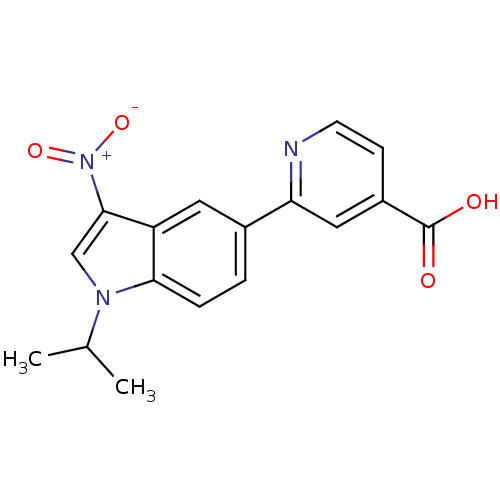 (US8729273, 27) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121542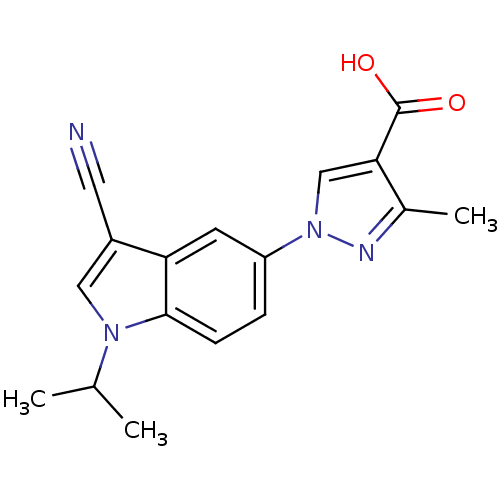 (US8729273, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11.6 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121544 (US8729273, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121548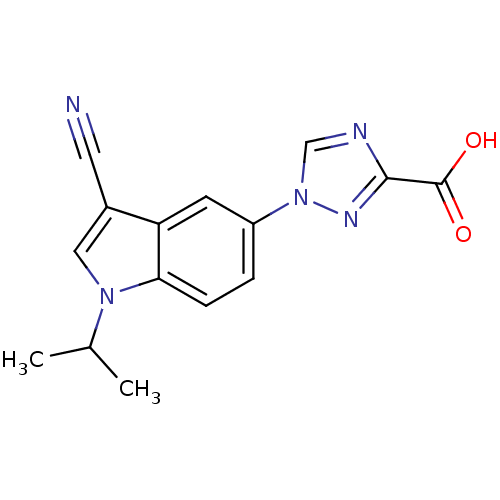 (US8729273, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121560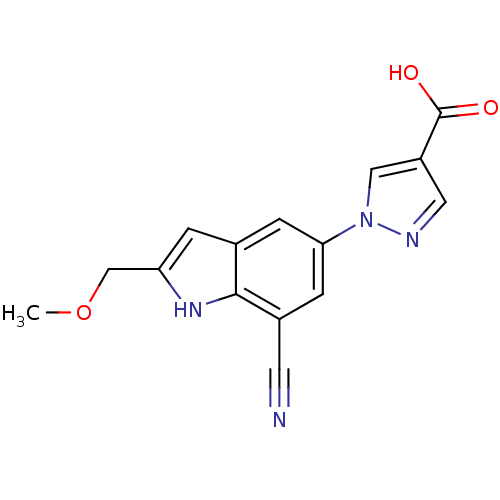 (US8729273, 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121549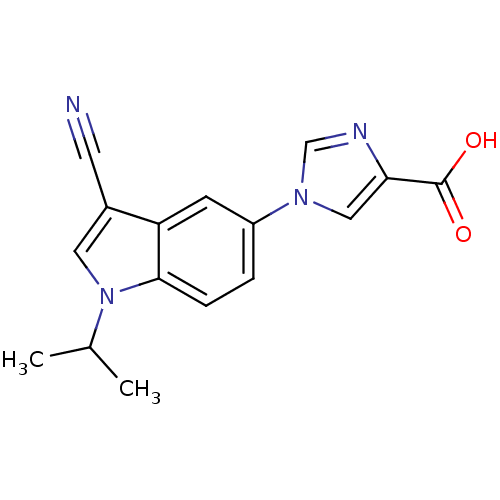 (US8729273, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121558 (US8729273, 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121561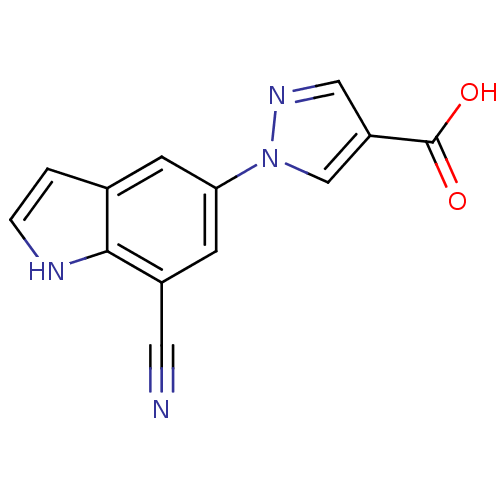 (US8729273, 31) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University Curated by ChEMBL | Assay Description Inhibition of COX2 assessed as inhibition of PGE2 production using arachidonic acid as substrate after 10 mins by ELISA | Bioorg Med Chem Lett 22: 793-6 (2012) Article DOI: 10.1016/j.bmcl.2011.12.072 BindingDB Entry DOI: 10.7270/Q2VT1SJQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using pNPB as substrate measured after 30 mins | Bioorg Med Chem Lett 29: 2079-2084 (2019) Article DOI: 10.1016/j.bmcl.2019.07.008 BindingDB Entry DOI: 10.7270/Q23B63K4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50262486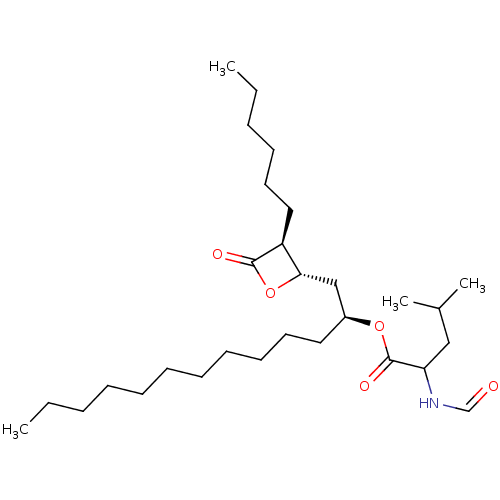 (CHEMBL2354678) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food Science and Biotechnology, Daegu University, Gyeongsan 38453, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate pretreated for 15 mins followed by substrate addition measured afte... | Bioorg Med Chem Lett 27: 4889-4892 (2017) Article DOI: 10.1016/j.bmcl.2017.09.035 BindingDB Entry DOI: 10.7270/Q23J3GFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Atomic Energy Research Institute Curated by ChEMBL | Assay Description Inhibition of pig pancreatic lipase assessed as hydrolysis of p-nitrophenylbutyrate to p-nitrophenol | Bioorg Med Chem Lett 23: 1099-103 (2013) Article DOI: 10.1016/j.bmcl.2012.12.003 BindingDB Entry DOI: 10.7270/Q2P55PTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu Haany University Curated by ChEMBL | Assay Description Inhibition of pig pancreatic lipase assessed as p-NPB hydrolysis by ELISA | Bioorg Med Chem Lett 21: 1512-4 (2011) Article DOI: 10.1016/j.bmcl.2010.12.122 BindingDB Entry DOI: 10.7270/Q2GQ6Z1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 600 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Korea Atomic Energy Research Institute US Patent | Assay Description Particularly, pancreatic lipase inhibiting activity was measured by the conventional method known to those in the art (Kim, J. H.; Kim, H. J.; Park, ... | US Patent US9328123 (2016) BindingDB Entry DOI: 10.7270/Q2Q81BXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50391834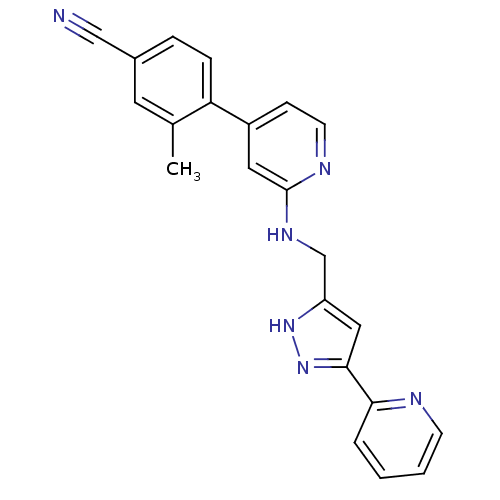 (CHEMBL2147024) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human ERG channel IKr expressed in CHO cells by patch clamp assay | ACS Med Chem Lett 3: 678-682 (2012) Article DOI: 10.1021/ml300146q BindingDB Entry DOI: 10.7270/Q23J3F11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50391830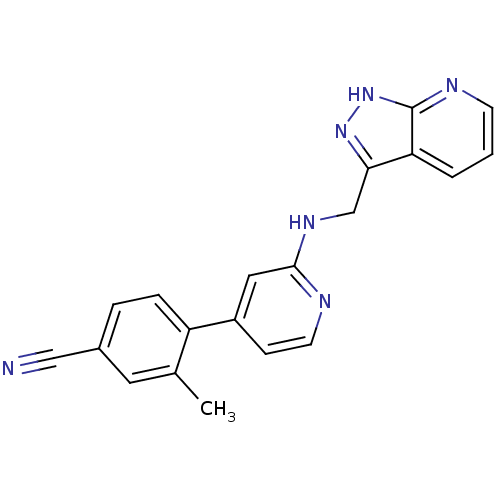 (CHEMBL2147020) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human ERG channel IKr expressed in CHO cells by patch clamp assay | ACS Med Chem Lett 3: 678-682 (2012) Article DOI: 10.1021/ml300146q BindingDB Entry DOI: 10.7270/Q23J3F11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50391831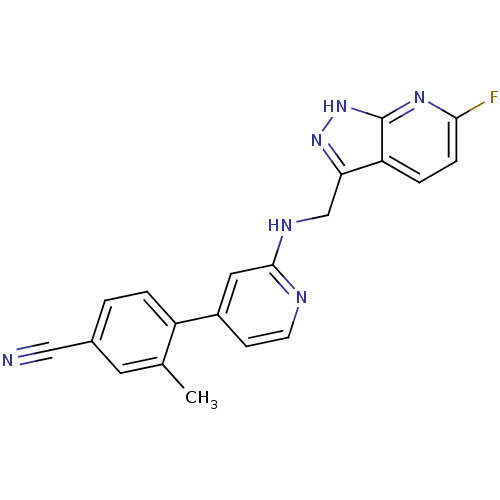 (CHEMBL2147021) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human ERG channel IKr expressed in CHO cells by patch clamp assay | ACS Med Chem Lett 3: 678-682 (2012) Article DOI: 10.1021/ml300146q BindingDB Entry DOI: 10.7270/Q23J3F11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121556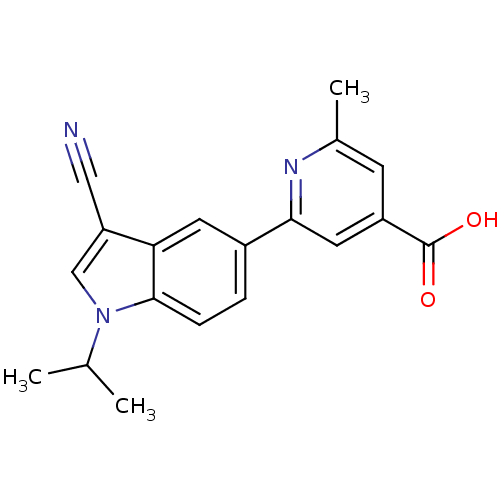 (US8729273, 26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50391832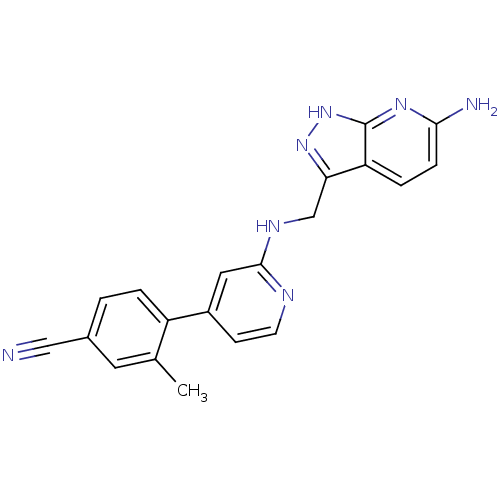 (CHEMBL2147022) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human ERG channel IKr expressed in CHO cells by patch clamp assay | ACS Med Chem Lett 3: 678-682 (2012) Article DOI: 10.1021/ml300146q BindingDB Entry DOI: 10.7270/Q23J3F11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50262488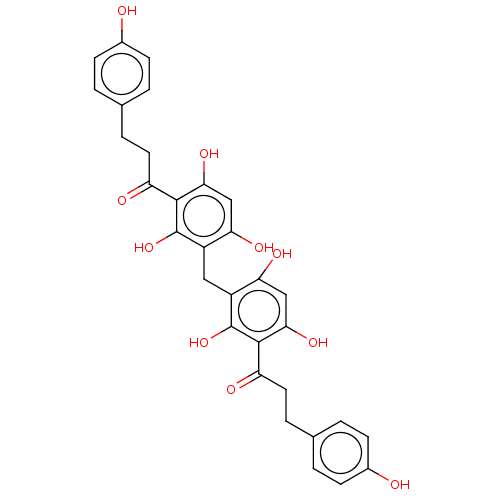 (CHEMBL4067236) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food Science and Biotechnology, Daegu University, Gyeongsan 38453, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate pretreated for 15 mins followed by substrate addition measured afte... | Bioorg Med Chem Lett 27: 4889-4892 (2017) Article DOI: 10.1016/j.bmcl.2017.09.035 BindingDB Entry DOI: 10.7270/Q23J3GFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50036635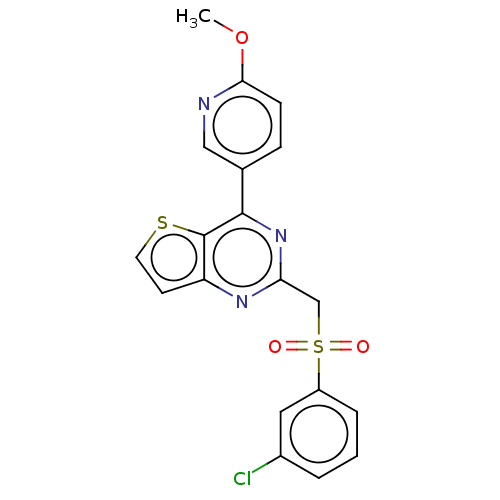 (CHEMBL3354313) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea Curated by ChEMBL | Assay Description Inhibition of recombinant CYP2C9 (unknown origin) after 10 mins by LC-MS/MS analysis | Bioorg Med Chem Lett 24: 5473-7 (2015) Article DOI: 10.1016/j.bmcl.2014.10.007 BindingDB Entry DOI: 10.7270/Q2F191B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 88 total ) | Next | Last >> |