 Found 8312 hits with Last Name = 'koltun' and Initial = 'es'
Found 8312 hits with Last Name = 'koltun' and Initial = 'es' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104963
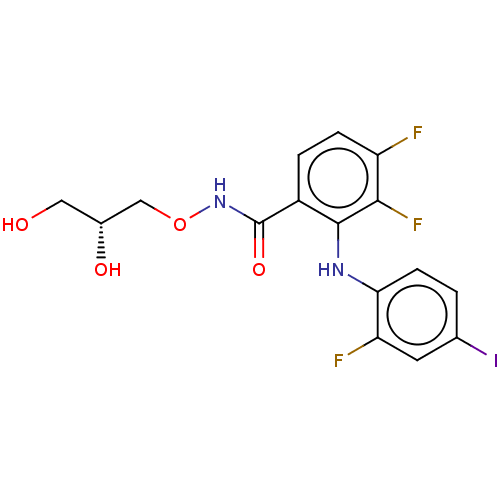
(CHEMBL507361 | US11147816, PD0325901 | US11701360,...)Show SMILES OC[C@@H](O)CONC(=O)c1ccc(F)c(F)c1Nc1ccc(I)cc1F |r| Show InChI InChI=1S/C16H14F3IN2O4/c17-11-3-2-10(16(25)22-26-7-9(24)6-23)15(14(11)19)21-13-4-1-8(20)5-12(13)18/h1-5,9,21,23-24H,6-7H2,(H,22,25)/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK1-mediated ERK2 T202/Y204 phosphorylation using biotinylated MBP as substrate preincubated for 30 mins measured after 100 mins by cR... |
ACS Med Chem Lett 3: 416-421 (2012)
Article DOI: 10.1021/ml300049d
BindingDB Entry DOI: 10.7270/Q2GT5P80 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402421
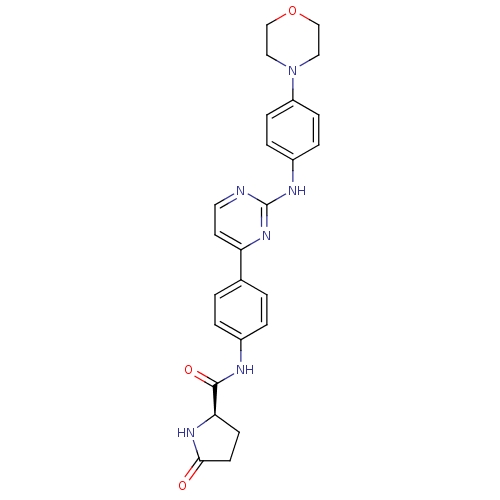
(CHEMBL2208035)Show SMILES O=C(Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)[C@H]1CCC(=O)N1 |r| Show InChI InChI=1S/C25H26N6O3/c32-23-10-9-22(29-23)24(33)27-18-3-1-17(2-4-18)21-11-12-26-25(30-21)28-19-5-7-20(8-6-19)31-13-15-34-16-14-31/h1-8,11-12,22H,9-10,13-16H2,(H,27,33)(H,29,32)(H,26,28,30)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50391802
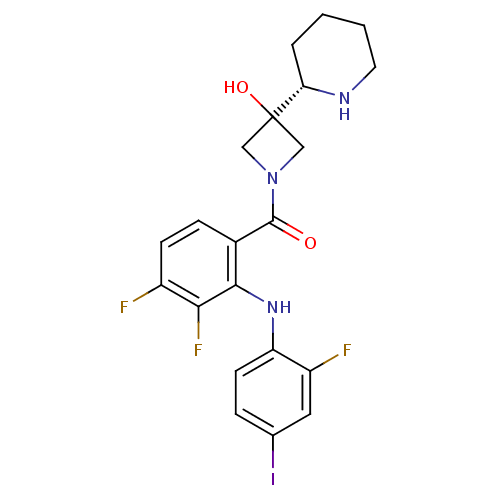
((3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl...)Show SMILES OC1(CN(C1)C(=O)c1ccc(F)c(F)c1Nc1ccc(I)cc1F)[C@@H]1CCCCN1 |r| Show InChI InChI=1S/C21H21F3IN3O2/c22-14-6-5-13(19(18(14)24)27-16-7-4-12(25)9-15(16)23)20(29)28-10-21(30,11-28)17-3-1-2-8-26-17/h4-7,9,17,26-27,30H,1-3,8,10-11H2/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK1-mediated ERK2 T202/Y204 phosphorylation using biotinylated MBP as substrate preincubated for 30 mins measured after 100 mins by cR... |
ACS Med Chem Lett 3: 416-421 (2012)
Article DOI: 10.1021/ml300049d
BindingDB Entry DOI: 10.7270/Q2GT5P80 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM527814
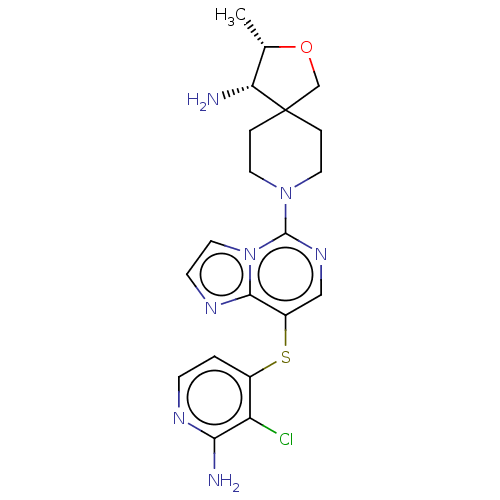
((3S,4S)-8-(8-((2- amino-3- chloropyridin-4- yl)thi...)Show SMILES C[C@@H]1OCC2(CCN(CC2)c2ncc(Sc3ccnc(N)c3Cl)c3nccn23)[C@@H]1N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24X5CRG |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50272192
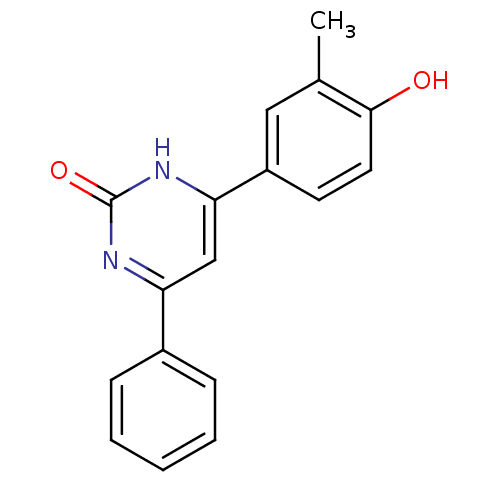
(4-(4-hydroxy-3-methylphenyl)-6-phenylpyrimidin-2(1...)Show InChI InChI=1S/C17H14N2O2/c1-11-9-13(7-8-16(11)20)15-10-14(18-17(21)19-15)12-5-3-2-4-6-12/h2-10,20H,1H3,(H,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402413

(CHEMBL2208032)Show SMILES C[C@@H](N)C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C23H26N6O2/c1-16(24)22(30)26-18-4-2-17(3-5-18)21-10-11-25-23(28-21)27-19-6-8-20(9-7-19)29-12-14-31-15-13-29/h2-11,16H,12-15,24H2,1H3,(H,26,30)(H,25,27,28)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50391804
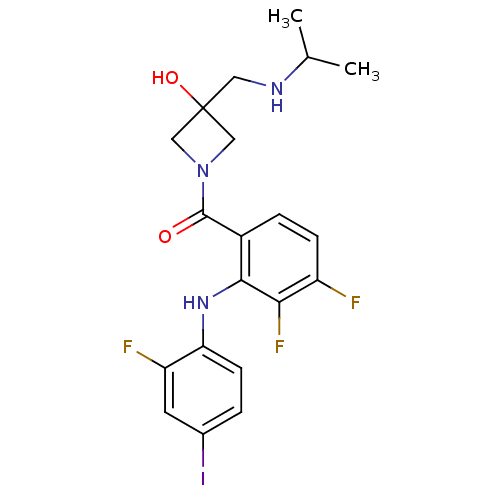
(CHEMBL2146893)Show SMILES CC(C)NCC1(O)CN(C1)C(=O)c1ccc(F)c(F)c1Nc1ccc(I)cc1F Show InChI InChI=1S/C20H21F3IN3O2/c1-11(2)25-8-20(29)9-27(10-20)19(28)13-4-5-14(21)17(23)18(13)26-16-6-3-12(24)7-15(16)22/h3-7,11,25-26,29H,8-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK1-mediated ERK2 T202/Y204 phosphorylation using biotinylated MBP as substrate preincubated for 30 mins measured after 100 mins by cR... |
ACS Med Chem Lett 3: 416-421 (2012)
Article DOI: 10.1021/ml300049d
BindingDB Entry DOI: 10.7270/Q2GT5P80 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394804
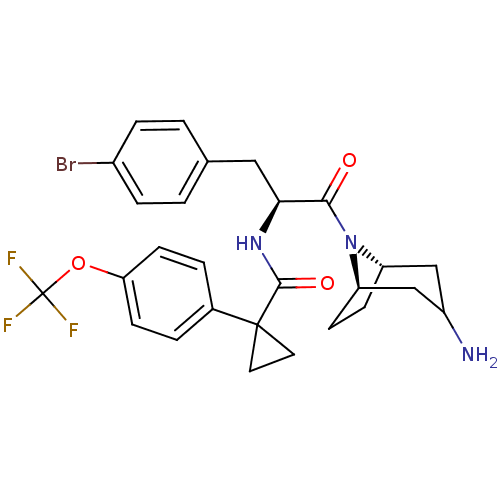
(CHEMBL2163828)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(OC(F)(F)F)cc1 |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C27H29BrF3N3O3/c28-18-5-1-16(2-6-18)13-23(24(35)34-20-7-8-21(34)15-19(32)14-20)33-25(36)26(11-12-26)17-3-9-22(10-4-17)37-27(29,30)31/h1-6,9-10,19-21,23H,7-8,11-15,32H2,(H,33,36)/t19?,20-,21+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394812
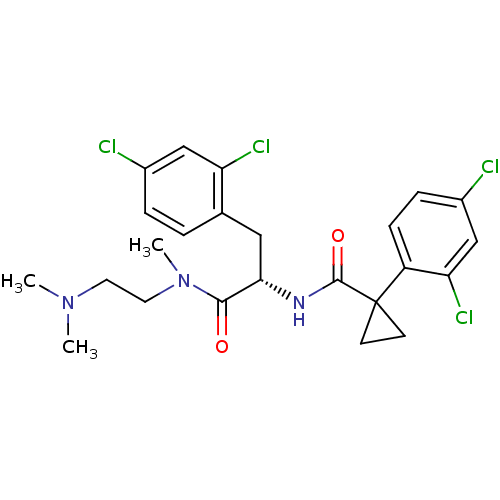
(CHEMBL2163838)Show SMILES CN(C)CCN(C)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H27Cl4N3O2/c1-30(2)10-11-31(3)22(32)21(12-15-4-5-16(25)13-19(15)27)29-23(33)24(8-9-24)18-7-6-17(26)14-20(18)28/h4-7,13-14,21H,8-12H2,1-3H3,(H,29,33)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394820
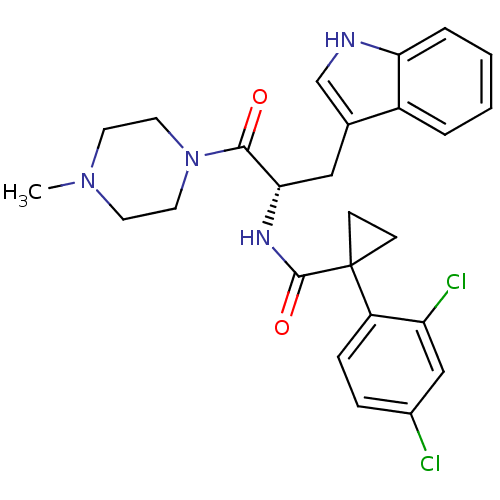
(CHEMBL2163829)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O2/c1-31-10-12-32(13-11-31)24(33)23(14-17-16-29-22-5-3-2-4-19(17)22)30-25(34)26(8-9-26)20-7-6-18(27)15-21(20)28/h2-7,15-16,23,29H,8-14H2,1H3,(H,30,34)/t23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394807
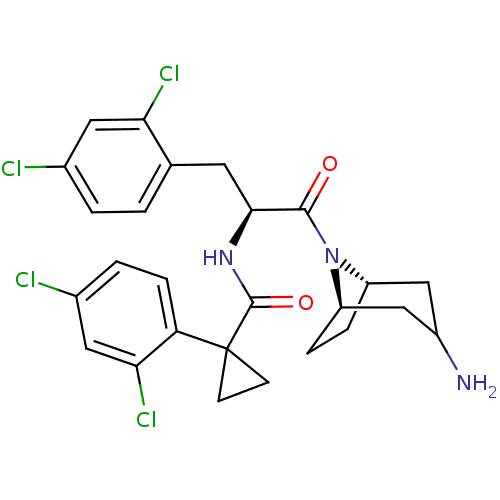
(CHEMBL2163825)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H27Cl4N3O2/c27-15-2-1-14(21(29)10-15)9-23(24(34)33-18-4-5-19(33)13-17(31)12-18)32-25(35)26(7-8-26)20-6-3-16(28)11-22(20)30/h1-3,6,10-11,17-19,23H,4-5,7-9,12-13,31H2,(H,32,35)/t17?,18-,19+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356090
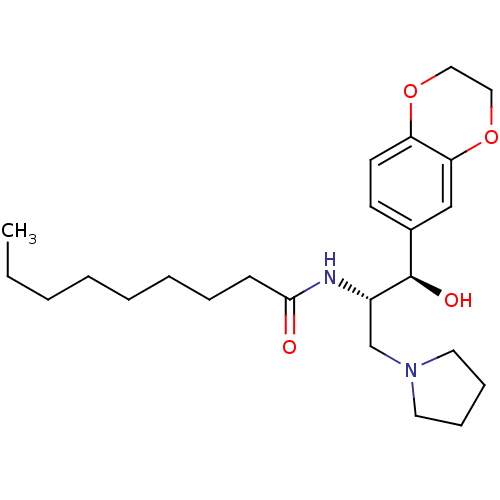
(CHEMBL1911678)Show SMILES CCCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C24H38N2O4/c1-2-3-4-5-6-7-10-23(27)25-20(18-26-13-8-9-14-26)24(28)19-11-12-21-22(17-19)30-16-15-29-21/h11-12,17,20,24,28H,2-10,13-16,18H2,1H3,(H,25,27)/t20-,24+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356091
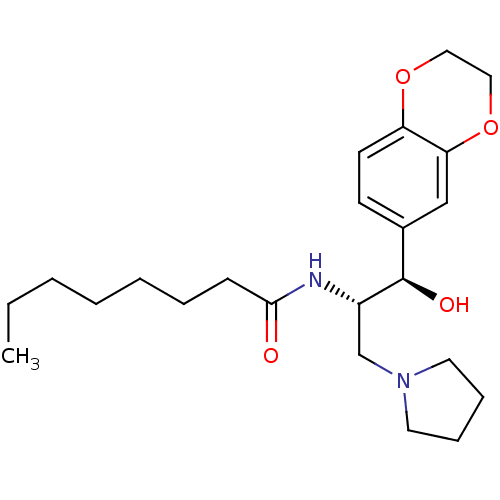
(CHEMBL1911679)Show SMILES CCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C23H36N2O4/c1-2-3-4-5-6-9-22(26)24-19(17-25-12-7-8-13-25)23(27)18-10-11-20-21(16-18)29-15-14-28-20/h10-11,16,19,23,27H,2-9,12-15,17H2,1H3,(H,24,26)/t19-,23+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402409
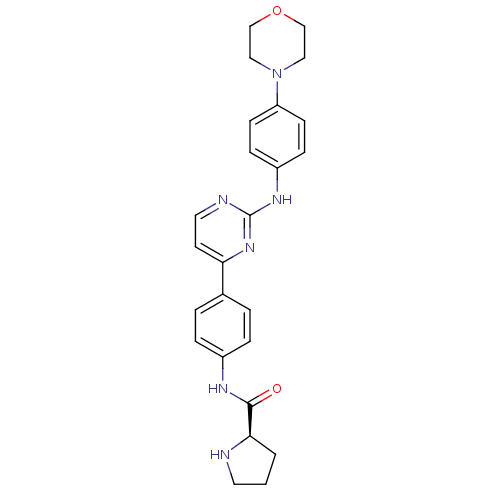
(CHEMBL2208034)Show SMILES O=C(Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)[C@H]1CCCN1 |r| Show InChI InChI=1S/C25H28N6O2/c32-24(23-2-1-12-26-23)28-19-5-3-18(4-6-19)22-11-13-27-25(30-22)29-20-7-9-21(10-8-20)31-14-16-33-17-15-31/h3-11,13,23,26H,1-2,12,14-17H2,(H,28,32)(H,27,29,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402412
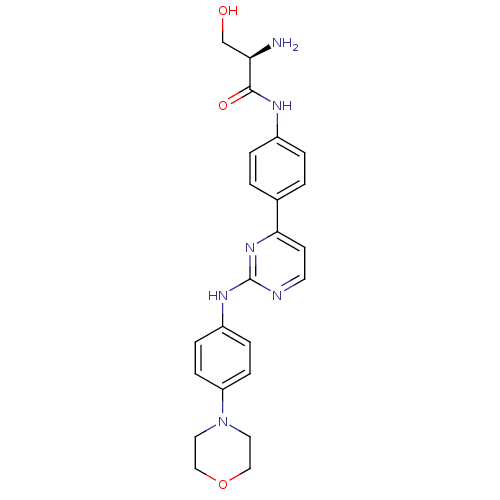
(CHEMBL2208033)Show SMILES N[C@H](CO)C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C23H26N6O3/c24-20(15-30)22(31)26-17-3-1-16(2-4-17)21-9-10-25-23(28-21)27-18-5-7-19(8-6-18)29-11-13-32-14-12-29/h1-10,20,30H,11-15,24H2,(H,26,31)(H,25,27,28)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385084

(CHEMBL2035636)Show SMILES CN1C[C@@H]2CC[C@H]1CN2Cc1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 |r| Show InChI InChI=1S/C18H19BrN4O2/c1-22-7-12-4-3-11(22)8-23(12)9-15-20-16-13-6-10(19)2-5-14(13)25-17(16)18(24)21-15/h2,5-6,11-12H,3-4,7-9H2,1H3,(H,20,21,24)/t11-,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385075

(CHEMBL2035626)Show SMILES NCc1ccc(c(Cl)c1)-c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 Show InChI InChI=1S/C17H11BrClN3O2/c18-9-2-4-13-11(6-9)14-15(24-13)17(23)22-16(21-14)10-3-1-8(7-20)5-12(10)19/h1-6H,7,20H2,(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385078
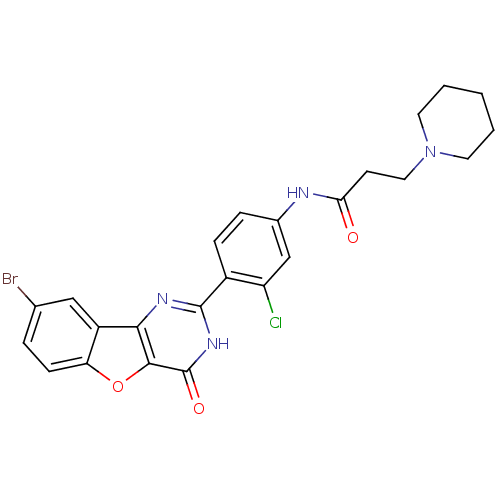
(CHEMBL2035629)Show SMILES Clc1cc(NC(=O)CCN2CCCCC2)ccc1-c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 Show InChI InChI=1S/C24H22BrClN4O3/c25-14-4-7-19-17(12-14)21-22(33-19)24(32)29-23(28-21)16-6-5-15(13-18(16)26)27-20(31)8-11-30-9-2-1-3-10-30/h4-7,12-13H,1-3,8-11H2,(H,27,31)(H,28,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394809
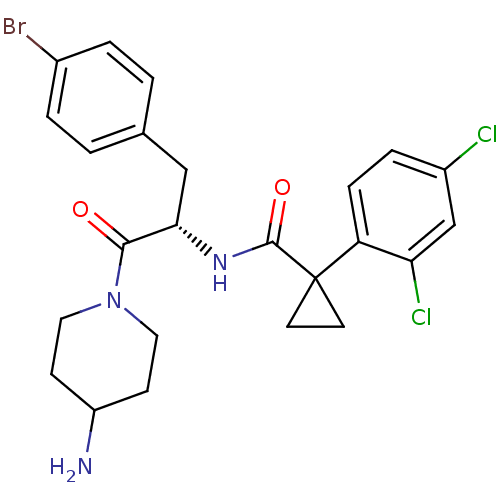
(CHEMBL2163823)Show SMILES NC1CCN(CC1)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H26BrCl2N3O2/c25-16-3-1-15(2-4-16)13-21(22(31)30-11-7-18(28)8-12-30)29-23(32)24(9-10-24)19-6-5-17(26)14-20(19)27/h1-6,14,18,21H,7-13,28H2,(H,29,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394806

(CHEMBL2163826)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H28BrCl2N3O2/c27-16-3-1-15(2-4-16)11-23(24(33)32-19-6-7-20(32)14-18(30)13-19)31-25(34)26(9-10-26)21-8-5-17(28)12-22(21)29/h1-5,8,12,18-20,23H,6-7,9-11,13-14,30H2,(H,31,34)/t18?,19-,20+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394805
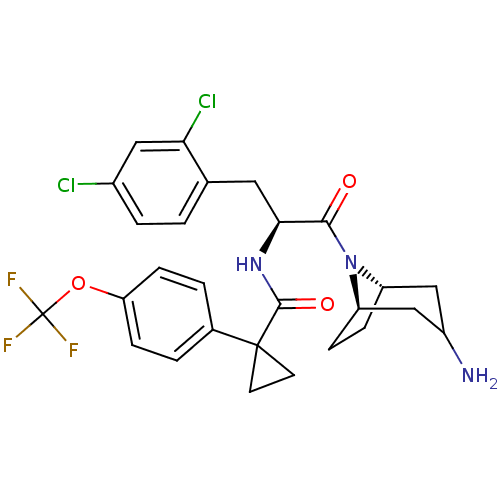
(CHEMBL2163827)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(OC(F)(F)F)cc1 |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C27H28Cl2F3N3O3/c28-17-4-1-15(22(29)12-17)11-23(24(36)35-19-5-6-20(35)14-18(33)13-19)34-25(37)26(9-10-26)16-2-7-21(8-3-16)38-27(30,31)32/h1-4,7-8,12,18-20,23H,5-6,9-11,13-14,33H2,(H,34,37)/t18?,19-,20+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394816
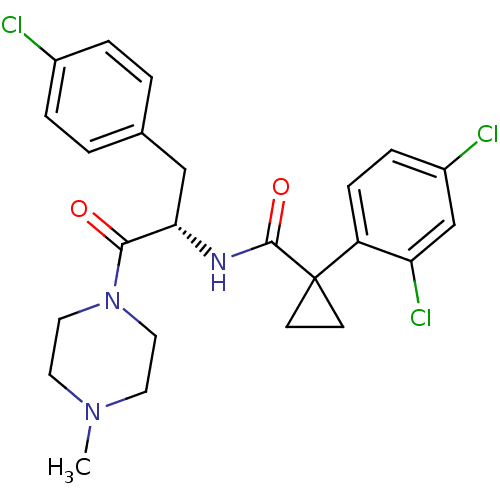
(CHEMBL2163833)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H26Cl3N3O2/c1-29-10-12-30(13-11-29)22(31)21(14-16-2-4-17(25)5-3-16)28-23(32)24(8-9-24)19-7-6-18(26)15-20(19)27/h2-7,15,21H,8-14H2,1H3,(H,28,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383714
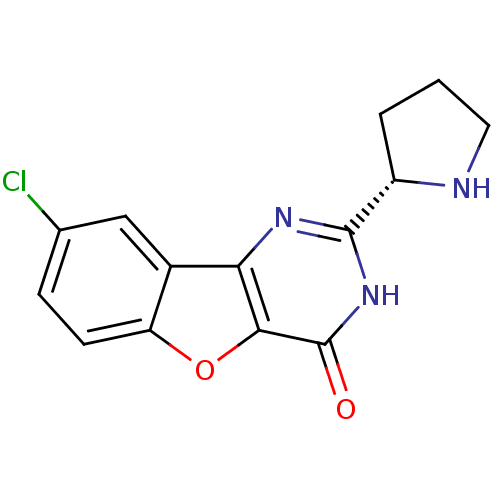
(CHEMBL2030402)Show SMILES Clc1ccc2oc3c(nc([nH]c3=O)[C@@H]3CCCN3)c2c1 |r| Show InChI InChI=1S/C14H12ClN3O2/c15-7-3-4-10-8(6-7)11-12(20-10)14(19)18-13(17-11)9-2-1-5-16-9/h3-4,6,9,16H,1-2,5H2,(H,17,18,19)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Son of sevenless homolog 1 [564-1049]
() | BDBM525146

(US11168102, Example 32-31.)Show SMILES CO[C@@]1(CCN([C@@H](C)C1)C(C)=O)c1cc2c(N[C@H](C)c3cccc(C(F)F)c3F)ncnc2n(C)c1=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21V5J3D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50310998

(CHEMBL1077458 | N-(4-(2-(4-morpholinophenylamino)p...)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 Show InChI InChI=1S/C22H23N5O2/c1-16(28)24-18-4-2-17(3-5-18)21-10-11-23-22(26-21)25-19-6-8-20(9-7-19)27-12-14-29-15-13-27/h2-11H,12-15H2,1H3,(H,24,28)(H,23,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383736
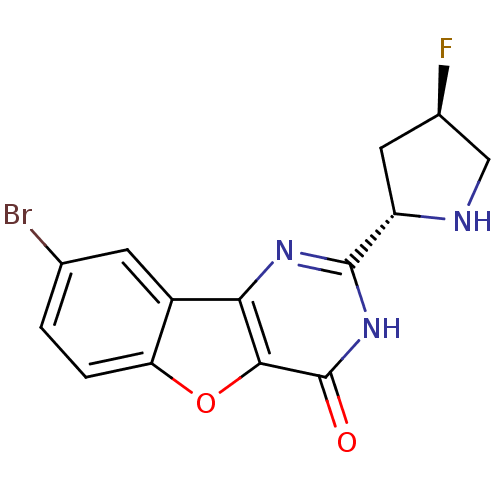
(CHEMBL2030400)Show SMILES F[C@H]1CN[C@@H](C1)c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 |r| Show InChI InChI=1S/C14H11BrFN3O2/c15-6-1-2-10-8(3-6)11-12(21-10)14(20)19-13(18-11)9-4-7(16)5-17-9/h1-3,7,9,17H,4-5H2,(H,18,19,20)/t7-,9+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383727
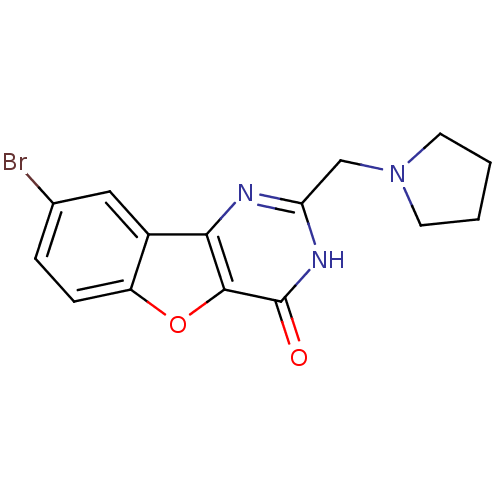
(CHEMBL2030389)Show InChI InChI=1S/C15H14BrN3O2/c16-9-3-4-11-10(7-9)13-14(21-11)15(20)18-12(17-13)8-19-5-1-2-6-19/h3-4,7H,1-2,5-6,8H2,(H,17,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50383711
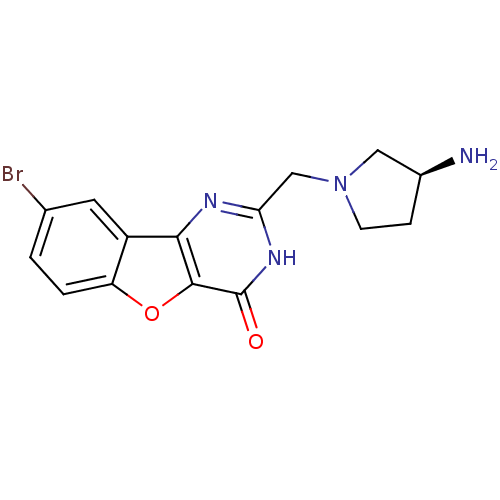
(CHEMBL2030387)Show SMILES N[C@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H15BrN4O2/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)19-12(18-13)7-20-4-3-9(17)6-20/h1-2,5,9H,3-4,6-7,17H2,(H,18,19,21)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by chemiluminescence assay |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383740
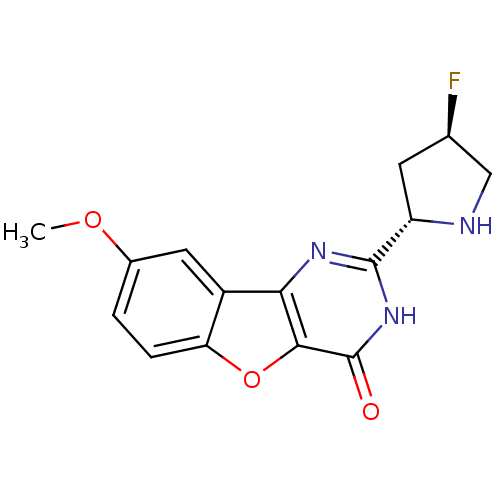
(CHEMBL2030405)Show SMILES COc1ccc2oc3c(nc([nH]c3=O)[C@@H]3C[C@@H](F)CN3)c2c1 |r| Show InChI InChI=1S/C15H14FN3O3/c1-21-8-2-3-11-9(5-8)12-13(22-11)15(20)19-14(18-12)10-4-7(16)6-17-10/h2-3,5,7,10,17H,4,6H2,1H3,(H,18,19,20)/t7-,10+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50383711

(CHEMBL2030387)Show SMILES N[C@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H15BrN4O2/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)19-12(18-13)7-20-4-3-9(17)6-20/h1-2,5,9H,3-4,6-7,17H2,(H,18,19,21)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385076

(CHEMBL2035627)Show SMILES Clc1cc(ccc1-c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1)C(=O)NCCN1CCCCC1 Show InChI InChI=1S/C24H22BrClN4O3/c25-15-5-7-19-17(13-15)20-21(33-19)24(32)29-22(28-20)16-6-4-14(12-18(16)26)23(31)27-8-11-30-9-2-1-3-10-30/h4-7,12-13H,1-3,8-11H2,(H,27,31)(H,28,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383739
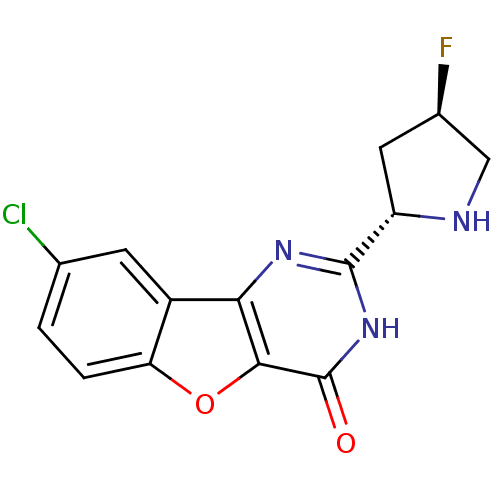
(CHEMBL2030404)Show SMILES F[C@H]1CN[C@@H](C1)c1nc2c3cc(Cl)ccc3oc2c(=O)[nH]1 |r| Show InChI InChI=1S/C14H11ClFN3O2/c15-6-1-2-10-8(3-6)11-12(21-10)14(20)19-13(18-11)9-4-7(16)5-17-9/h1-3,7,9,17H,4-5H2,(H,18,19,20)/t7-,9+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50383725
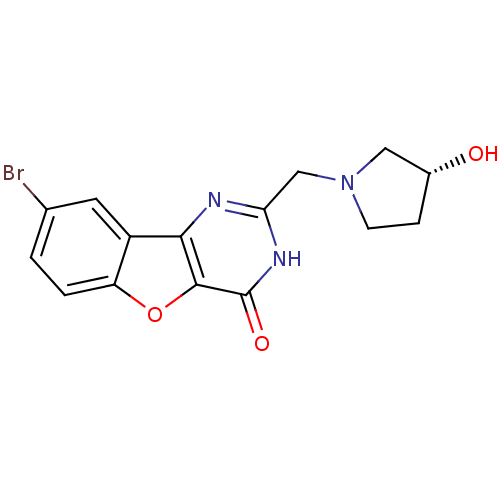
(CHEMBL2030386)Show SMILES O[C@@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H14BrN3O3/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)18-12(17-13)7-19-4-3-9(20)6-19/h1-2,5,9,20H,3-4,6-7H2,(H,17,18,21)/t9-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM3 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385077

(CHEMBL2035628)Show SMILES Clc1cc(CNC2CCNCC2)ccc1-c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 Show InChI InChI=1S/C22H20BrClN4O2/c23-13-2-4-18-16(10-13)19-20(30-18)22(29)28-21(27-19)15-3-1-12(9-17(15)24)11-26-14-5-7-25-8-6-14/h1-4,9-10,14,25-26H,5-8,11H2,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394817
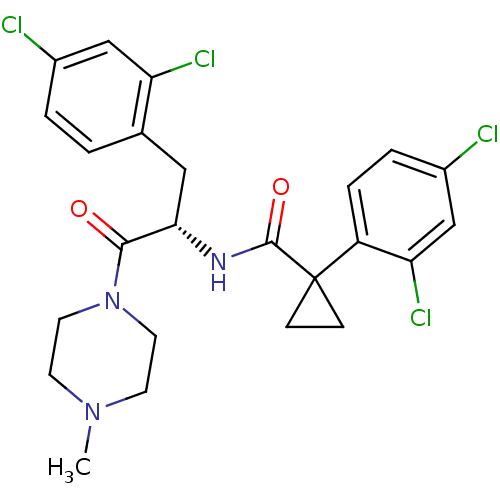
(CHEMBL2163832)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H25Cl4N3O2/c1-30-8-10-31(11-9-30)22(32)21(12-15-2-3-16(25)13-19(15)27)29-23(33)24(6-7-24)18-5-4-17(26)14-20(18)28/h2-5,13-14,21H,6-12H2,1H3,(H,29,33)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394807

(CHEMBL2163825)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H27Cl4N3O2/c27-15-2-1-14(21(29)10-15)9-23(24(34)33-18-4-5-19(33)13-17(31)12-18)32-25(35)26(7-8-26)20-6-3-16(28)11-22(20)30/h1-3,6,10-11,17-19,23H,4-5,7-9,12-13,31H2,(H,32,35)/t17?,18-,19+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394811
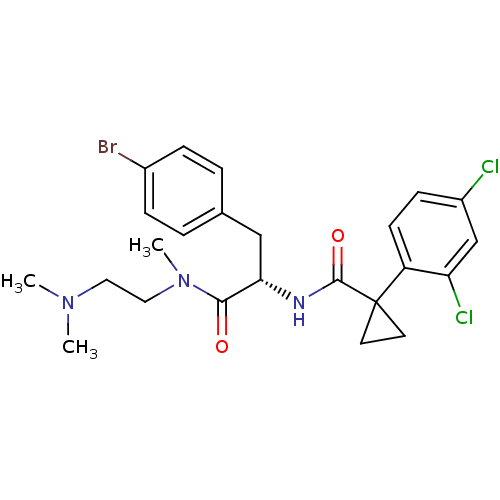
(CHEMBL2163839)Show SMILES CN(C)CCN(C)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H28BrCl2N3O2/c1-29(2)12-13-30(3)22(31)21(14-16-4-6-17(25)7-5-16)28-23(32)24(10-11-24)19-9-8-18(26)15-20(19)27/h4-9,15,21H,10-14H2,1-3H3,(H,28,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402416

(CHEMBL2208025)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2cccc(c2)N2CCOCC2)n1 Show InChI InChI=1S/C22H23N5O2/c1-16(28)24-18-7-5-17(6-8-18)21-9-10-23-22(26-21)25-19-3-2-4-20(15-19)27-11-13-29-14-12-27/h2-10,15H,11-14H2,1H3,(H,24,28)(H,23,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402424

(CHEMBL2208027)Show SMILES CC(=O)Nc1ccc(cc1)-c1nc(Nc2ccc(cc2)N2CCOCC2)ncc1F Show InChI InChI=1S/C22H22FN5O2/c1-15(29)25-17-4-2-16(3-5-17)21-20(23)14-24-22(27-21)26-18-6-8-19(9-7-18)28-10-12-30-13-11-28/h2-9,14H,10-13H2,1H3,(H,25,29)(H,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Son of sevenless homolog 1 [564-1049]
() | BDBM525147

(US11168102, Example 32-32.)Show SMILES COC1(CCN(CC1)C(C)=O)c1cc2c(N[C@H](C)c3cccc(C(F)F)c3F)nc(C)nc2n(C)c1=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q21V5J3D |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50391805
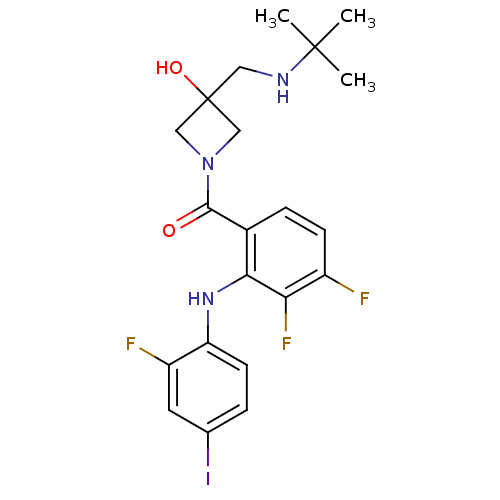
(CHEMBL2146894)Show SMILES CC(C)(C)NCC1(O)CN(C1)C(=O)c1ccc(F)c(F)c1Nc1ccc(I)cc1F Show InChI InChI=1S/C21H23F3IN3O2/c1-20(2,3)26-9-21(30)10-28(11-21)19(29)13-5-6-14(22)17(24)18(13)27-16-7-4-12(25)8-15(16)23/h4-8,26-27,30H,9-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK1-mediated ERK2 T202/Y204 phosphorylation using biotinylated MBP as substrate preincubated for 30 mins measured after 100 mins by cR... |
ACS Med Chem Lett 3: 416-421 (2012)
Article DOI: 10.1021/ml300049d
BindingDB Entry DOI: 10.7270/Q2GT5P80 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383730
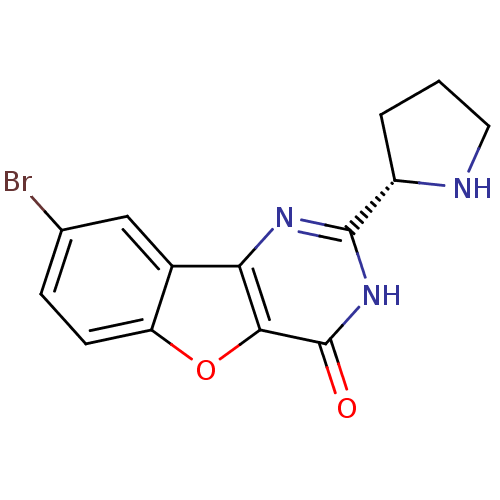
(CHEMBL2030393)Show SMILES Brc1ccc2oc3c(nc([nH]c3=O)[C@@H]3CCCN3)c2c1 |r| Show InChI InChI=1S/C14H12BrN3O2/c15-7-3-4-10-8(6-7)11-12(20-10)14(19)18-13(17-11)9-2-1-5-16-9/h3-4,6,9,16H,1-2,5H2,(H,17,18,19)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50383725

(CHEMBL2030386)Show SMILES O[C@@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H14BrN3O3/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)18-12(17-13)7-19-4-3-9(20)6-19/h1-2,5,9,20H,3-4,6-7H2,(H,17,18,21)/t9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50385078

(CHEMBL2035629)Show SMILES Clc1cc(NC(=O)CCN2CCCCC2)ccc1-c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 Show InChI InChI=1S/C24H22BrClN4O3/c25-14-4-7-19-17(12-14)21-22(33-19)24(32)29-23(28-21)16-6-5-15(13-18(16)26)27-20(31)8-11-30-9-2-1-3-10-30/h4-7,12-13H,1-3,8-11H2,(H,27,31)(H,28,29,32) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM3 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
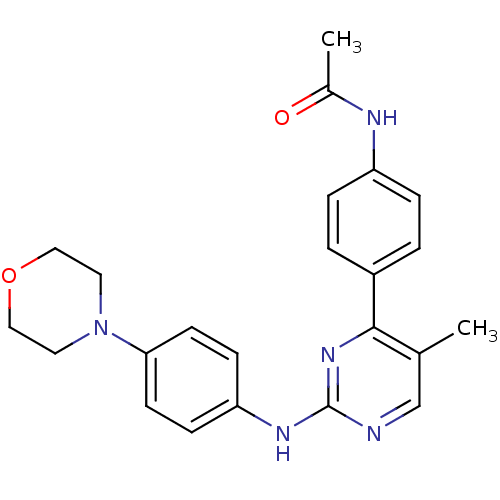
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402415
(CHEMBL2208028)Show SMILES CC(=O)Nc1ccc(cc1)-c1nc(Nc2ccc(cc2)N2CCOCC2)ncc1C Show InChI InChI=1S/C23H25N5O2/c1-16-15-24-23(27-22(16)18-3-5-19(6-4-18)25-17(2)29)26-20-7-9-21(10-8-20)28-11-13-30-14-12-28/h3-10,15H,11-14H2,1-2H3,(H,25,29)(H,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50391811

(CHEMBL2146895)Show SMILES CN(C)CC1(O)CN(C1)C(=O)c1ccc(F)c(F)c1Nc1ccc(I)cc1F Show InChI InChI=1S/C19H19F3IN3O2/c1-25(2)8-19(28)9-26(10-19)18(27)12-4-5-13(20)16(22)17(12)24-15-6-3-11(23)7-14(15)21/h3-7,24,28H,8-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK1-mediated ERK2 T202/Y204 phosphorylation using biotinylated MBP as substrate preincubated for 30 mins measured after 100 mins by cR... |
ACS Med Chem Lett 3: 416-421 (2012)
Article DOI: 10.1021/ml300049d
BindingDB Entry DOI: 10.7270/Q2GT5P80 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50391800

(CHEMBL2146898)Show SMILES C[C@H](N)C1(O)CN(C1)C(=O)c1ccc(F)c(F)c1Nc1ccc(I)cc1F |r| Show InChI InChI=1S/C18H17F3IN3O2/c1-9(23)18(27)7-25(8-18)17(26)11-3-4-12(19)15(21)16(11)24-14-5-2-10(22)6-13(14)20/h2-6,9,24,27H,7-8,23H2,1H3/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK1-mediated ERK2 T202/Y204 phosphorylation using biotinylated MBP as substrate preincubated for 30 mins measured after 100 mins by cR... |
ACS Med Chem Lett 3: 416-421 (2012)
Article DOI: 10.1021/ml300049d
BindingDB Entry DOI: 10.7270/Q2GT5P80 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402411

(CHEMBL2207759)Show SMILES CN1CCC[C@@H]1C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C26H30N6O2/c1-31-14-2-3-24(31)25(33)28-20-6-4-19(5-7-20)23-12-13-27-26(30-23)29-21-8-10-22(11-9-21)32-15-17-34-18-16-32/h4-13,24H,2-3,14-18H2,1H3,(H,28,33)(H,27,29,30)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383725

(CHEMBL2030386)Show SMILES O[C@@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H14BrN3O3/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)18-12(17-13)7-19-4-3-9(20)6-19/h1-2,5,9,20H,3-4,6-7H2,(H,17,18,21)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402420

(CHEMBL2207758)Show SMILES O=C(Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)[C@H]1CCNC1 |r| Show InChI InChI=1S/C25H28N6O2/c32-24(19-9-11-26-17-19)28-20-3-1-18(2-4-20)23-10-12-27-25(30-23)29-21-5-7-22(8-6-21)31-13-15-33-16-14-31/h1-8,10,12,19,26H,9,11,13-17H2,(H,28,32)(H,27,29,30)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data