 Found 260 hits with Last Name = 'kopcho' and Initial = 'lm'
Found 260 hits with Last Name = 'kopcho' and Initial = 'lm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119489
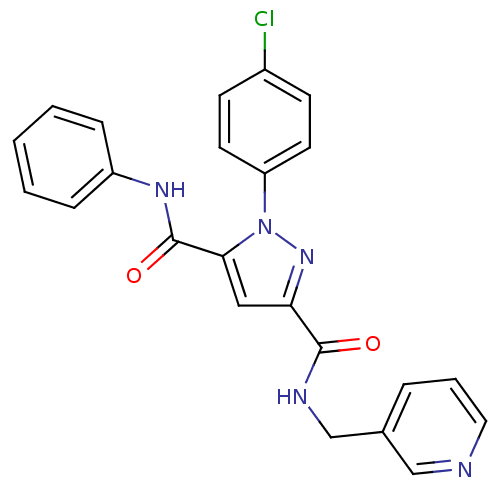
(1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...)Show SMILES Clc1ccc(cc1)-n1nc(cc1C(=O)Nc1ccccc1)C(=O)NCc1cccnc1 Show InChI InChI=1S/C23H18ClN5O2/c24-17-8-10-19(11-9-17)29-21(23(31)27-18-6-2-1-3-7-18)13-20(28-29)22(30)26-15-16-5-4-12-25-14-16/h1-14H,15H2,(H,26,30)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119488
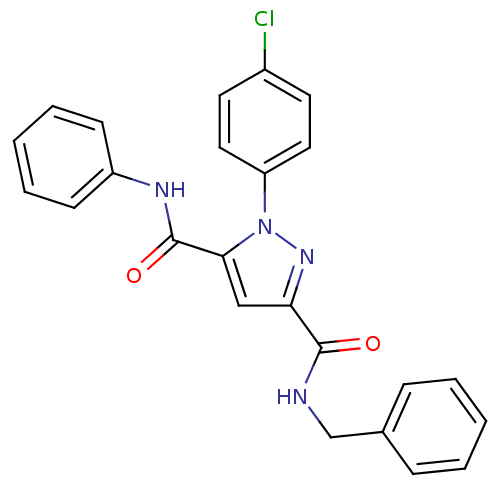
(CHEMBL143628 | X1-(4-Chloro-phenyl)-1H-pyrazole-3,...)Show SMILES Clc1ccc(cc1)-n1nc(cc1C(=O)Nc1ccccc1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H19ClN4O2/c25-18-11-13-20(14-12-18)29-22(24(31)27-19-9-5-2-6-10-19)15-21(28-29)23(30)26-16-17-7-3-1-4-8-17/h1-15H,16H2,(H,26,30)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119481

(1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...)Show SMILES NC(=O)c1ccc(NC(=O)c2cc(nn2-c2ccc(Cl)cc2)C(=O)NCc2ccccc2)cc1 Show InChI InChI=1S/C25H20ClN5O3/c26-18-8-12-20(13-9-18)31-22(25(34)29-19-10-6-17(7-11-19)23(27)32)14-21(30-31)24(33)28-15-16-4-2-1-3-5-16/h1-14H,15H2,(H2,27,32)(H,28,33)(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119483

(1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...)Show SMILES Clc1ccc(cc1)-n1nc(cc1C(=O)Nc1ccc(cc1)N1CCCCC1)C(=O)NCc1cccnc1 Show InChI InChI=1S/C28H27ClN6O2/c29-21-6-10-24(11-7-21)35-26(17-25(33-35)27(36)31-19-20-5-4-14-30-18-20)28(37)32-22-8-12-23(13-9-22)34-15-2-1-3-16-34/h4-14,17-18H,1-3,15-16,19H2,(H,31,36)(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119485
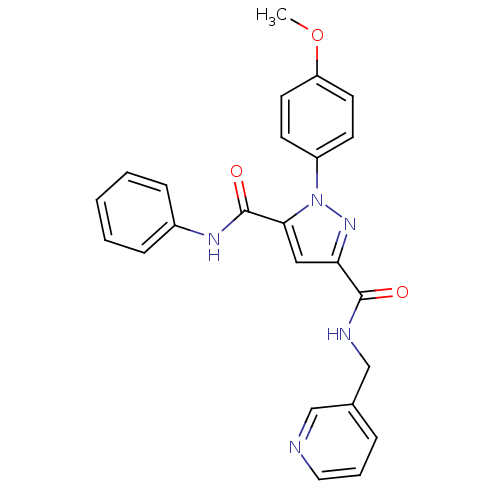
(1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Nc1ccccc1)C(=O)NCc1cccnc1 Show InChI InChI=1S/C24H21N5O3/c1-32-20-11-9-19(10-12-20)29-22(24(31)27-18-7-3-2-4-8-18)14-21(28-29)23(30)26-16-17-6-5-13-25-15-17/h2-15H,16H2,1H3,(H,26,30)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119490

(1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Nc1ccc(cc1)C(=O)N1CCCC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C30H29N5O4/c1-39-25-15-13-24(14-16-25)35-27(19-26(33-35)28(36)31-20-21-7-3-2-4-8-21)29(37)32-23-11-9-22(10-12-23)30(38)34-17-5-6-18-34/h2-4,7-16,19H,5-6,17-18,20H2,1H3,(H,31,36)(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119492

(1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...)Show SMILES Cc1ccc(CNC(=O)c2cc(C(=O)Nc3ccccc3)n(n2)-c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C25H21ClN4O2/c1-17-7-9-18(10-8-17)16-27-24(31)22-15-23(25(32)28-20-5-3-2-4-6-20)30(29-22)21-13-11-19(26)12-14-21/h2-15H,16H2,1H3,(H,27,31)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119487
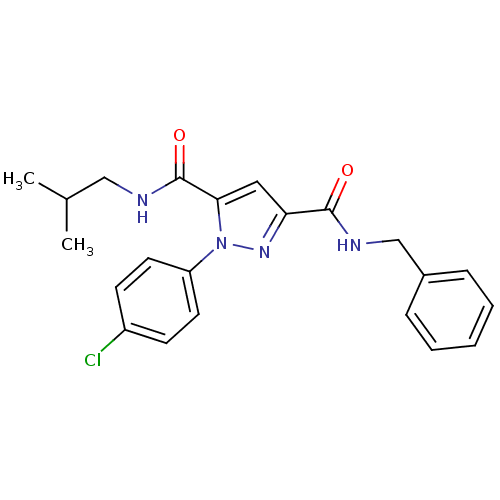
(1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...)Show SMILES CC(C)CNC(=O)c1cc(nn1-c1ccc(Cl)cc1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C22H23ClN4O2/c1-15(2)13-24-22(29)20-12-19(21(28)25-14-16-6-4-3-5-7-16)26-27(20)18-10-8-17(23)9-11-18/h3-12,15H,13-14H2,1-2H3,(H,24,29)(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119491
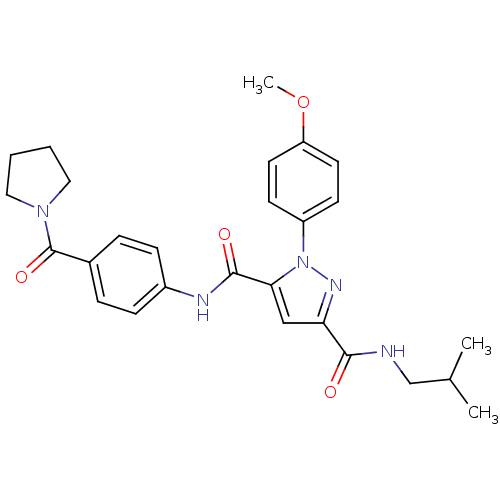
(1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Nc1ccc(cc1)C(=O)N1CCCC1)C(=O)NCC(C)C Show InChI InChI=1S/C27H31N5O4/c1-18(2)17-28-25(33)23-16-24(32(30-23)21-10-12-22(36-3)13-11-21)26(34)29-20-8-6-19(7-9-20)27(35)31-14-4-5-15-31/h6-13,16,18H,4-5,14-15,17H2,1-3H3,(H,28,33)(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119484

(1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Nc1ccc(cc1)C(=O)N1CCCC1)C(=O)NCC1CCCO1 Show InChI InChI=1S/C28H31N5O5/c1-37-22-12-10-21(11-13-22)33-25(17-24(31-33)26(34)29-18-23-5-4-16-38-23)27(35)30-20-8-6-19(7-9-20)28(36)32-14-2-3-15-32/h6-13,17,23H,2-5,14-16,18H2,1H3,(H,29,34)(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119482
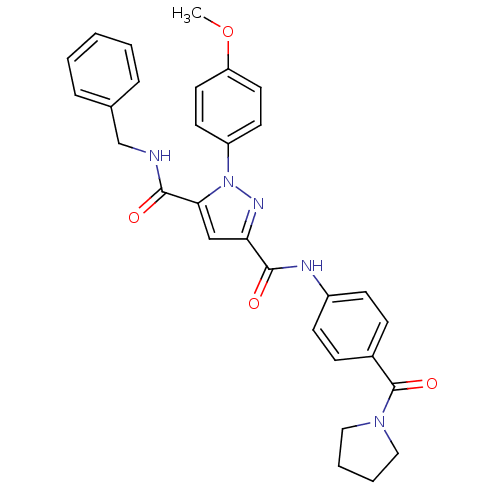
(1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)NCc1ccccc1)C(=O)Nc1ccc(cc1)C(=O)N1CCCC1 Show InChI InChI=1S/C30H29N5O4/c1-39-25-15-13-24(14-16-25)35-27(29(37)31-20-21-7-3-2-4-8-21)19-26(33-35)28(36)32-23-11-9-22(10-12-23)30(38)34-17-5-6-18-34/h2-4,7-16,19H,5-6,17-18,20H2,1H3,(H,31,37)(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119486
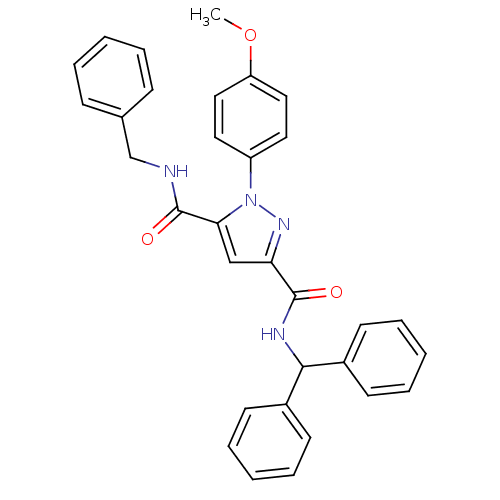
(1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)NCc1ccccc1)C(=O)NC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H28N4O3/c1-39-27-19-17-26(18-20-27)36-29(32(38)33-22-23-11-5-2-6-12-23)21-28(35-36)31(37)34-30(24-13-7-3-8-14-24)25-15-9-4-10-16-25/h2-21,30H,22H2,1H3,(H,33,38)(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of dihydroorotate dehydrogenase (DHODase) of Helicobacter pylori |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119485

(1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Nc1ccccc1)C(=O)NCc1cccnc1 Show InChI InChI=1S/C24H21N5O3/c1-32-20-11-9-19(10-12-20)29-22(24(31)27-18-7-3-2-4-8-18)14-21(28-29)23(30)26-16-17-6-5-13-25-15-17/h2-15H,16H2,1H3,(H,26,30)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human dihydroorotate dehydrogenase (DHODase) |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119484

(1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Nc1ccc(cc1)C(=O)N1CCCC1)C(=O)NCC1CCCO1 Show InChI InChI=1S/C28H31N5O5/c1-37-22-12-10-21(11-13-22)33-25(17-24(31-33)26(34)29-18-23-5-4-16-38-23)27(35)30-20-8-6-19(7-9-20)28(36)32-14-2-3-15-32/h6-13,17,23H,2-5,14-16,18H2,1H3,(H,29,34)(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human dihydroorotate dehydrogenase (DHODase) |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119486

(1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)NCc1ccccc1)C(=O)NC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H28N4O3/c1-39-27-19-17-26(18-20-27)36-29(32(38)33-22-23-11-5-2-6-12-23)21-28(35-36)31(37)34-30(24-13-7-3-8-14-24)25-15-9-4-10-16-25/h2-21,30H,22H2,1H3,(H,33,38)(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human dihydroorotate dehydrogenase (DHODase) |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119491

(1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Nc1ccc(cc1)C(=O)N1CCCC1)C(=O)NCC(C)C Show InChI InChI=1S/C27H31N5O4/c1-18(2)17-28-25(33)23-16-24(32(30-23)21-10-12-22(36-3)13-11-21)26(34)29-20-8-6-19(7-9-20)27(35)31-14-4-5-15-31/h6-13,16,18H,4-5,14-15,17H2,1-3H3,(H,28,33)(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human dihydroorotate dehydrogenase (DHODase) |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119487

(1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...)Show SMILES CC(C)CNC(=O)c1cc(nn1-c1ccc(Cl)cc1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C22H23ClN4O2/c1-15(2)13-24-22(29)20-12-19(21(28)25-14-16-6-4-3-5-7-16)26-27(20)18-10-8-17(23)9-11-18/h3-12,15H,13-14H2,1-2H3,(H,24,29)(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human dihydroorotate dehydrogenase (DHODase) |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119490

(1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Nc1ccc(cc1)C(=O)N1CCCC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C30H29N5O4/c1-39-25-15-13-24(14-16-25)35-27(19-26(33-35)28(36)31-20-21-7-3-2-4-8-21)29(37)32-23-11-9-22(10-12-23)30(38)34-17-5-6-18-34/h2-4,7-16,19H,5-6,17-18,20H2,1H3,(H,31,36)(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human dihydroorotate dehydrogenase (DHODase) |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119489

(1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...)Show SMILES Clc1ccc(cc1)-n1nc(cc1C(=O)Nc1ccccc1)C(=O)NCc1cccnc1 Show InChI InChI=1S/C23H18ClN5O2/c24-17-8-10-19(11-9-17)29-21(23(31)27-18-6-2-1-3-7-18)13-20(28-29)22(30)26-15-16-5-4-12-25-14-16/h1-14H,15H2,(H,26,30)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human dihydroorotate dehydrogenase (DHODase) |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119488

(CHEMBL143628 | X1-(4-Chloro-phenyl)-1H-pyrazole-3,...)Show SMILES Clc1ccc(cc1)-n1nc(cc1C(=O)Nc1ccccc1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H19ClN4O2/c25-18-11-13-20(14-12-18)29-22(24(31)27-19-9-5-2-6-10-19)15-21(28-29)23(30)26-16-17-7-3-1-4-8-17/h1-15H,16H2,(H,26,30)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human dihydroorotate dehydrogenase (DHODase) |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119483

(1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...)Show SMILES Clc1ccc(cc1)-n1nc(cc1C(=O)Nc1ccc(cc1)N1CCCCC1)C(=O)NCc1cccnc1 Show InChI InChI=1S/C28H27ClN6O2/c29-21-6-10-24(11-7-21)35-26(17-25(33-35)27(36)31-19-20-5-4-14-30-18-20)28(37)32-22-8-12-23(13-9-22)34-15-2-1-3-16-34/h4-14,17-18H,1-3,15-16,19H2,(H,31,36)(H,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human dihydroorotate dehydrogenase (DHODase) |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119492

(1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...)Show SMILES Cc1ccc(CNC(=O)c2cc(C(=O)Nc3ccccc3)n(n2)-c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C25H21ClN4O2/c1-17-7-9-18(10-8-17)16-27-24(31)22-15-23(25(32)28-20-5-3-2-4-6-20)30(29-22)21-13-11-19(26)12-14-21/h2-15H,16H2,1H3,(H,27,31)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human dihydroorotate dehydrogenase (DHODase) |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119481

(1-(4-Chloro-phenyl)-1H-pyrazole-3,5-dicarboxylic a...)Show SMILES NC(=O)c1ccc(NC(=O)c2cc(nn2-c2ccc(Cl)cc2)C(=O)NCc2ccccc2)cc1 Show InChI InChI=1S/C25H20ClN5O3/c26-18-8-12-20(13-9-18)31-22(25(34)29-19-10-6-17(7-11-19)23(27)32)14-21(30-31)24(33)28-15-16-4-2-1-3-5-16/h1-14H,15H2,(H2,27,32)(H,28,33)(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human dihydroorotate dehydrogenase (DHODase) |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50119482

(1-(4-Methoxy-phenyl)-1H-pyrazole-3,5-dicarboxylic ...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)NCc1ccccc1)C(=O)Nc1ccc(cc1)C(=O)N1CCCC1 Show InChI InChI=1S/C30H29N5O4/c1-39-25-15-13-24(14-16-25)35-27(29(37)31-20-21-7-3-2-4-8-21)19-26(33-35)28(36)32-23-11-9-22(10-12-23)30(38)34-17-5-6-18-34/h2-4,7-16,19H,5-6,17-18,20H2,1H3,(H,31,37)(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human dihydroorotate dehydrogenase (DHODase) |
J Med Chem 45: 4669-78 (2002)
BindingDB Entry DOI: 10.7270/Q2C24VSW |
More data for this
Ligand-Target Pair | |
Hepatic triacylglycerol lipase
(Homo sapiens (Human)) | BDBM50525871

(CHEMBL4460999)Show SMILES O=C(CNC(=O)C(C#N)c1nc2ccc(cc2s1)-c1ccc(NC(=O)OCc2ccccc2)cc1)NC1CC1 Show InChI InChI=1S/C29H25N5O4S/c30-15-23(27(36)31-16-26(35)32-21-11-12-21)28-34-24-13-8-20(14-25(24)39-28)19-6-9-22(10-7-19)33-29(37)38-17-18-4-2-1-3-5-18/h1-10,13-14,21,23H,11-12,16-17H2,(H,31,36)(H,32,35)(H,33,37) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of hepatic lipase (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126673
BindingDB Entry DOI: 10.7270/Q2DN48G0 |
More data for this
Ligand-Target Pair | |
Endothelial lipase
(Homo sapiens (Human)) | BDBM50525871

(CHEMBL4460999)Show SMILES O=C(CNC(=O)C(C#N)c1nc2ccc(cc2s1)-c1ccc(NC(=O)OCc2ccccc2)cc1)NC1CC1 Show InChI InChI=1S/C29H25N5O4S/c30-15-23(27(36)31-16-26(35)32-21-11-12-21)28-34-24-13-8-20(14-25(24)39-28)19-6-9-22(10-7-19)33-29(37)38-17-18-4-2-1-3-5-18/h1-10,13-14,21,23H,11-12,16-17H2,(H,31,36)(H,32,35)(H,33,37) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of endothelial lipase (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126673
BindingDB Entry DOI: 10.7270/Q2DN48G0 |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50554034
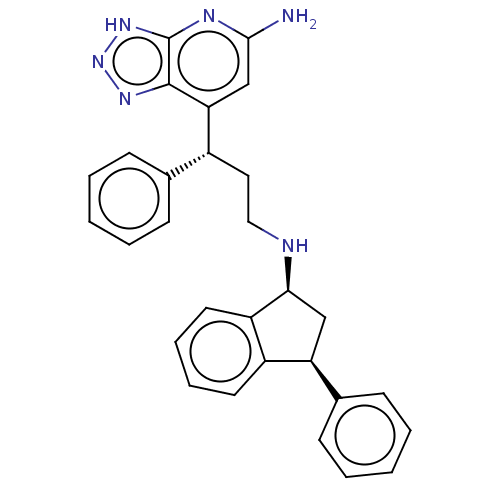
(CHEMBL4747269)Show SMILES Nc1cc([C@H](CCN[C@H]2C[C@H](c3ccccc23)c2ccccc2)c2ccccc2)c2nn[nH]c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115723
BindingDB Entry DOI: 10.7270/Q21Z4829 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50554035
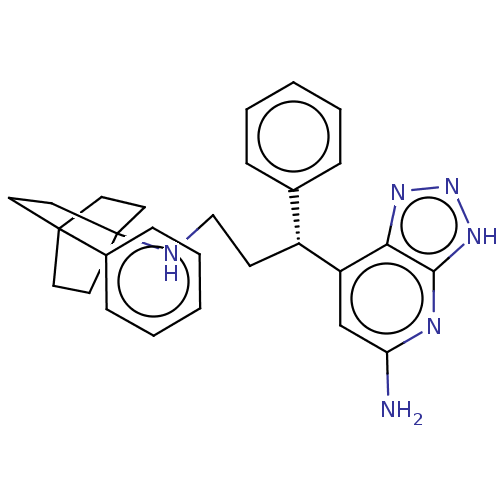
(CHEMBL4790231)Show SMILES Nc1cc([C@H](CCNC23CCC(CC2)(CC3)c2ccccc2)c2ccccc2)c2nn[nH]c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115723
BindingDB Entry DOI: 10.7270/Q21Z4829 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50554052
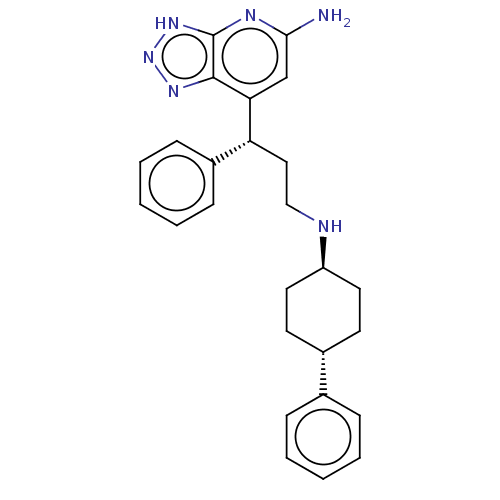
(CHEMBL4763667)Show SMILES Nc1cc([C@H](CCN[C@H]2CC[C@@H](CC2)c2ccccc2)c2ccccc2)c2nn[nH]c2n1 |r,wU:4.4,11.14,wD:8.7,(28.35,-39.89,;29.68,-39.12,;29.69,-37.58,;31.01,-36.8,;31,-35.26,;32.34,-34.49,;33.67,-35.25,;35.01,-34.48,;36.35,-35.25,;36.34,-36.78,;37.67,-37.55,;39.01,-36.78,;39.01,-35.24,;37.67,-34.47,;40.33,-37.55,;40.33,-39.1,;41.66,-39.87,;43,-39.1,;43,-37.55,;41.66,-36.78,;29.67,-34.5,;29.67,-32.96,;28.34,-32.19,;27,-32.97,;27.01,-34.52,;28.35,-35.27,;32.35,-37.57,;33.81,-37.09,;34.72,-38.34,;33.81,-39.59,;32.35,-39.11,;31.01,-39.89,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115723
BindingDB Entry DOI: 10.7270/Q21Z4829 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50554050
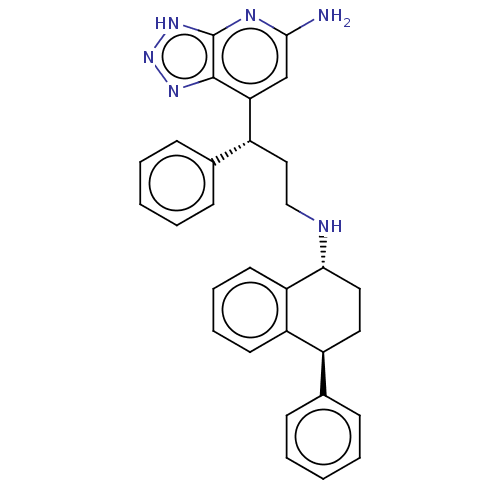
(CHEMBL4752248)Show SMILES Nc1cc([C@H](CCN[C@@H]2CC[C@@H](c3ccccc3)c3ccccc23)c2ccccc2)c2nn[nH]c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115723
BindingDB Entry DOI: 10.7270/Q21Z4829 |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50554057
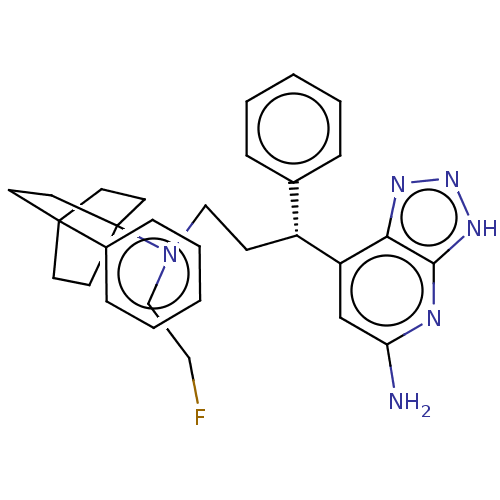
(CHEMBL4740830)Show SMILES Nc1cc([C@H](CCN(CCF)C23CCC(CC2)(CC3)c2ccccc2)c2ccccc2)c2nn[nH]c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115723
BindingDB Entry DOI: 10.7270/Q21Z4829 |
More data for this
Ligand-Target Pair | |
Endothelial lipase
(Homo sapiens (Human)) | BDBM50525879
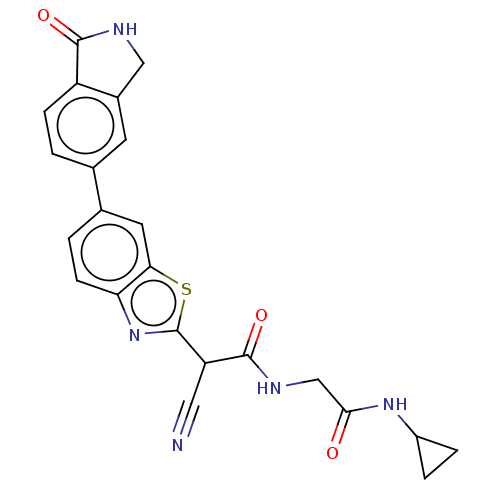
(CHEMBL4541404)Show SMILES O=C(CNC(=O)C(C#N)c1nc2ccc(cc2s1)-c1ccc2C(=O)NCc2c1)NC1CC1 Show InChI InChI=1S/C23H19N5O3S/c24-9-17(22(31)26-11-20(29)27-15-3-4-15)23-28-18-6-2-13(8-19(18)32-23)12-1-5-16-14(7-12)10-25-21(16)30/h1-2,5-8,15,17H,3-4,10-11H2,(H,25,30)(H,26,31)(H,27,29) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of endothelial lipase (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126673
BindingDB Entry DOI: 10.7270/Q2DN48G0 |
More data for this
Ligand-Target Pair | |
Hepatic triacylglycerol lipase
(Homo sapiens (Human)) | BDBM50525869

(CHEMBL4587475)Show SMILES O=C(CNC(=O)C(C#N)c1nc2ccc(cc2s1)-c1ccccc1)NC1CC1 Show InChI InChI=1S/C21H18N4O2S/c22-11-16(20(27)23-12-19(26)24-15-7-8-15)21-25-17-9-6-14(10-18(17)28-21)13-4-2-1-3-5-13/h1-6,9-10,15-16H,7-8,12H2,(H,23,27)(H,24,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of hepatic lipase (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126673
BindingDB Entry DOI: 10.7270/Q2DN48G0 |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50554056
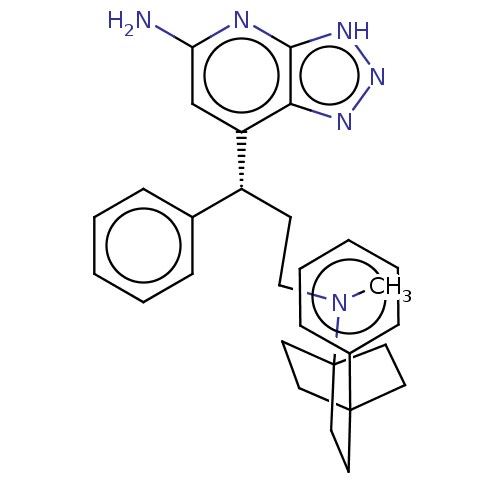
(CHEMBL4782307)Show SMILES CN(CC[C@H](c1ccccc1)c1cc(N)nc2[nH]nnc12)C12CCC(CC1)(CC2)c1ccccc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115723
BindingDB Entry DOI: 10.7270/Q21Z4829 |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50554054
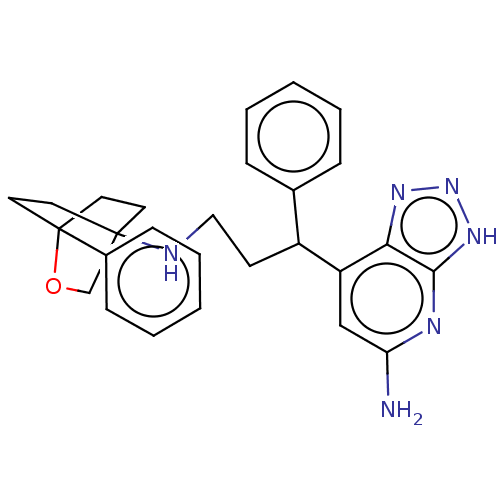
(CHEMBL4751166)Show SMILES Nc1cc(C(CCNC23CCC(CC2)(OC3)c2ccccc2)c2ccccc2)c2nn[nH]c2n1 |(4.37,-54.45,;5.71,-53.68,;5.71,-52.14,;7.04,-51.36,;7.04,-49.82,;8.37,-49.05,;9.71,-49.81,;11.04,-49.04,;12.38,-49.81,;12.38,-51.34,;13.7,-52.11,;15.05,-51.34,;13.82,-51.06,;13.7,-50.02,;15.04,-49.8,;13.7,-49.02,;16.37,-52.11,;16.37,-53.66,;17.71,-54.43,;19.04,-53.66,;19.04,-52.11,;17.71,-51.34,;5.7,-49.06,;5.7,-47.52,;4.36,-46.75,;3.03,-47.52,;3.04,-49.07,;4.38,-49.83,;8.38,-52.13,;9.85,-51.65,;10.76,-52.9,;9.85,-54.15,;8.38,-53.67,;7.04,-54.45,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115723
BindingDB Entry DOI: 10.7270/Q21Z4829 |
More data for this
Ligand-Target Pair | |
Endothelial lipase
(Homo sapiens (Human)) | BDBM50525869

(CHEMBL4587475)Show SMILES O=C(CNC(=O)C(C#N)c1nc2ccc(cc2s1)-c1ccccc1)NC1CC1 Show InChI InChI=1S/C21H18N4O2S/c22-11-16(20(27)23-12-19(26)24-15-7-8-15)21-25-17-9-6-14(10-18(17)28-21)13-4-2-1-3-5-13/h1-6,9-10,15-16H,7-8,12H2,(H,23,27)(H,24,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of endothelial lipase (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126673
BindingDB Entry DOI: 10.7270/Q2DN48G0 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50552364
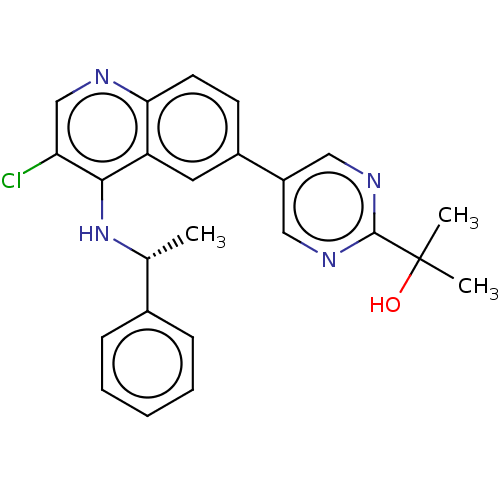
(CHEMBL4781606)Show SMILES C[C@@H](Nc1c(Cl)cnc2ccc(cc12)-c1cnc(nc1)C(C)(C)O)c1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent labeled probe from human recombinant His-tagged TNFalpha (77 to 233 residues) expressed in Escherichia coli preincubated ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01732
BindingDB Entry DOI: 10.7270/Q2S46WM7 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50552381

(CHEMBL4787433)Show SMILES C[C@@H](Nc1c(Cl)cnc2ccc(cc12)-c1ccc(nc1)S(C)(=O)=O)c1ccccc1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent labeled probe from human recombinant His-tagged TNFalpha (77 to 233 residues) expressed in Escherichia coli preincubated ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01732
BindingDB Entry DOI: 10.7270/Q2S46WM7 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50552380
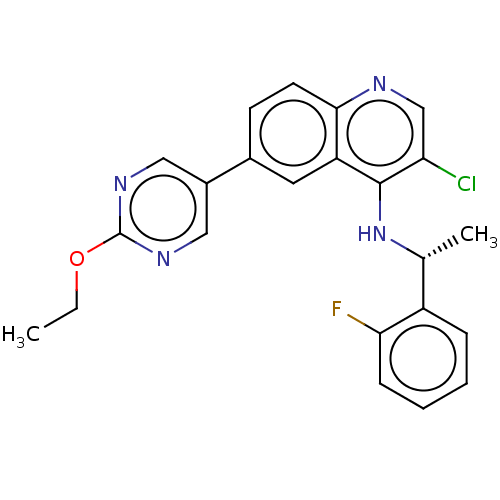
(CHEMBL4797699)Show SMILES CCOc1ncc(cn1)-c1ccc2ncc(Cl)c(N[C@H](C)c3ccccc3F)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent labeled probe from human recombinant His-tagged TNFalpha (77 to 233 residues) expressed in Escherichia coli preincubated ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01732
BindingDB Entry DOI: 10.7270/Q2S46WM7 |
More data for this
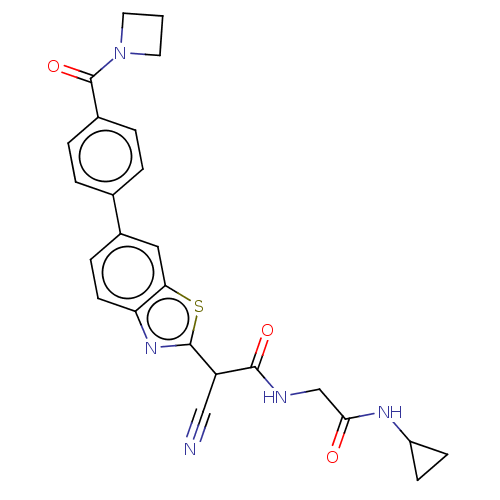
Ligand-Target Pair | |
Endothelial lipase
(Homo sapiens (Human)) | BDBM50525868
(CHEMBL4557646)Show SMILES O=C(CNC(=O)C(C#N)c1nc2ccc(cc2s1)-c1ccc(cc1)C(=O)N1CCC1)NC1CC1 Show InChI InChI=1S/C25H23N5O3S/c26-13-19(23(32)27-14-22(31)28-18-7-8-18)24-29-20-9-6-17(12-21(20)34-24)15-2-4-16(5-3-15)25(33)30-10-1-11-30/h2-6,9,12,18-19H,1,7-8,10-11,14H2,(H,27,32)(H,28,31) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of endothelial lipase (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126673
BindingDB Entry DOI: 10.7270/Q2DN48G0 |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50554051
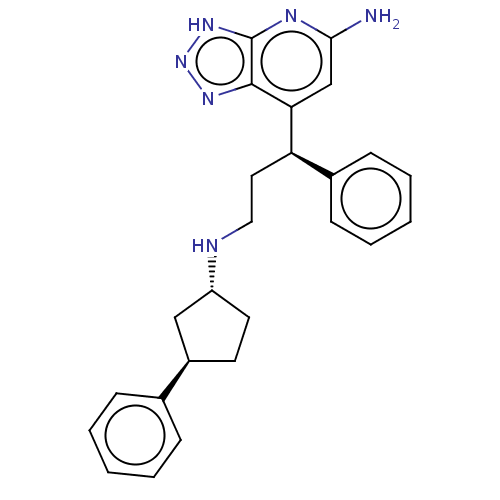
(CHEMBL4795827)Show SMILES Nc1cc([C@H](CCN[C@@H]2CC[C@H](C2)c2ccccc2)c2ccccc2)c2nn[nH]c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115723
BindingDB Entry DOI: 10.7270/Q21Z4829 |
More data for this
Ligand-Target Pair | |
Endothelial lipase
(Homo sapiens (Human)) | BDBM50525878
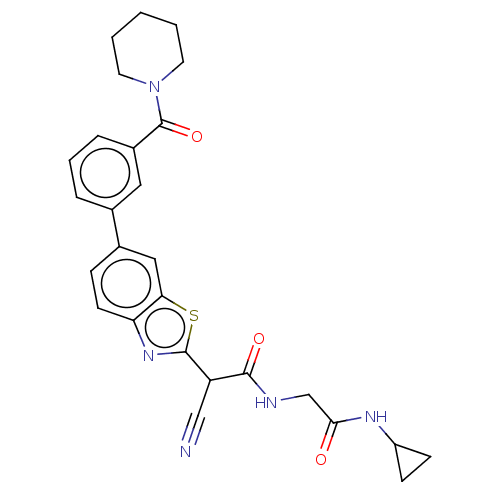
(CHEMBL4581408)Show SMILES O=C(CNC(=O)C(C#N)c1nc2ccc(cc2s1)-c1cccc(c1)C(=O)N1CCCCC1)NC1CC1 Show InChI InChI=1S/C27H27N5O3S/c28-15-21(25(34)29-16-24(33)30-20-8-9-20)26-31-22-10-7-18(14-23(22)36-26)17-5-4-6-19(13-17)27(35)32-11-2-1-3-12-32/h4-7,10,13-14,20-21H,1-3,8-9,11-12,16H2,(H,29,34)(H,30,33) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of endothelial lipase (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126673
BindingDB Entry DOI: 10.7270/Q2DN48G0 |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM357630
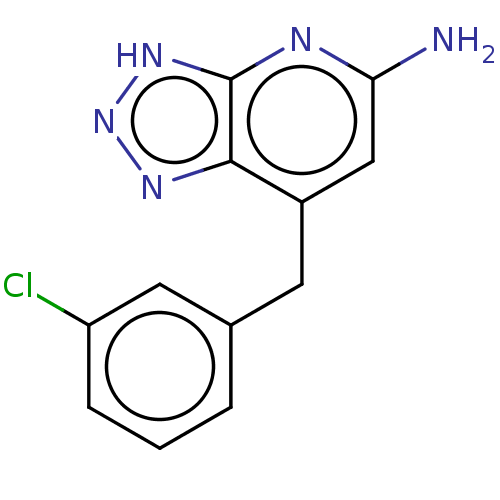
(7-(3- chlorobenzyl)- 3H- [1,2,3]triazolo [4,5-b]py...)Show InChI InChI=1S/C12H10ClN5/c13-9-3-1-2-7(5-9)4-8-6-10(14)15-12-11(8)16-18-17-12/h1-3,5-6H,4H2,(H3,14,15,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MPO incubated for 10 mins in presence of 240 mM NaCl and 10 uM H2O2 by aminophenyl fluorescein based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115723
BindingDB Entry DOI: 10.7270/Q21Z4829 |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50554036
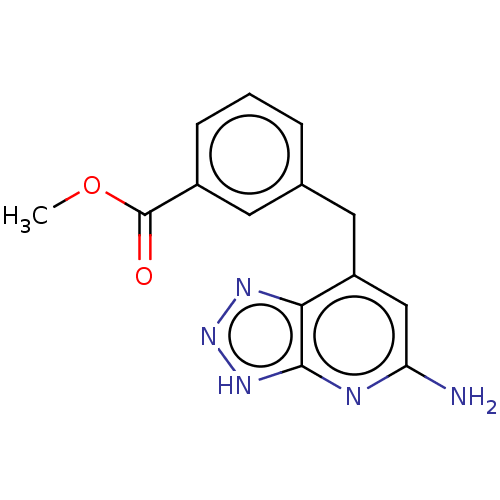
(CHEMBL4764237) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MPO incubated for 10 mins by amplex red dye based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115723
BindingDB Entry DOI: 10.7270/Q21Z4829 |
More data for this
Ligand-Target Pair | |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM357653
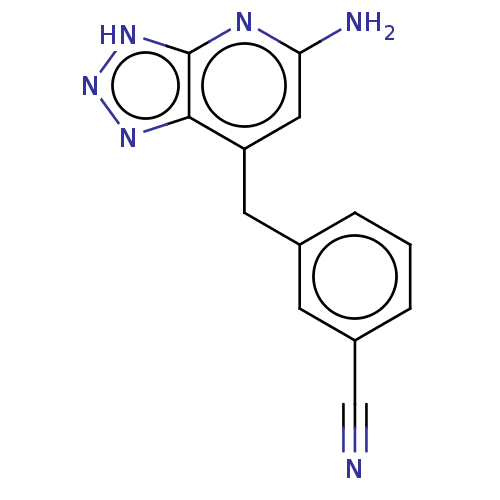
(3-((5-amino- 3H- [1,2,3]triazolo [4,5-b] pyridin- ...)Show InChI InChI=1S/C13H10N6/c14-7-9-3-1-2-8(4-9)5-10-6-11(15)16-13-12(10)17-19-18-13/h1-4,6H,5H2,(H3,15,16,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MPO incubated for 10 mins in presence of 240 mM NaCl and 10 uM H2O2 by aminophenyl fluorescein based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115723
BindingDB Entry DOI: 10.7270/Q21Z4829 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50552368

(CHEMBL4787001)Show SMILES C[C@@H](Nc1c(Cl)cnc2ccc(cc12)-c1cnc(nc1)C(C)(C)O)c1ccccc1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent labeled probe from human recombinant His-tagged TNFalpha (77 to 233 residues) expressed in Escherichia coli preincubated ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01732
BindingDB Entry DOI: 10.7270/Q2S46WM7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Myeloperoxidase
(Homo sapiens (Human)) | BDBM50554055
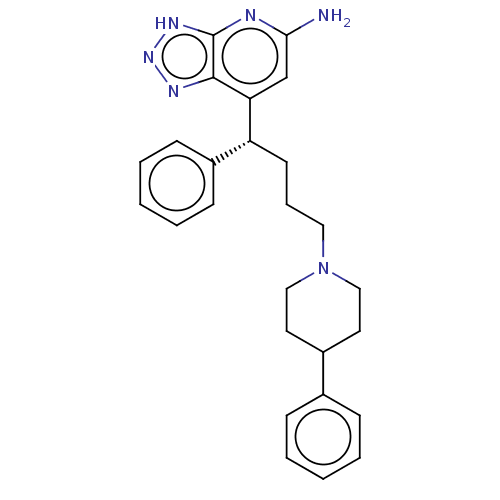
(CHEMBL4755252)Show SMILES Nc1cc([C@H](CCCN2CCC(CC2)c2ccccc2)c2ccccc2)c2nn[nH]c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115723
BindingDB Entry DOI: 10.7270/Q21Z4829 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50552388
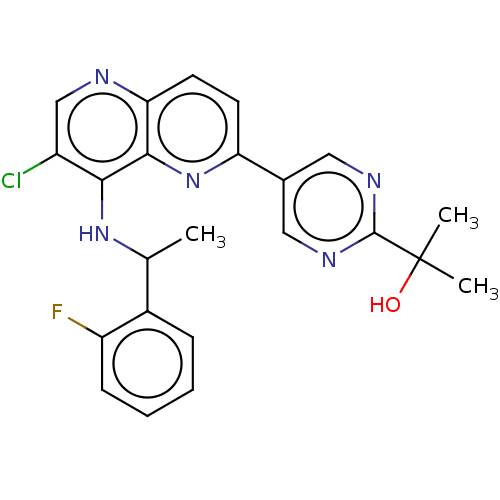
(CHEMBL4742065)Show SMILES CC(Nc1c(Cl)cnc2ccc(nc12)-c1cnc(nc1)C(C)(C)O)c1ccccc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent labeled probe from human recombinant His-tagged TNFalpha (77 to 233 residues) expressed in Escherichia coli preincubated ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01732
BindingDB Entry DOI: 10.7270/Q2S46WM7 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50552388

(CHEMBL4742065)Show SMILES CC(Nc1c(Cl)cnc2ccc(nc12)-c1cnc(nc1)C(C)(C)O)c1ccccc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent labeled probe from human recombinant His-tagged TNFalpha (77 to 233 residues) expressed in Escherichia coli preincubated ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01732
BindingDB Entry DOI: 10.7270/Q2S46WM7 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM50552388

(CHEMBL4742065)Show SMILES CC(Nc1c(Cl)cnc2ccc(nc12)-c1cnc(nc1)C(C)(C)O)c1ccccc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of fluorescent labeled probe from human recombinant His-tagged TNFalpha (77 to 233 residues) expressed in Escherichia coli preincubated ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01732
BindingDB Entry DOI: 10.7270/Q2S46WM7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data