

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50263773 (1-(2-naphthoyl)-3-((2R,3R,4S,5S,6R)-3,4,5-trihydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50295843 (4-methyl-N-((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50263768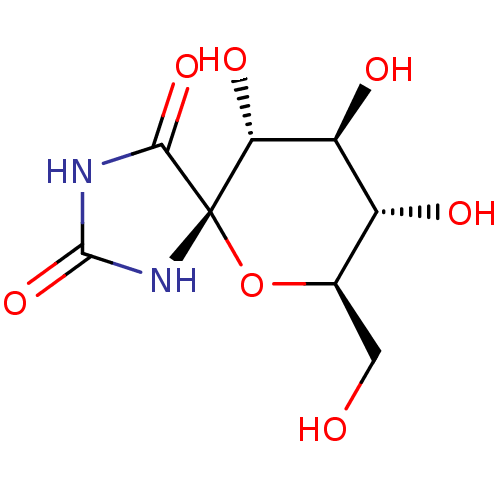 ((5S,7R,8S,9S,10R)-8,9,10-Trihydroxy-7-hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50363878 (CHEMBL1947268) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50295844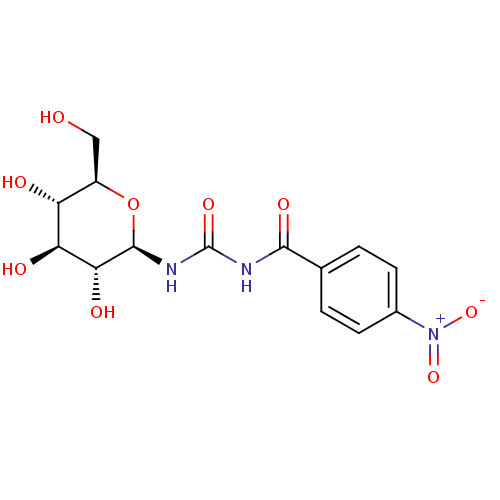 (4-nitro-N-((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50363876 (CHEMBL1232637) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153698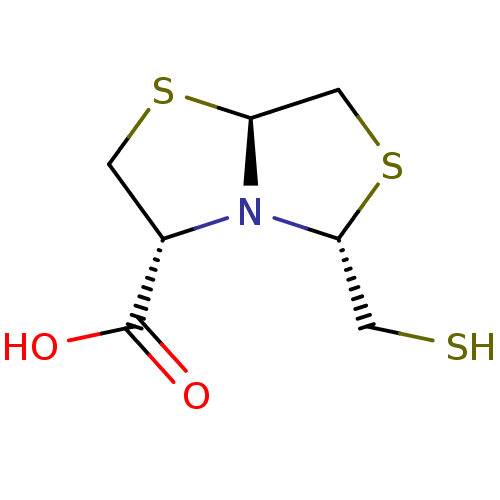 (L-CS319) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153700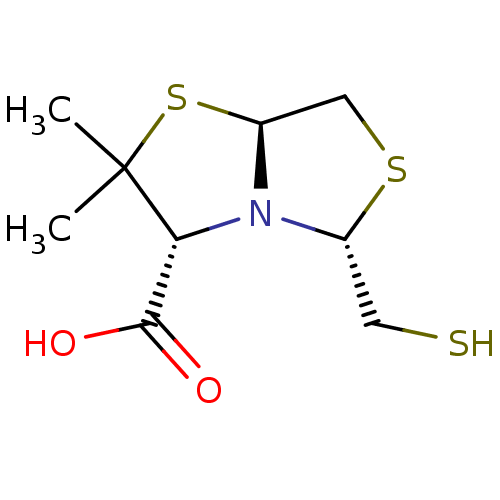 (L-VC26) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50363879 (CHEMBL1947269) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50363877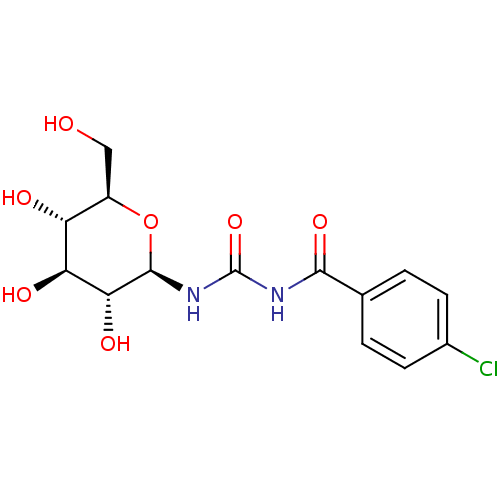 (CHEMBL1232636) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50263771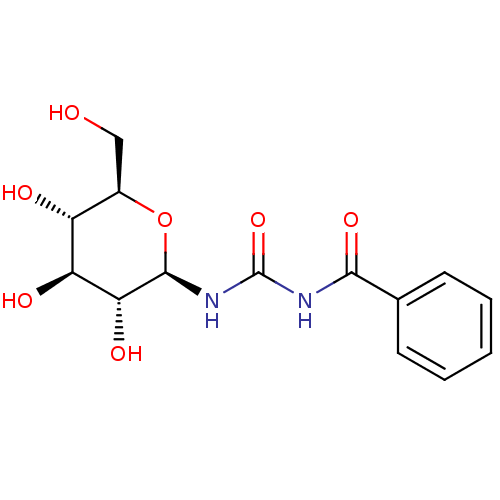 (1-benzoyl-3-((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153700 (L-VC26) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50263769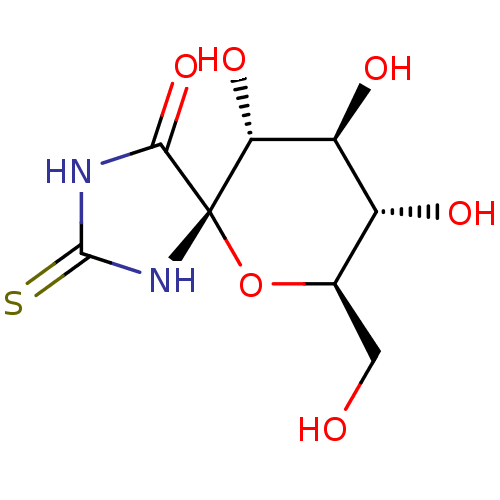 ((5S,7R,8S,9S,10R)-8,9,10-Trihydroxy-7-hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153699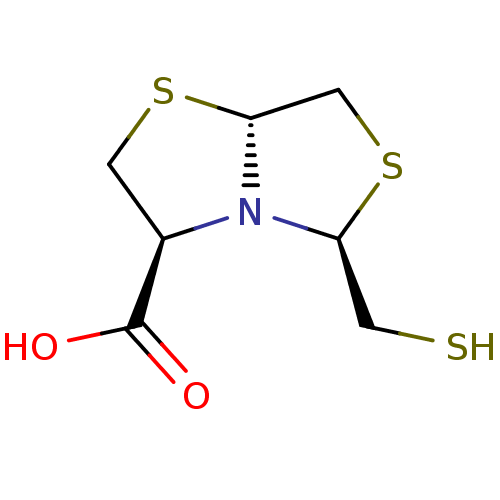 (D-CS319) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153698 (L-CS319) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50295845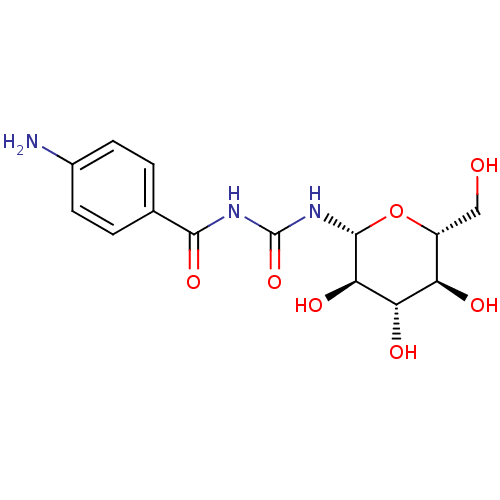 (4-amino-N-((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50295846 (4-hydroxy-N-((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50263723 (CHEMBL488967 | N-((2R,3R,4S,5S,6R)-3,4,5-trihydrox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153699 (D-CS319) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| VIM-24 (Klebsiella pneumoniae (Enterobacteria)) | BDBM153701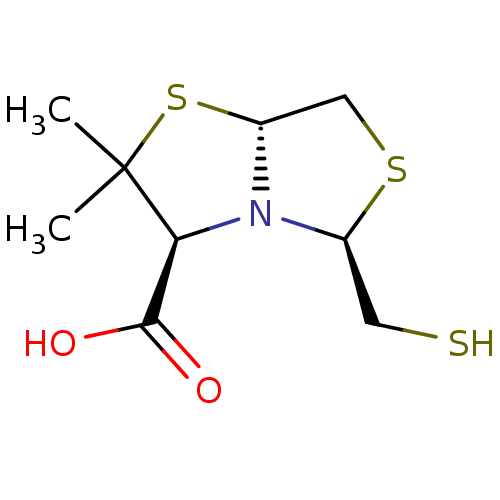 (D-VC26) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase VIM-2 (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM153701 (D-VC26) | PDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Veterans Affairs Medical Center | Assay Description The Ki for each inhibitor was determined by direct competition assays under steady-state conditions. The initial velocity was measured in the presenc... | Biochemistry 54: 3183-96 (2015) Article DOI: 10.1021/acs.biochem.5b00106 BindingDB Entry DOI: 10.7270/Q2513WZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50363872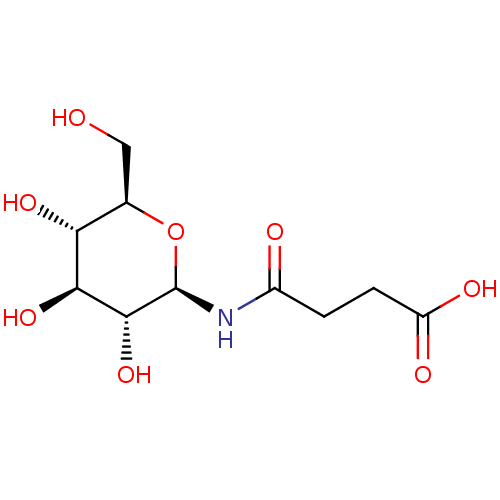 (CHEMBL1946930) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50240802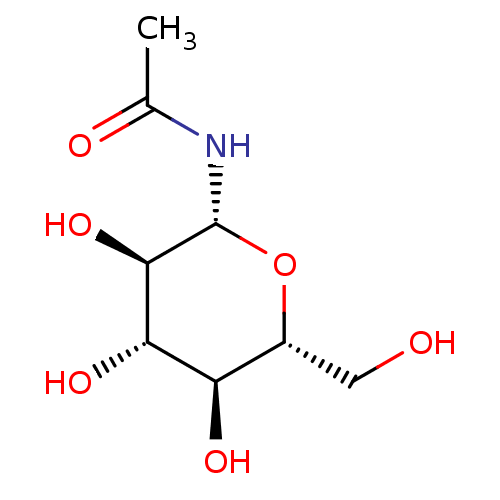 (1-N-ACETYL-BETA-D-GLUCOSAMINE | CHEMBL335315 | N-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50363871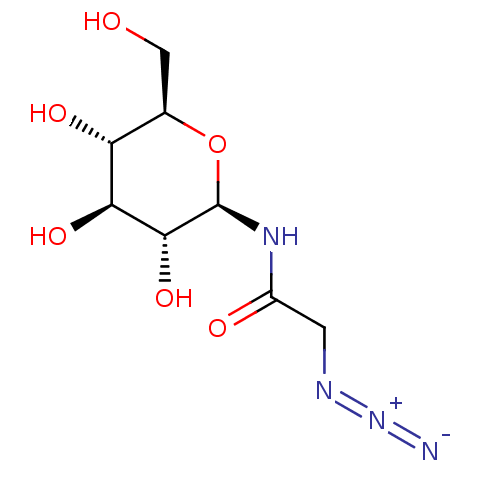 (CHEMBL1232255) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50363869 (CHEMBL1234850) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50240801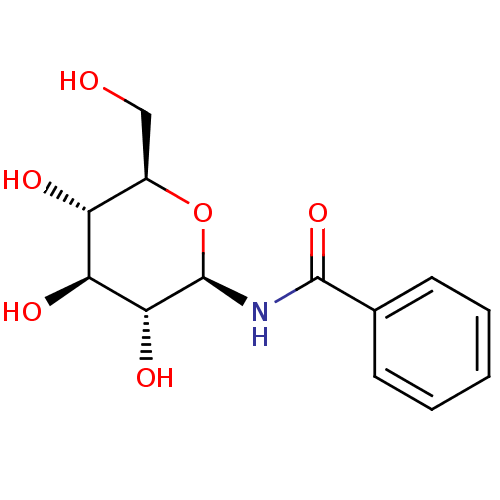 (CHEMBL131967 | N-((2R,3R,4S,5S,6R)-3,4,5-trihydrox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50363881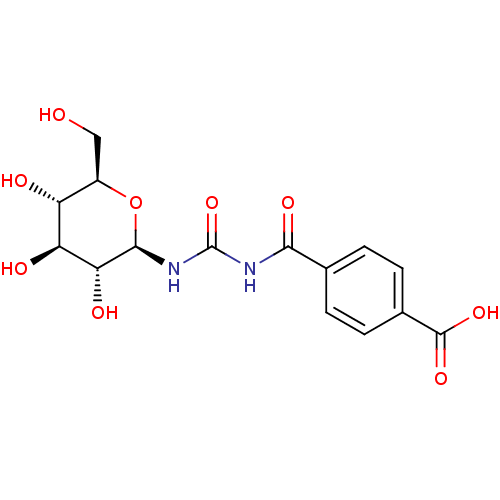 (CHEMBL1947271) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50363875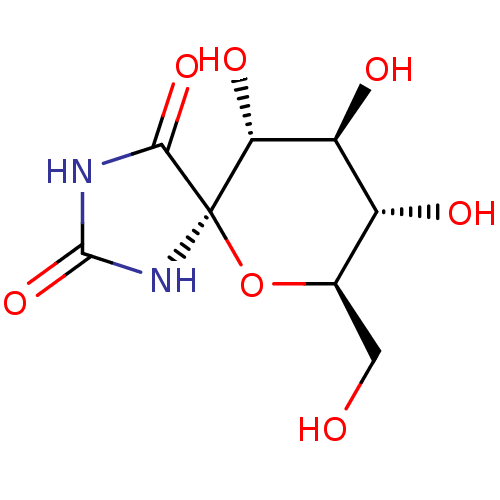 (CHEMBL595439) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Similars | DrugBank Article PubMed | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50363870 (CHEMBL132020) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50363880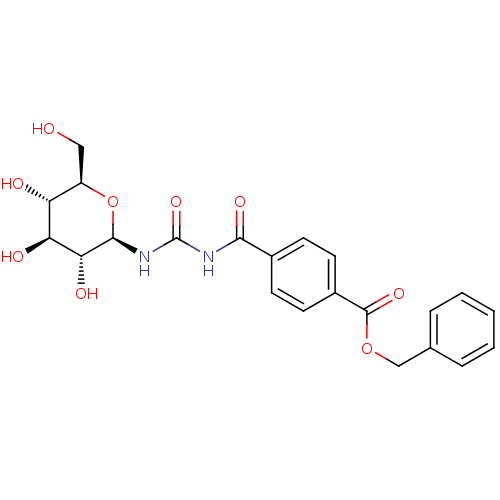 (CHEMBL1947270) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50363873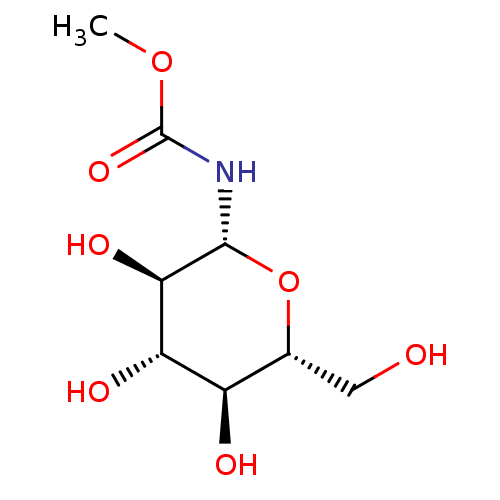 (CHEMBL134529) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50263770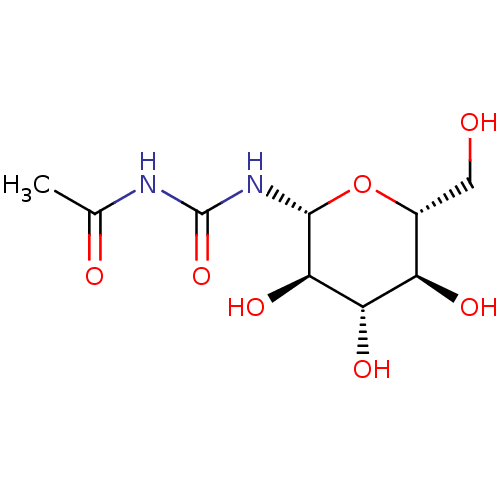 (1-acetyl-3-((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50363874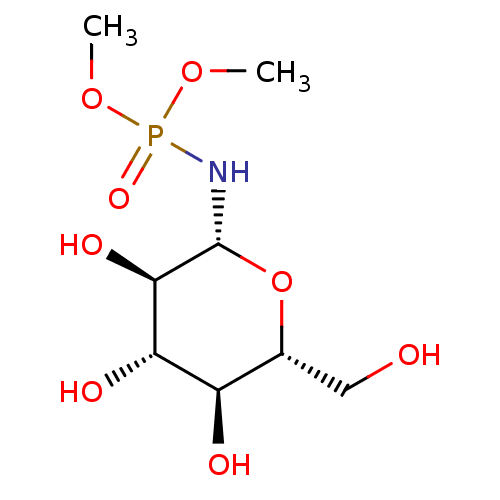 (CHEMBL1233057) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | 5.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Debrecen Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase b assessed as inorganic phosphate release at pH 6.8 | Bioorg Med Chem 20: 1801-16 (2012) Article DOI: 10.1016/j.bmc.2011.12.059 BindingDB Entry DOI: 10.7270/Q2SN09DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50443493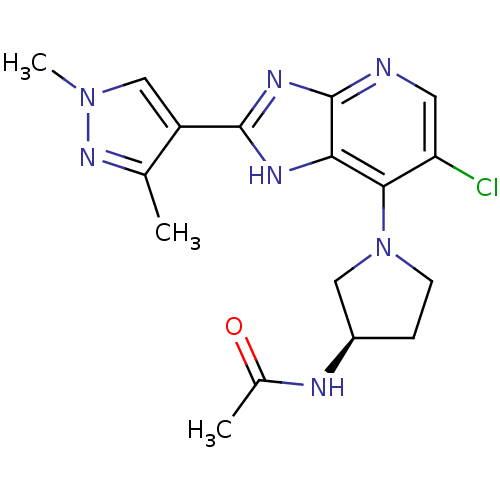 (CHEMBL3087777) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full-length aurora-B (unknown origin) using 5FAMLRRASLG-CONH2 as substrate after 60 mins | J Med Chem 56: 9122-35 (2013) Article DOI: 10.1021/jm401115g BindingDB Entry DOI: 10.7270/Q2R78GPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50318595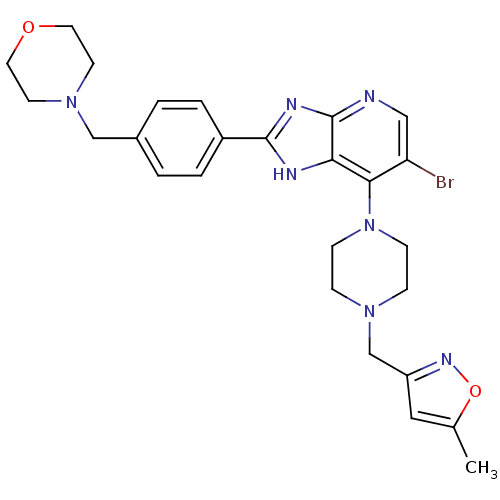 (6-Bromo-7-[4-(5-methyl-isoxazol-3-ylmethyl)-pipera...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant Aurora A expressed in baculovirus system | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50318610 (6-Bromo-7-[4-(5-methyl-isoxazol-3-ylmethyl)-pipera...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant Aurora A expressed in baculovirus system | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50318575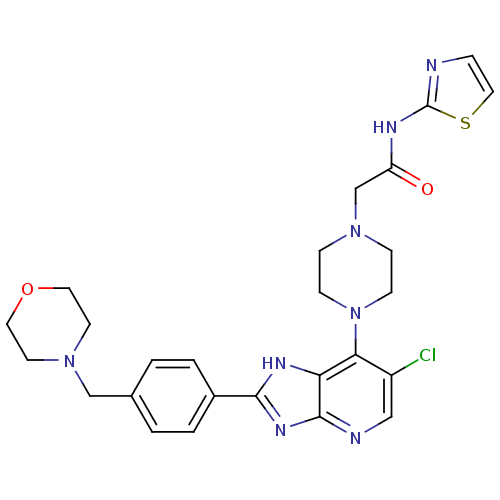 (2-{4-[6-Chloro-2-(4-morpholin-4-ylmethylphenyl)-3H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant Aurora A expressed in baculovirus system | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50443493 (CHEMBL3087777) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of N-terminal HIS-tagged aurora-A (unknown origin) using 5FAM-LRRASLG-CONH2 as substrate after 60 mins | J Med Chem 56: 9122-35 (2013) Article DOI: 10.1021/jm401115g BindingDB Entry DOI: 10.7270/Q2R78GPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50318601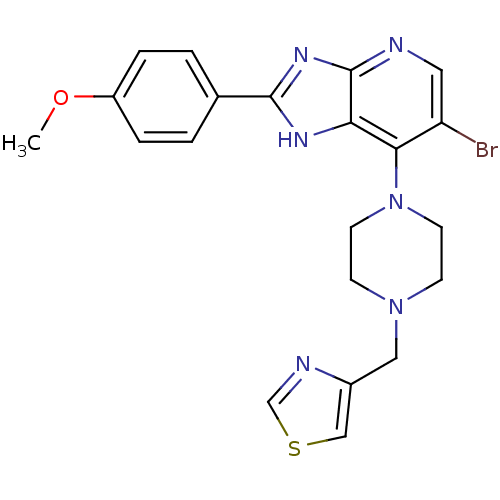 (4-((4-(6-Bromo-2-(4-methoxyphenyl)-3H-imidazo[4,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant Aurora A expressed in baculovirus system | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50401448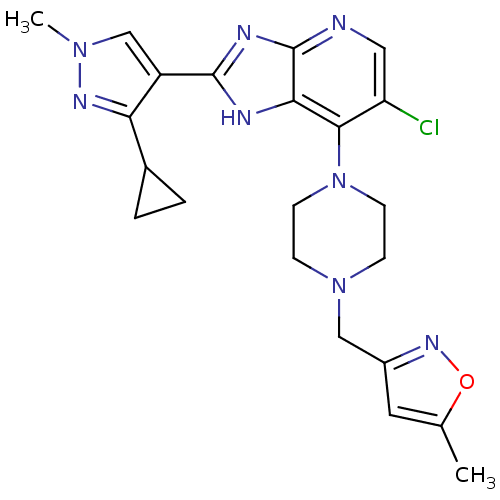 (CHEMBL2203764) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to Aurora A kinase | J Med Chem 55: 8721-34 (2012) Article DOI: 10.1021/jm300952s BindingDB Entry DOI: 10.7270/Q22J6D2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50443510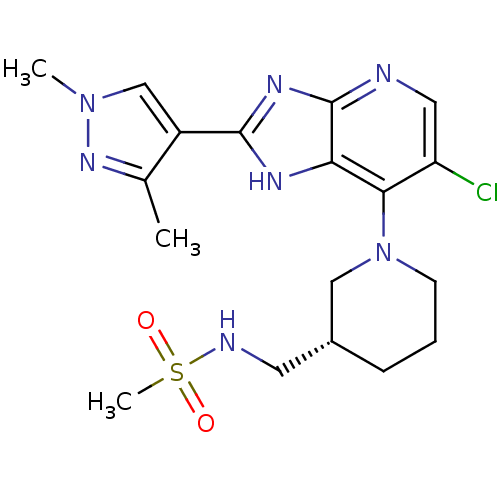 (CHEMBL3087779) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of N-terminal HIS-tagged aurora-A (unknown origin) using 5FAM-LRRASLG-CONH2 as substrate after 60 mins | J Med Chem 56: 9122-35 (2013) Article DOI: 10.1021/jm401115g BindingDB Entry DOI: 10.7270/Q2R78GPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50443508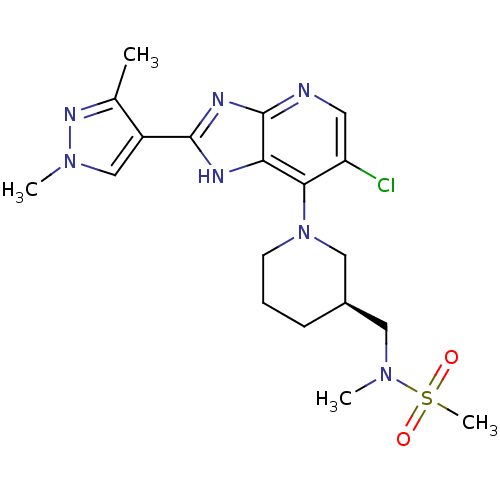 (CHEMBL3087781) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of N-terminal HIS-tagged aurora-A (unknown origin) using 5FAM-LRRASLG-CONH2 as substrate after 60 mins | J Med Chem 56: 9122-35 (2013) Article DOI: 10.1021/jm401115g BindingDB Entry DOI: 10.7270/Q2R78GPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50318588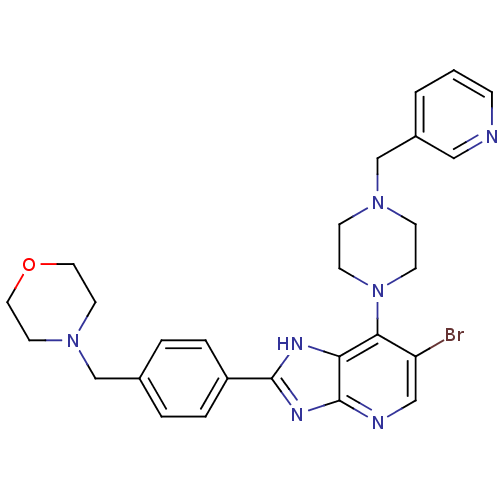 (6-Bromo-2-(4-morpholin-4-ylmethyl-phenyl)-7-(4-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant Aurora A expressed in baculovirus system | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50318602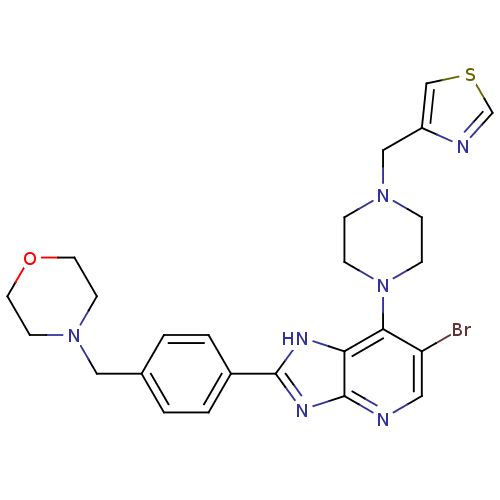 (4-(4-(6-Bromo-7-(4-(thiazol-4-ylmethyl)piperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant Aurora A expressed in baculovirus system | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50318590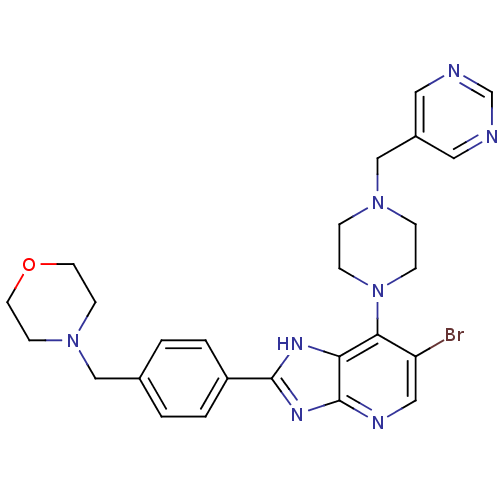 (4-(4-(6-Bromo-7-(4-(pyrimidin-5-ylmethyl)piperazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant Aurora A expressed in baculovirus system | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50401447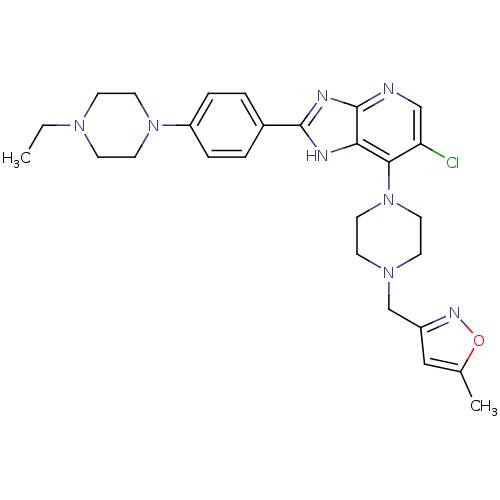 (CHEMBL2203767) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to Aurora A kinase | J Med Chem 55: 8721-34 (2012) Article DOI: 10.1021/jm300952s BindingDB Entry DOI: 10.7270/Q22J6D2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50401446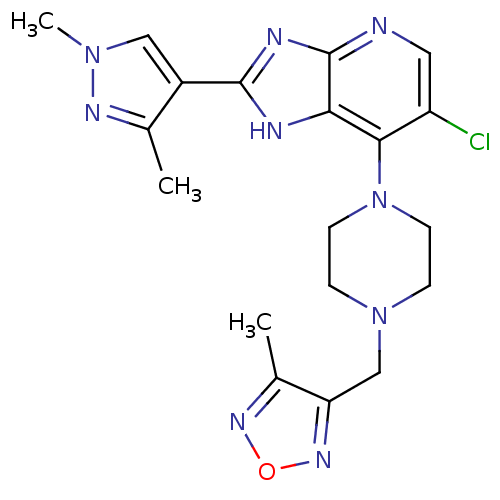 (CHEMBL2207499) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to Aurora A kinase | J Med Chem 55: 8721-34 (2012) Article DOI: 10.1021/jm300952s BindingDB Entry DOI: 10.7270/Q22J6D2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50443492 (CHEMBL3087778) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full-length aurora-B (unknown origin) using 5FAMLRRASLG-CONH2 as substrate after 60 mins | J Med Chem 56: 9122-35 (2013) Article DOI: 10.1021/jm401115g BindingDB Entry DOI: 10.7270/Q2R78GPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50401445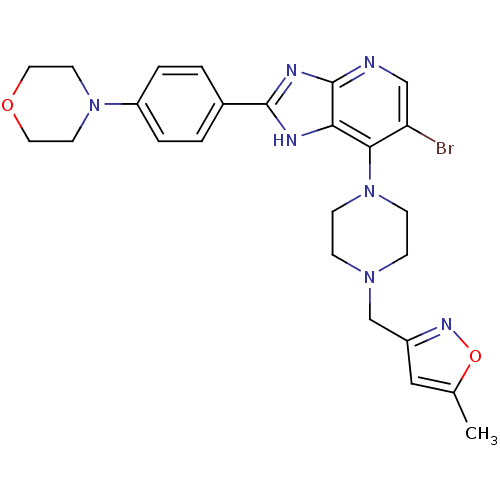 (CHEMBL2203765) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to Aurora A kinase | J Med Chem 55: 8721-34 (2012) Article DOI: 10.1021/jm300952s BindingDB Entry DOI: 10.7270/Q22J6D2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50443509 (CHEMBL3087780) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of N-terminal HIS-tagged aurora-A (unknown origin) using 5FAM-LRRASLG-CONH2 as substrate after 60 mins | J Med Chem 56: 9122-35 (2013) Article DOI: 10.1021/jm401115g BindingDB Entry DOI: 10.7270/Q2R78GPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 309 total ) | Next | Last >> |