

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159366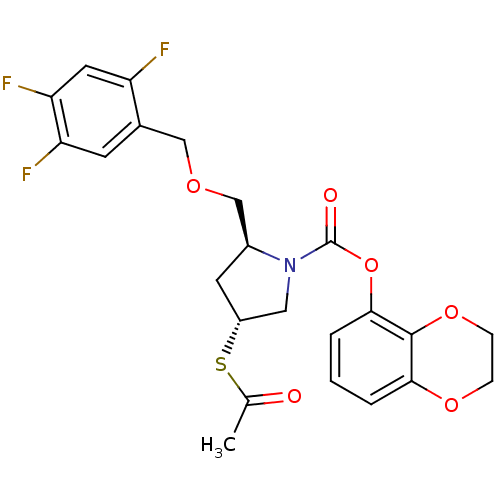 ((2S,4R)-4-Acetylsulfanyl-2-(2,4,5-trifluoro-benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 by RIA | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159365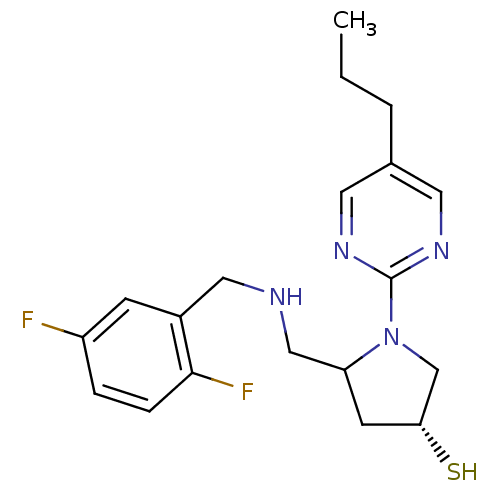 ((3R,5S)-5-{[(2,5-difluorobenzyl)amino]methyl}-1-(5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 by RIA | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50098118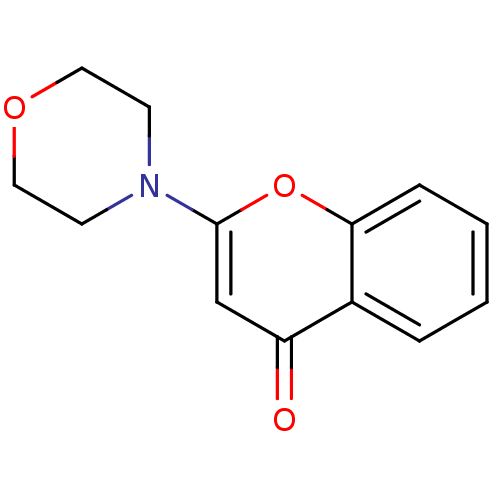 ((2S,4R)-4-Mercapto-2-(2,4,5-trifluoro-benzyloxymet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 by RIA | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159364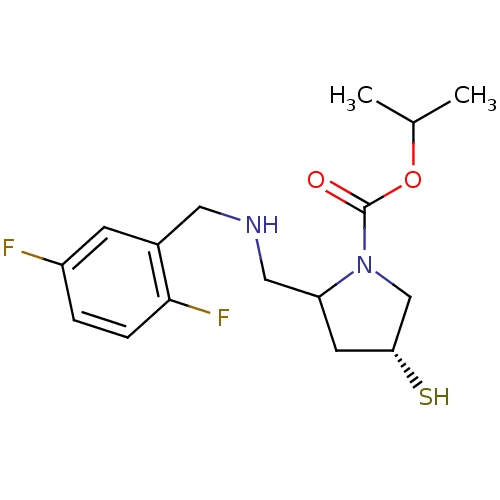 (CHEMBL192441 | isopropyl (2S,4R)-2-{[(2,5-difluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 by RIA | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159367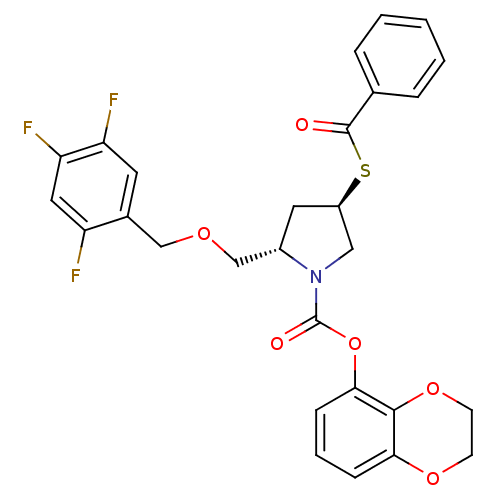 ((2S,4R)-4-Benzoylsulfanyl-2-(2,4,5-trifluoro-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49.7 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 by RIA | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159368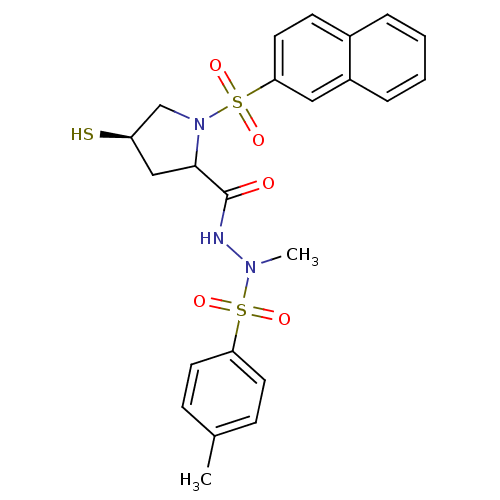 ((4R)-N'-methyl-N'-[(4-methylbenzene)sulfonyl]-1-(n...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61.3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 by RIA | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50098118 ((2S,4R)-4-Mercapto-2-(2,4,5-trifluoro-benzyloxymet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 expressed in MDCK cells | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159365 ((3R,5S)-5-{[(2,5-difluorobenzyl)amino]methyl}-1-(5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72.9 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 expressed in MDCK cells | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159366 ((2S,4R)-4-Acetylsulfanyl-2-(2,4,5-trifluoro-benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 expressed in MDCK cells | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159363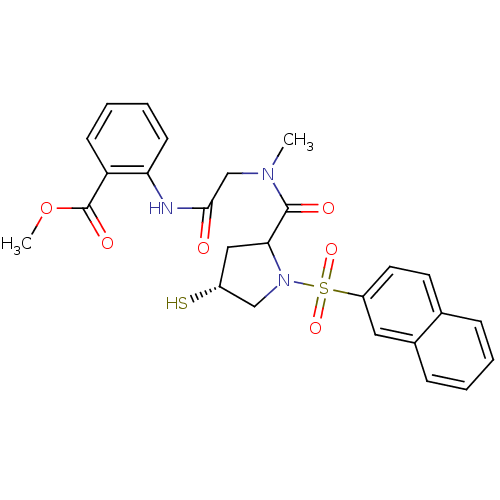 (CHEMBL177000 | methyl 2-(2-{N-methyl-1-[(4R)-1-(na...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80.8 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 by RIA | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114917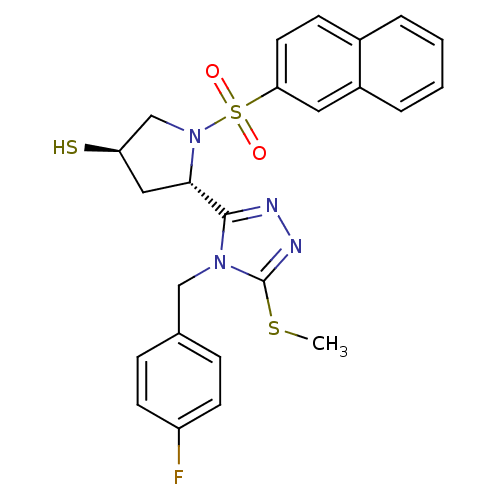 ((3R,5S)-5-[4-(4-Fluoro-benzyl)-5-methylsulfanyl-4H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114905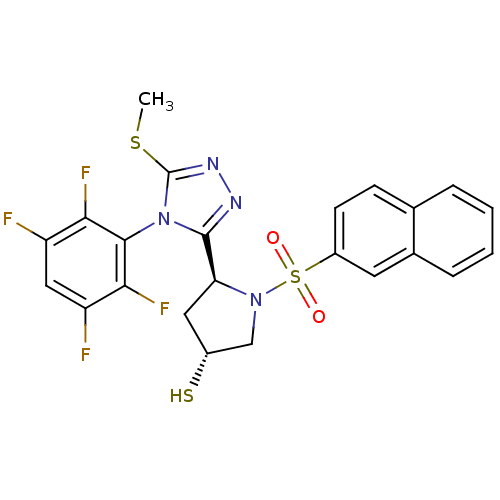 ((3R,5S)-5-[5-Methylsulfanyl-4-(2,3,5,6-tetrafluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114911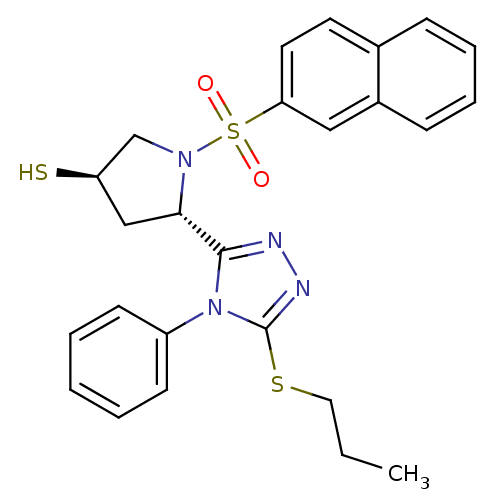 ((3R,5S)-1-(Naphthalene-2-sulfonyl)-5-(4-phenyl-5-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114908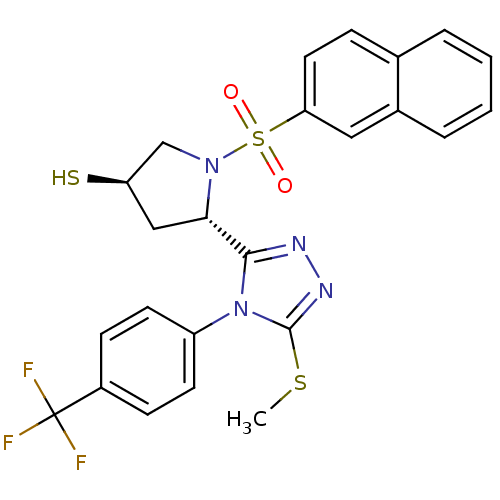 ((3R,5S)-5-[5-Methylsulfanyl-4-(4-trifluoromethyl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159367 ((2S,4R)-4-Benzoylsulfanyl-2-(2,4,5-trifluoro-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 481 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 expressed in MDCK cells | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114915 ((3R,5S)-5-(5-Methylsulfanyl-4-phenyl-4H-[1,2,4]tri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114904 ((3R,5S)-5-(5-Cyclopropylmethylsulfanyl-4-phenyl-4H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114914 ((3R,5S)-5-(5-Isobutylsulfanyl-4-phenyl-4H-[1,2,4]t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 by RIA | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159364 (CHEMBL192441 | isopropyl (2S,4R)-2-{[(2,5-difluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 expressed in MDCK cells | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114916 ((3R,5S)-5-[4-(3-Chloro-4-fluoro-phenyl)-5-methylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114919 ((3R,5S)-5-(5-Methylsulfanyl-4-phenyl-4H-[1,2,4]tri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114910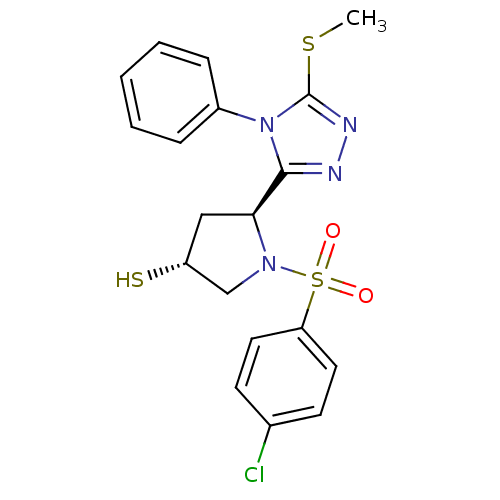 ((3R,5S)-1-(4-Chloro-benzenesulfonyl)-5-(5-methylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114906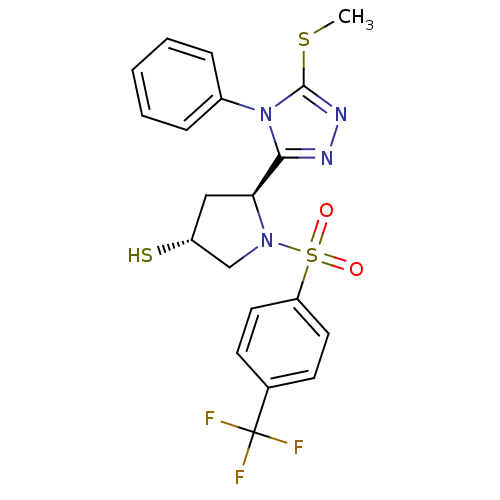 ((3R,5S)-5-(5-Methylsulfanyl-4-phenyl-4H-[1,2,4]tri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159368 ((4R)-N'-methyl-N'-[(4-methylbenzene)sulfonyl]-1-(n...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 expressed in MDCK cells | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114912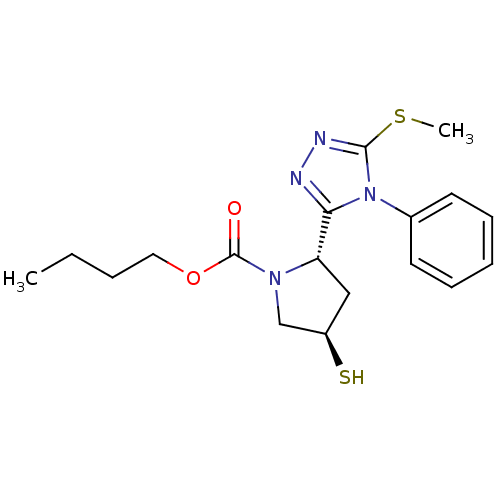 ((2S,4R)-4-Mercapto-2-(5-methylsulfanyl-4-phenyl-4H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114909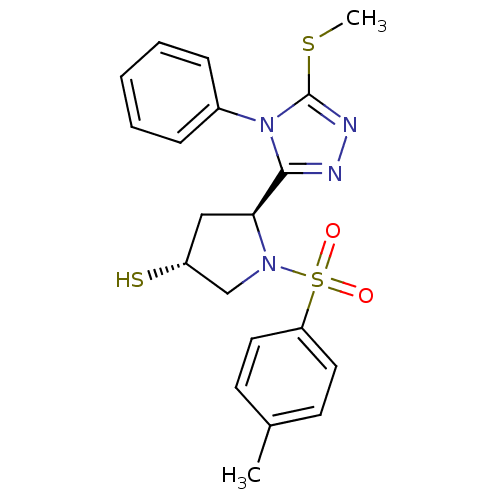 ((3R,5S)-5-(5-Methylsulfanyl-4-phenyl-4H-[1,2,4]tri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114907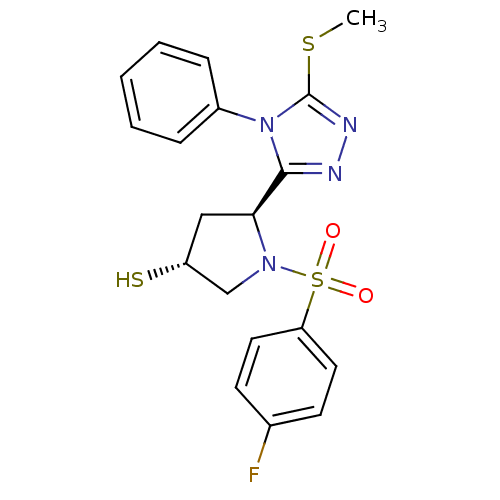 ((3R,5S)-1-(4-Fluoro-benzenesulfonyl)-5-(5-methylsu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159363 (CHEMBL177000 | methyl 2-(2-{N-methyl-1-[(4R)-1-(na...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 expressed in MDCK cells | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50098118 ((2S,4R)-4-Mercapto-2-(2,4,5-trifluoro-benzyloxymet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human angiotensin I converting enzyme | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50159368 ((4R)-N'-methyl-N'-[(4-methylbenzene)sulfonyl]-1-(n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human angiotensin I converting enzyme | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114918 ((3R,5S)-1-Methanesulfonyl-5-(5-methylsulfanyl-4-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50114913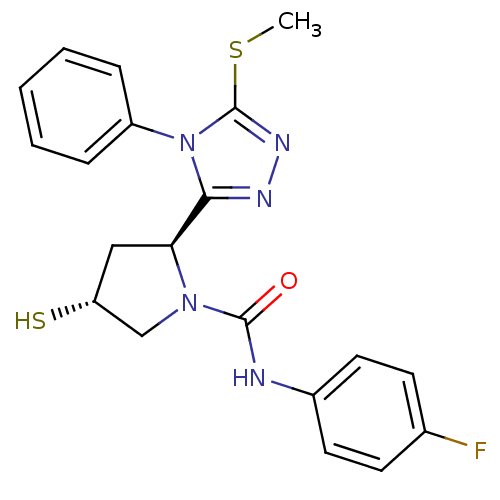 ((2S,4R)-4-Mercapto-2-(5-methylsulfanyl-4-phenyl-4H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Tested for inhibitory concentration against human Endothelin-converting enzyme 1 at 10 microM | Bioorg Med Chem Lett 12: 1727-30 (2002) BindingDB Entry DOI: 10.7270/Q2XP748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159369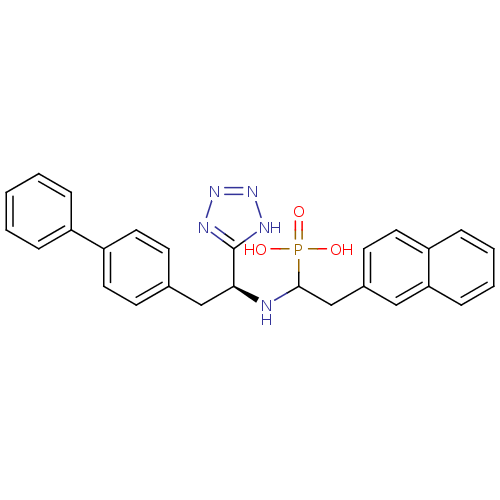 (CGS-31,4447 | CHEMBL415967 | {1-[(S)-2-Biphenyl-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 by RIA | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50159365 ((3R,5S)-5-{[(2,5-difluorobenzyl)amino]methyl}-1-(5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human angiotensin I converting enzyme | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50159363 (CHEMBL177000 | methyl 2-(2-{N-methyl-1-[(4R)-1-(na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human angiotensin I converting enzyme | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50159368 ((4R)-N'-methyl-N'-[(4-methylbenzene)sulfonyl]-1-(n...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against Neprilysin | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50159363 (CHEMBL177000 | methyl 2-(2-{N-methyl-1-[(4R)-1-(na...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against Neprilysin | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50159366 ((2S,4R)-4-Acetylsulfanyl-2-(2,4,5-trifluoro-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human angiotensin I converting enzyme | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50159364 (CHEMBL192441 | isopropyl (2S,4R)-2-{[(2,5-difluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human angiotensin I converting enzyme | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50159367 ((2S,4R)-4-Benzoylsulfanyl-2-(2,4,5-trifluoro-benzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human angiotensin I converting enzyme | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50159365 ((3R,5S)-5-{[(2,5-difluorobenzyl)amino]methyl}-1-(5...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against Neprilysin | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50098118 ((2S,4R)-4-Mercapto-2-(2,4,5-trifluoro-benzyloxymet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against Neprilysin | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50159367 ((2S,4R)-4-Benzoylsulfanyl-2-(2,4,5-trifluoro-benzy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against Neprilysin | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50159366 ((2S,4R)-4-Acetylsulfanyl-2-(2,4,5-trifluoro-benzyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against Neprilysin | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50159364 (CHEMBL192441 | isopropyl (2S,4R)-2-{[(2,5-difluoro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against Neprilysin | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||