

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor receptor superfamily member 16 (Homo sapiens (Human)) | BDBM50029495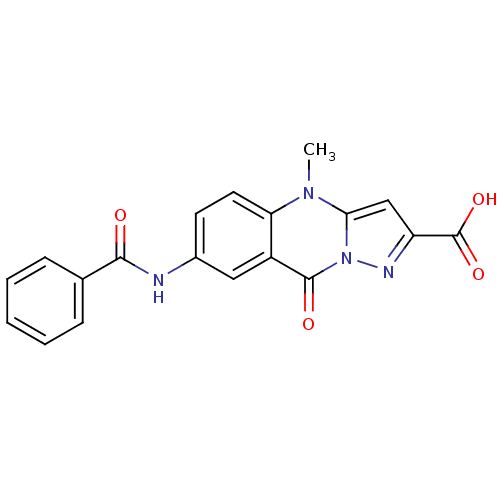 (7-Benzoylamino-4-methyl-9-oxo-4,9-dihydro-pyrazolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]- NGF binding to extracellular domain of p75 receptor | J Med Chem 38: 4439-45 (1995) BindingDB Entry DOI: 10.7270/Q2W37V9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human PBMC cells assessed as inhibition of CCL2-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human PBMC cells assessed as inhibition of CCL4-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339635 (1-(1,3-Dimethyl-1H-pyrazolo[3,4-b]pyridine-5-carbo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339635 (1-(1,3-Dimethyl-1H-pyrazolo[3,4-b]pyridine-5-carbo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50230839 (CHEMBL15087) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 754 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition against DNA gyrase | J Med Chem 36: 871-82 (1993) Article DOI: 10.1021/jm00059a012 BindingDB Entry DOI: 10.7270/Q22R3VDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339630 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(3-isopropox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50469854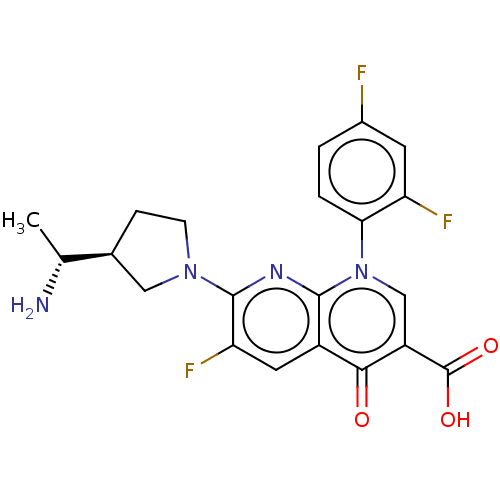 (CHEMBL2110225) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition against DNA gyrase | J Med Chem 36: 871-82 (1993) Article DOI: 10.1021/jm00059a012 BindingDB Entry DOI: 10.7270/Q22R3VDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor receptor superfamily member 16 (Homo sapiens (Human)) | BDBM50029497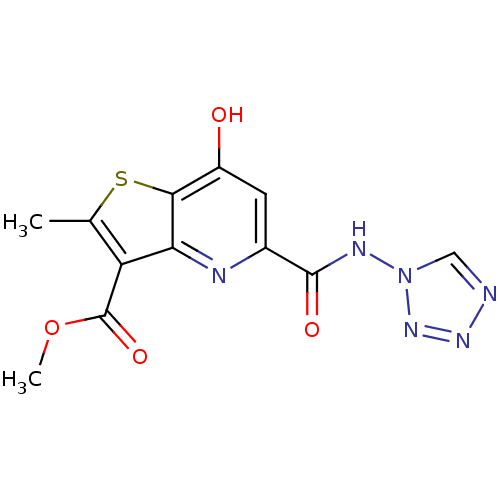 (2-Methyl-7-oxo-5-(tetrazol-1-ylcarbamoyl)-4,7-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]- NGF binding to extracellular domain of p75 receptor | J Med Chem 38: 4439-45 (1995) BindingDB Entry DOI: 10.7270/Q2W37V9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50469849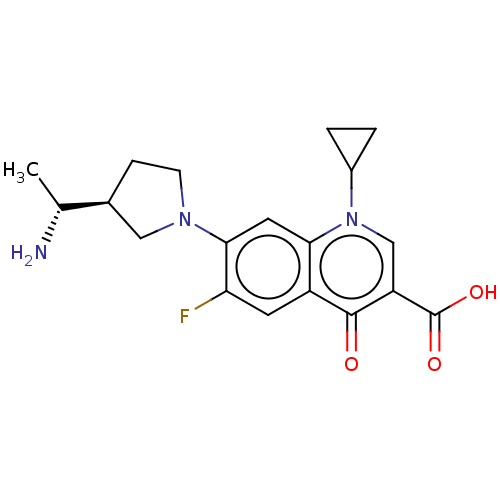 (CHEMBL2110220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition against DNA gyrase | J Med Chem 36: 871-82 (1993) Article DOI: 10.1021/jm00059a012 BindingDB Entry DOI: 10.7270/Q22R3VDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50469849 (CHEMBL2110220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition against DNA gyrase | J Med Chem 36: 871-82 (1993) Article DOI: 10.1021/jm00059a012 BindingDB Entry DOI: 10.7270/Q22R3VDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Mus musculus) | BDBM50339635 (1-(1,3-Dimethyl-1H-pyrazolo[3,4-b]pyridine-5-carbo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in mouse spleen cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50469845 (CHEMBL15296) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition against DNA gyrase | J Med Chem 36: 871-82 (1993) Article DOI: 10.1021/jm00059a012 BindingDB Entry DOI: 10.7270/Q22R3VDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339628 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(3,4-dichlor...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339629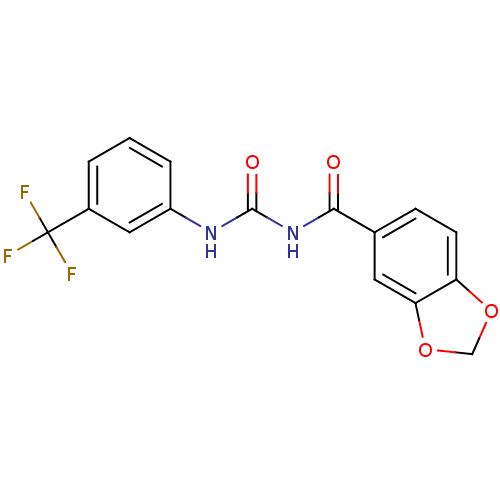 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(3-(trifluor...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339627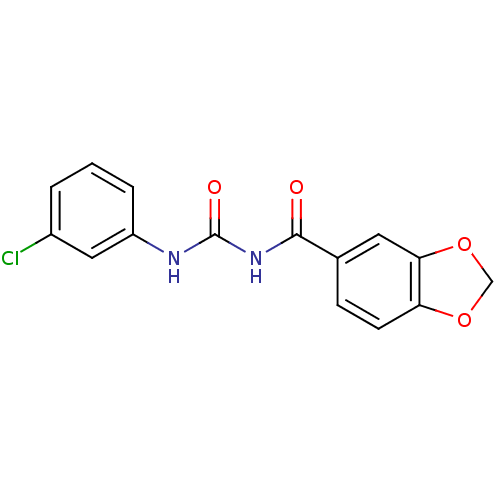 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(3-chlorophe...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50469855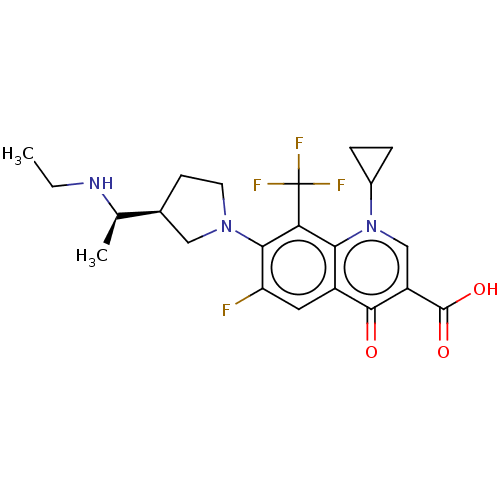 (CHEMBL2110328) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition against DNA gyrase | J Med Chem 36: 871-82 (1993) Article DOI: 10.1021/jm00059a012 BindingDB Entry DOI: 10.7270/Q22R3VDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50469846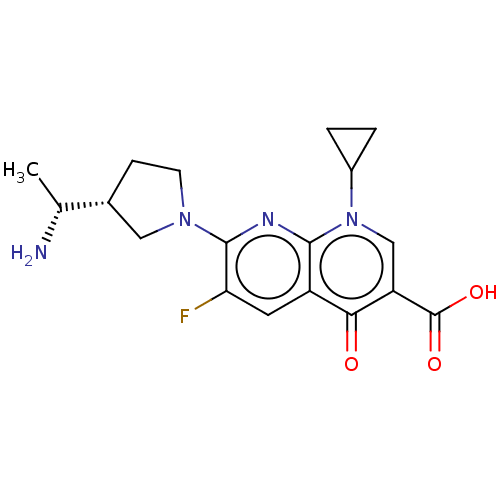 (CHEMBL2110221) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition against DNA gyrase | J Med Chem 36: 871-82 (1993) Article DOI: 10.1021/jm00059a012 BindingDB Entry DOI: 10.7270/Q22R3VDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50469848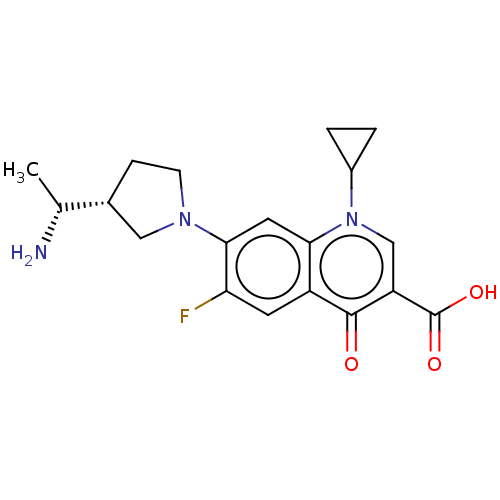 (CHEMBL3350260) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition against DNA gyrase | J Med Chem 36: 871-82 (1993) Article DOI: 10.1021/jm00059a012 BindingDB Entry DOI: 10.7270/Q22R3VDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50469853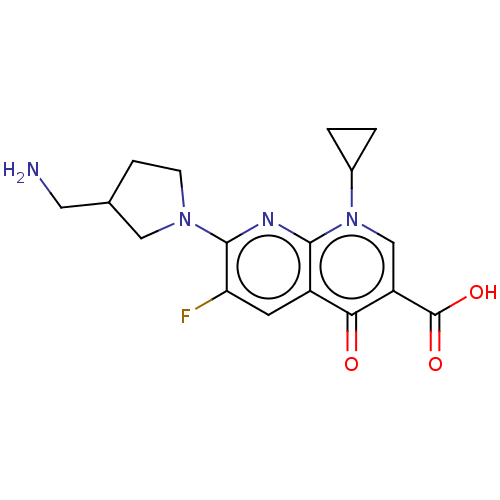 (CHEMBL15314) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition against DNA gyrase | J Med Chem 36: 871-82 (1993) Article DOI: 10.1021/jm00059a012 BindingDB Entry DOI: 10.7270/Q22R3VDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50230833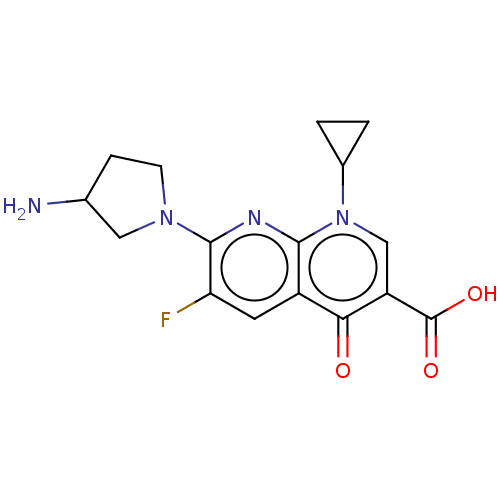 (CHEMBL276583) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition against DNA gyrase | J Med Chem 36: 871-82 (1993) Article DOI: 10.1021/jm00059a012 BindingDB Entry DOI: 10.7270/Q22R3VDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor receptor superfamily member 16 (Homo sapiens (Human)) | BDBM50029494 (2-Methyl-7-oxo-4,7-dihydro-thieno[3,2-b]pyridine-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]- NGF binding to extracellular domain of p75 receptor | J Med Chem 38: 4439-45 (1995) BindingDB Entry DOI: 10.7270/Q2W37V9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50230842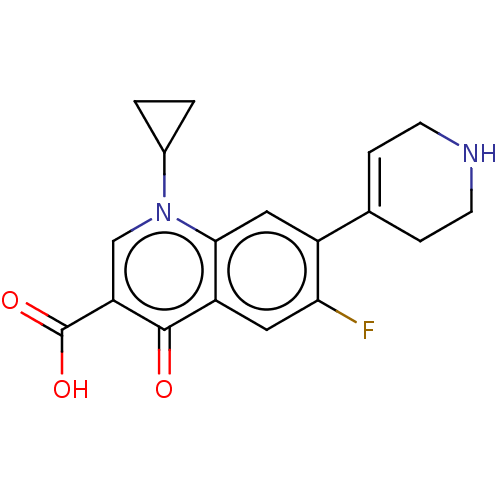 (CHEMBL58226) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of the gyrase mediated cleavage of supercoiled DNA | J Med Chem 36: 1964-70 (1993) Article DOI: 10.1021/jm00066a005 BindingDB Entry DOI: 10.7270/Q2F191XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50469847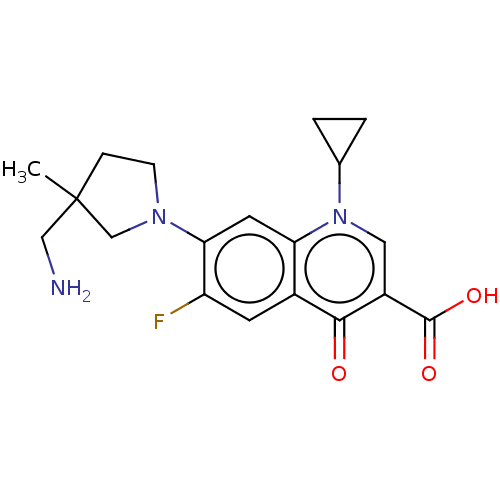 (CHEMBL278648) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition against DNA gyrase | J Med Chem 36: 871-82 (1993) Article DOI: 10.1021/jm00059a012 BindingDB Entry DOI: 10.7270/Q22R3VDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339635 (1-(1,3-Dimethyl-1H-pyrazolo[3,4-b]pyridine-5-carbo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human PBMC cells assessed as inhibition of CCL4-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor receptor superfamily member 16 (Homo sapiens (Human)) | BDBM50029496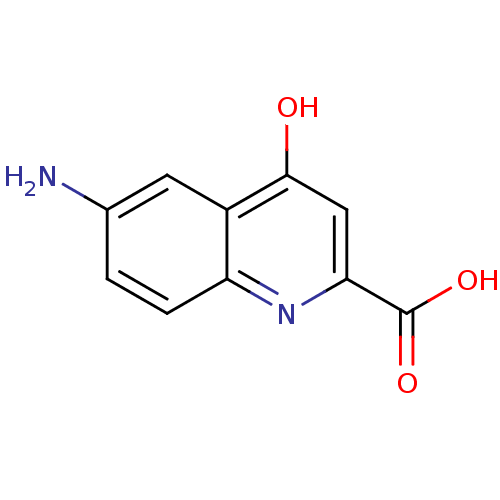 (6-Amino-4-oxo-1,4-dihydro-quinoline-2-carboxylic a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]- NGF binding to extracellular domain of p75 receptor | J Med Chem 38: 4439-45 (1995) BindingDB Entry DOI: 10.7270/Q2W37V9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339626 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(3-chloro-4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50153034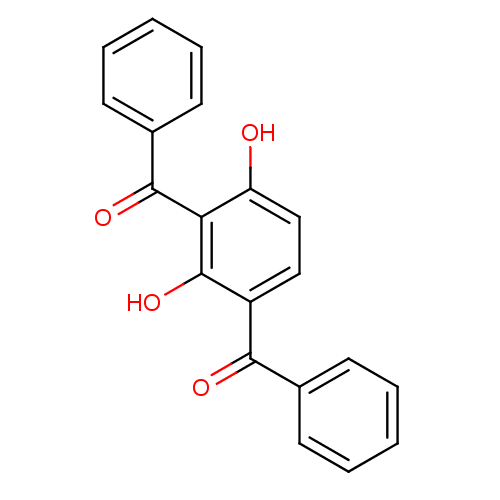 ((3-Benzoyl-2,6-dihydroxy-phenyl)-phenyl-methanone ...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against ovine cyclooxygenase-1 (COX-1) | J Med Chem 47: 4875-80 (2004) Article DOI: 10.1021/jm049950b BindingDB Entry DOI: 10.7270/Q24X578J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50469850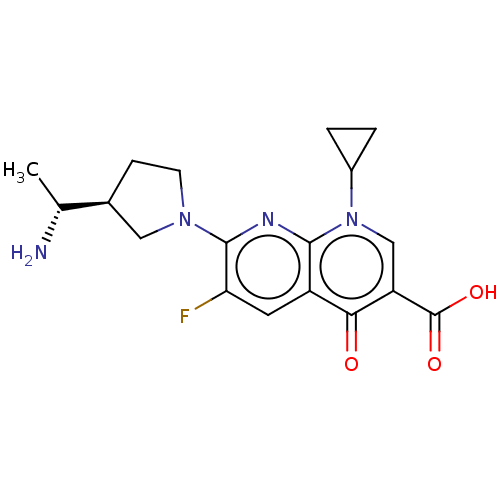 (CHEMBL2110321) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition against DNA gyrase | J Med Chem 36: 871-82 (1993) Article DOI: 10.1021/jm00059a012 BindingDB Entry DOI: 10.7270/Q22R3VDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50469850 (CHEMBL2110321) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition against DNA gyrase | J Med Chem 36: 871-82 (1993) Article DOI: 10.1021/jm00059a012 BindingDB Entry DOI: 10.7270/Q22R3VDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50469844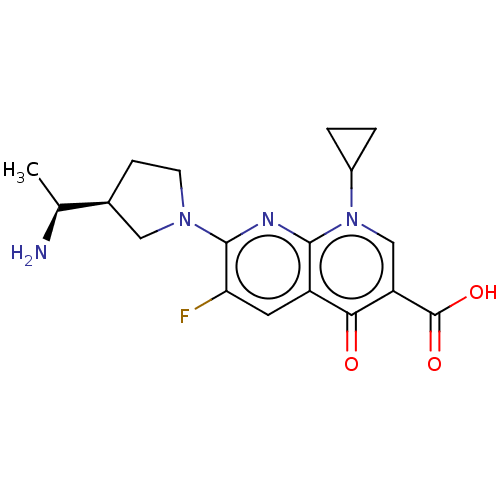 (CHEMBL3350261) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition against DNA gyrase | J Med Chem 36: 871-82 (1993) Article DOI: 10.1021/jm00059a012 BindingDB Entry DOI: 10.7270/Q22R3VDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50230834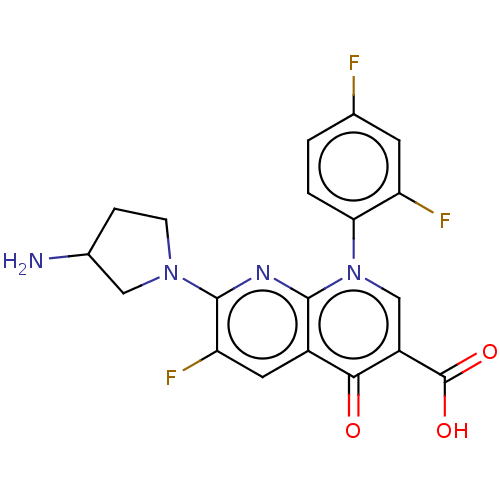 (ABBOTT-61827 | CHEBI:77581 | Ozex | Tosufloxacin |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of the gyrase mediated cleavage of supercoiled DNA | J Med Chem 36: 1964-70 (1993) Article DOI: 10.1021/jm00066a005 BindingDB Entry DOI: 10.7270/Q2F191XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50230839 (CHEMBL15087) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of the gyrase mediated cleavage of supercoiled DNA | J Med Chem 36: 1964-70 (1993) Article DOI: 10.1021/jm00066a005 BindingDB Entry DOI: 10.7270/Q2F191XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50230840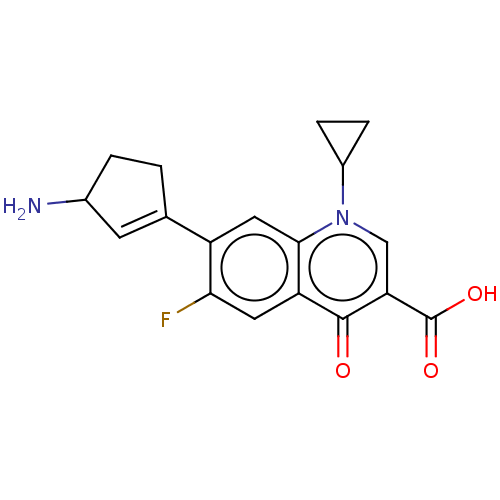 (CHEMBL293970) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of the gyrase mediated cleavage of supercoiled DNA | J Med Chem 36: 1964-70 (1993) Article DOI: 10.1021/jm00066a005 BindingDB Entry DOI: 10.7270/Q2F191XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50230833 (CHEMBL276583) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of the gyrase mediated cleavage of supercoiled DNA | J Med Chem 36: 1964-70 (1993) Article DOI: 10.1021/jm00066a005 BindingDB Entry DOI: 10.7270/Q2F191XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50339635 (1-(1,3-Dimethyl-1H-pyrazolo[3,4-b]pyridine-5-carbo...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in transfected in THP-1 cells assessed as inhibition of CCL17-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in transfected in THP-1 cells assessed as inhibition of CCL17-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50339635 (1-(1,3-Dimethyl-1H-pyrazolo[3,4-b]pyridine-5-carbo...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CXCR1 in human PMN cells assessed as inhibition of CXCL8-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CXCR1 in human PMN cells assessed as inhibition of CXCL8-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 in human PMN cells assessed as inhibition of CXCL8-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Rattus norvegicus) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in rat spleen cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339637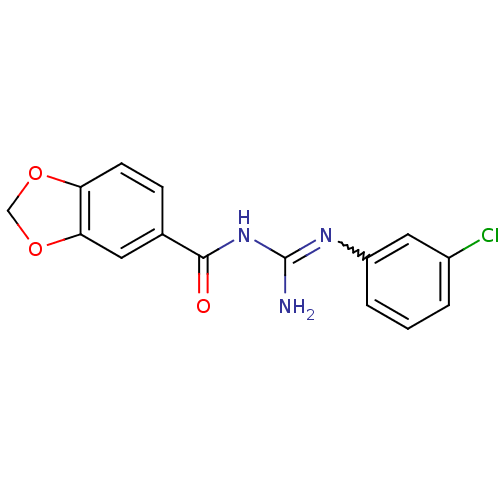 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(3-chlorophe...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339638 (5-(Benzo[d][1,3]dioxol-5-yl)-N-(3,4-dichlorophenyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339639 (1-(Benzo[d][1,3]dioxol-5-yl)-3-(3-chlorobenzoyl)ur...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 in eosinophil cells assessed as inhibition of CCL11-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR1 in eosinophil cells assessed as inhibition of CCL11-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human PBMC cells assessed as inhibition of CCL5-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 in human PBMC cells assessed as inhibition of CCL5-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 72 total ) | Next | Last >> |